Documente Academic
Documente Profesional
Documente Cultură
NBCIE - Fuminwang PDF
Încărcat de
Asip HussinTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
NBCIE - Fuminwang PDF
Încărcat de
Asip HussinDrepturi de autor:
Formate disponibile
J Med Sci 2009;29(3):147-149
http://jms.ndmctsgh.edu.tw/2903147.pdf
Copyright 2009 JMS
Fu-Min Wang, et al.
Nonbullous Congenital Ichthyosiform Erythroderma in a Neonate
Fu-Min Wang, Chih-Chien Wang, Chuen-Ming Le, and Wen-Tsung Lo*
Department of Pediatrics, Tri-Service General Hospital, National Defense Medical Center,
Taipei, Taiwan, Republic of China.
Nonbullous congenital ichthyosiform erythroderma (NBCIE) in newborns usually presents with a transparent covering that
desquamates over 10 to 14 days. We describe a patient admitted to our intensive care unit with a generalized exfoliative
erythroderma and clinical signs and biopsy specimen findings consistent with NBCIE. The patient was born as a collodion
baby and has remarkably improved after antibiotic treatment and application of topical emollients without severe side effects.
Keywords: Nonbullous congenital ichthyosiform erythroderma, staphylococcal scalded skin syndrome, lamellar ichthyosis
INTRODUCTION
Neonatal nonbullous congenital ichthyosiform erythroderma (NBCIE) is an autosomal recessive form of inherited ichthyosis. The incidence of this disorder is about 1 in
300,000 births. Clinically, NBCIE appears as generalized
erythroderma with fine white scales that gradually replace
the collodion membrane. Other associations include
ectropion, eclabium, scalp alopecia, decreased sweating
with heat intolerance, and nail dystrophy. Treatment is
based on the severity of the disease. Topical treatments
including emollients, keratolytics, lactic acid, and propylene glycol are effective, but their application is limited
because of their adverse effect of local irritation. Systemic
use of retinoid is also applicable for the treatment of severe
NBCIE. We describe a premature male newborn who
developed NBCIE with methicillin-resistant Staphylococcus infection, and discuss relevant pathology and issues in
clinical management.
CASE REPORT
A male neonate, weighing 2365 g, was born at GA 36 5/
7 weeks by cesarean section because of preterm premature
rupture of membranes. The Apgar score was 7 and 9 at one
and five minutes, respectively. The mother, who works in
Received: January 21, 2009; Revised: April 2, 2009;
Accepted: April 13, 2009
*
Corresponding author: Wen-Tsung Lo, Department of
Pediatrics, Tri-Service General Hospital, National Defense
Medical Center, No. 325, Sec.2, Cheng-Gong Rd, Taipei
114, Taiwan, Republic of China. Tel: +886-2-87927025;
FAX: +886-2-87927293; E-mail: drluoped@yahoo.com.tw
the surgical intensive care unit of our hospital, was healthy
and had no family history of any hereditary skin disease.
Adequate antibiotic coverage was given to the mother
before delivery. After delivery, the baby was noted to have
a cling-film-like membrane all over his skin. The typical
features of collodion baby were noted. After birth, generalized erythroderma with some epidermal peeling over the
face, trunk and the four limbs was noticed (Figure 1), and
hypothermia (35.7 oC), tachypnea (pulse 170 beats/min),
respiratory retraction and frequent desaturation (SPO2 7080%) were found with suspicions of neonatal sepsis. The
Nikolsky sign was positive. The mucous membranes of the
mouth and anus were unaffected and hair was sparse. There
was no ectropion or eclabium.
Pinnae and nasal passages were normal. No constriction
bands or blisters were noted. Laboratory tests obtained
after delivery showed a normal blood count. Chromosomal
studies revealed a male karyotype (46, XY) with no apparent abnormalities. Cultures from the scalded-skin lesion
and the bloodstream were obtained at day 1 of life. The
culture of the scalded-skin lesion grew methicillin-resistant Staphylococcus aureus (MRSA), whereas the bloodstream culture was sterile. The MRSA grown from the skin
produced toxic shock syndrome toxin-1 (TSST-1) but was
negative for the exfoliative toxins A (ETA) and B (ETB).
A skin biopsy performed at day 10 of life showed no
splitting at the granular layer of the epidermis and no
inflammatory infiltrates, with mild thickening of the
orthokeratotic cornified layer and no epidermolytic
hyperkeratosis. The granular cell layer was slightly
increased. There were no cholesterol crystals found (Figure
2).
Treatment included administration of intravenous antibiotics and fluids. Infection control measures were immediately implemented and included isolation of the infected
147
Nonbullous Congenital Ichthyosiform Erythroderma in a Neonate
Fig. 2 Histologic examination of a skin biopsy specimen
from the left leg lesion shows mild thickening of the
orthokeratotic cornified layer with no epidermolytic
hyperkeratosis (arrows). No characteristic splitting
at the granular layer of the epidermis and no inflammatory infiltrates were found. The granular cell layer
is normal and no cholesterol crystals were found.
(Hematoxylin eosin stain; original magnification
200.)
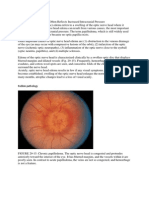
Fig. 1 Clinical photograph showing the extensive areas of
peeling skin characteristic of nonbullous congenital
ichthyosiform erythroderma. A.face, B.axillary, C.
leg .
infant. Vancomycin was administered intravenously for 14
days in accordance with the culture sensitivity report.
Supportive management included Vaseline application for
skin care. Improvement was noted with time as the collodion membrane shed, leaving generalized erythroderma.
After the collodion membrane was shed, progressive improvement was noted with frequent emollient applications
alone. Resolution was near complete three months later,
with only mild residual ichthyosis at the sacral area.
DISCUSSION
Erythroderma is defined as an inflammatory skin disorder affecting more than 90% of the body surface1. NBCIE
is a rare nonblistering disorder characterized by fine grayish-white scales and erythroderma. The disorder is inherited as an autosomal recessive trait except for a few cases
caused by a dominant trait. An affected newborn is frequently born as a collodion baby (covered with oiled
parchment-like shiny skin)2. Other symptoms include
alopecia, deep skin fissures and nail dystrophy but the teeth
and mucosal surfaces are normal3. Hyperkeratosis is par148
ticularly noticeable around the knees, elbows, and ankles.
The face is often markedly involved, including ectropion,
flattening of the ears and nose, and fixation of the lips in an
O-shaped configuration. The disorder is present soon or
shortly after birth and is the most common form of ichthyosis to present as a collodion baby with a glistening membrane resembling sausage skin4. Our patient was covered at
birth in a thick, taut membrane resembling collodion. After
shedding this collodion membrane, generalized scaly erythroderma was apparent and persisted for several weeks.
Since the 1980s, nonbullous autosomal recessive ichthyoses have been divided into two major clinical entities,
NBCIE and lamellar ichthyosis (LI)5. The nature of scaling
and intensity of erythroderma are important clinical features that distinguish NBCIE and LI. After shedding of the
collodion membrane, generalized erythroderma with fine,
white or gray scales, different from the large, dark scales
seen in LI, was evident in our patient6. In some families
with LI, transglutaminase 1 gene mutations have been
identified as causative genetic defects, and transglutaminase
1 is thought to be one of the candidate molecules for
NBCIE7.
NBCIE is unlike bullous congenital ichthyosiform erythroderma (BCIE), which is a severe autosomal dominant
congenital ichthyosis which exhibits widespread blisters
Fu-Min Wang, et al.
and erosions in the erythrodermic skin at birth4. The entire
skin is rarely involved and the scales of epidermolytic
hyperkeratosis are thick, gray-brown, and often verrucous
in areas of flexion over the knees and elbows. Histologically,
predominant vacuolation of the cells is observed in the
middle and upper spinous layers and granular layers. The
vacuolated keratinocytes have large and irregularly shaped
granules. The light microscopic findings are characterized
by hyperkeratosis, papillomatosis, acanthosis and vacuolization of the granular and upper prickle cell layers.
In addition, exfoliative skin diseases are rare in neonates.
When causation by S. aureus is a possibility, scalded-skin
diseases such as staphylococcal scalded-skin syndrome
(SSSS) may be considered. Differential diagnosis of general erythroderma and infectious disease, SSSS and toxic
shock syndrome should be considered. SSSS is caused by
the circulation of ETA and ETB produced by staphylococci in focal infections such as conjunctivitis, omphalitis,
or rhinitis8. Some congenital and neonatal cases (following
chorioamnionitis) have been described9. Within one to two
days, the patients develop a generalized macular and
subsequently erythrodermic rash, which is accompanied
by increased skin tenderness. Skin biopsy of SSSS reveals
a split in the granular layer of the skin, induced proteolysis,
and might exhibit evidence of superantigen activities, such
as epidermolysis and lymphocyte mitogenicity. This precedes the formation of subcorneal blisters, exudation,
crusting, and, finally, generalized exfoliation. In our patient,
SSSS is ruled out by negative findings for ETA and ETB
in microbial studies and the skin biopsy result. Although
TSST-1 was positive, this infant did not fulfill the criteria
for toxic shock syndrome10.
Treatment is symptomatic with a high-humidity
environment, and application of nonocclusive lubricants
may facilitate shedding of the membrane. Emollients remain the mainstay of treatment. Generous and frequent
applications of emollients and keratolytic agents such as
lactic or glycolic acid (5%), urea (10-40%), and retinoic
acid (0.1% cream) may lessen the scaling to some extent,
although these agents produce stinging if applied to fissured skin11,12. The long-term risks of these compounds
(teratogenic effects and toxicity to bone) may limit their
usefulness. Prevention of sunburn and sunstroke is
important. Ectropion requires ophthalmologic care, and, at
times, plastic procedures.
Neonatal morbidity and deaths may be caused by cutaneous infection, aspiration pneumonia (squamous material),
hypothermia, or hypernatremic dehydration from excessive transcutaneous fluid losses resulting from increased
skin permeability.
In conclusion, neonatal exfoliative erythroderma is a
rare condition and can be potentially life threatening irrespective of the cause, and must be carefully differentiated
from ichthyosiform disorders. It is usually difficult to
establish a diagnosis of the condition because of the poor
specificity of the clinical and histological signs, but certain
parameters may be helpful in making the diagnosis. Careful monitoring and management of the patients may improve the outcome.
REFERENCES
1. Burton JL. Eczema, lichenification, prurigo and
erythroderma. In: Champion RH, Burton JL, Ebling
FJG, eds. Rook/Wilkinson/Ebling Textbook of
Dermatology. 5th ed. Vol 1. Oxford: Blackwell Scientific Publications, 1992;537-588.
2. Larregue
M, Gharbi R, Daniel J, Le Marec Y, Civatte
collodion: evolution
J. Le bebe
a propos de 29 cas. Ann
Dermatol Syphiligr 1976;103:31-56.
3. Reed WB, Herwick RP, Harville D, Poster PS, Conant
M. Lamellar ichthyosis of the newborn: a distinct
clinical entity. Its comparison to other ichthyosiform
erythrodermis. Arch Dermatot 1972;105:394-399.
4. Judge MR, Harper JI. The ichthyoses. In: Harper JI, ed.
Inherited Skin Diseases. The Genodermatoses. Oxford:
Butterworth, 1996;69-96.
5. Akiyama M. Severe congenital ichthyosis of the
neonate. Int J Dermatol 1998;37:722-728.
6. Williams ML, Elias PM. Heterogeneity in autosomal
recessive ichthyosis. Arch Dermatol 1985;121:477488.
7. Akiyama M. The pathogenesis of severe congenital
ichthyosis. J Dermatol Sci 1999;21:96-104.
8. Hoeger PH, Harper JI. Neonatal erythroderma: differential diagnosis and management of the red baby.
Arch Dis Child 1998;79:186-191.
9. Loughead JL. Congenital staphylococcal scalded skin
syndrome: report of a case. Pediatr Infect Dis J 1992;
11:413-414.
10. Reingold AL, Hargrett NT, Shands KN. Toxic shock
syndrome surveillance in the United States, 1980 to
1981. Am J Emerg Med 1982;96:875-880.
11. Van Scott EJ, Yee RJ. Control of keratinization with
-hydroxy acid and related compounds. I. Topical
treatment of ichthyotic disorders. Arch Dermatol 1974;
110:586-590.
12. Goldsmith LA, Baden HP. Management and treatment
of ichthyosis. N Engl J Med 1972;286:821-823.
149
S-ar putea să vă placă și
- 1237 3585 1 PBDocument6 pagini1237 3585 1 PBUli Kartika SihalohoÎncă nu există evaluări
- Status Gizi Vs Tensi MahasiswaDocument7 paginiStatus Gizi Vs Tensi MahasiswaAsip HussinÎncă nu există evaluări
- Fashoin ManiaDocument3 paginiFashoin ManiaAsip HussinÎncă nu există evaluări
- Nbcie - GhaziDocument2 paginiNbcie - GhaziAsip HussinÎncă nu există evaluări
- Bullous Congenital Ichthyosiform Erythroderma Case ReportDocument5 paginiBullous Congenital Ichthyosiform Erythroderma Case ReportAsip HussinÎncă nu există evaluări
- 05Document4 pagini05Fauziah RusliÎncă nu există evaluări
- Bullous Congenital Ichthyosiform Erythroderma Case ReportDocument5 paginiBullous Congenital Ichthyosiform Erythroderma Case ReportAsip HussinÎncă nu există evaluări
- TORCH InfectionsDocument37 paginiTORCH InfectionsEylin Halim Rahardjo100% (1)
- Bowel CancerDocument5 paginiBowel CancerAsip HussinÎncă nu există evaluări
- 2011 Combat Infections Guideline J TraumaDocument25 pagini2011 Combat Infections Guideline J TraumaAsip HussinÎncă nu există evaluări
- Breast CancerDocument11 paginiBreast CancerAsip HussinÎncă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5784)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (72)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Group Project: Project Guide:-Prof. Lissy JoseDocument14 paginiGroup Project: Project Guide:-Prof. Lissy JoseAditya ManeÎncă nu există evaluări
- B V DPP (2000) 2 AC 428 House of LordsDocument3 paginiB V DPP (2000) 2 AC 428 House of LordsDevika KhanÎncă nu există evaluări
- Chapter 3 ResearchDocument2 paginiChapter 3 ResearchFrez DejuanÎncă nu există evaluări
- Group Action of PilesDocument19 paginiGroup Action of PilesRachit Khandelwal0% (1)
- UPSC Civil Services Preliminary Exam 2014 SyllabusDocument66 paginiUPSC Civil Services Preliminary Exam 2014 Syllabusvishnuvr143Încă nu există evaluări
- NEJM Review Mar 2018 Autoimmune Polyendocrine SyndromesDocument10 paginiNEJM Review Mar 2018 Autoimmune Polyendocrine SyndromesBrian Almodovar BuenoÎncă nu există evaluări
- Changing Modes of Production in Indian AgricultureDocument2 paginiChanging Modes of Production in Indian Agricultureabhijit488Încă nu există evaluări
- Manual 7pxrDocument204 paginiManual 7pxrMario GomesÎncă nu există evaluări
- Crime Detection and Investigation Ultimate Final CoachingDocument6 paginiCrime Detection and Investigation Ultimate Final CoachingArnel Sali-otÎncă nu există evaluări
- Persona 5 Royal WalkthroughDocument91 paginiPersona 5 Royal WalkthroughKill YourselfÎncă nu există evaluări
- Ejercicios Con El Verbo To BeDocument3 paginiEjercicios Con El Verbo To BeSeabiscuit NygmaÎncă nu există evaluări
- Corporate Agent License DetailsDocument980 paginiCorporate Agent License DetailsskpakaleÎncă nu există evaluări
- Matthews, 2021.treatments For Morton's NeuromaDocument20 paginiMatthews, 2021.treatments For Morton's NeuromaFarhan RendyÎncă nu există evaluări
- 16 4 2019Document3 pagini16 4 2019asma .sassi100% (1)
- Business Project Weight LossDocument18 paginiBusiness Project Weight Lossshubh_bra100% (1)
- Human Physiology PRACTICE SCENERIO QUESTIONDocument8 paginiHuman Physiology PRACTICE SCENERIO QUESTIONThao NguyenÎncă nu există evaluări
- Making Health Policy Book PDFDocument213 paginiMaking Health Policy Book PDFtasiambd100% (4)
- Housekeeping QuestionDocument3 paginiHousekeeping QuestionFredo QuijanaÎncă nu există evaluări
- Motor Octane Number of Spark-Ignition Engine Fuel: Standard Test Method ForDocument56 paginiMotor Octane Number of Spark-Ignition Engine Fuel: Standard Test Method ForAMANDA BARRERA100% (1)
- Miotonicna Distrofija Tip 1Document6 paginiMiotonicna Distrofija Tip 1Katarina VladusicÎncă nu există evaluări
- Coupling GuardsDocument10 paginiCoupling Guards최승원Încă nu există evaluări
- Ats - Geist Power - Atrn102-101r15st-1 PDFDocument2 paginiAts - Geist Power - Atrn102-101r15st-1 PDFCastellon DonaldÎncă nu există evaluări
- Johan Fleischer - Chemical MoonshineDocument18 paginiJohan Fleischer - Chemical MoonshinetravellerfellowÎncă nu există evaluări
- Assistant Human Resources Officer - Human Resources Officer - Well Core Limited - Hong Kong - JobsDBDocument2 paginiAssistant Human Resources Officer - Human Resources Officer - Well Core Limited - Hong Kong - JobsDBBerberber YffÎncă nu există evaluări
- Constitutional Provisions Relating To Labour LawsDocument2 paginiConstitutional Provisions Relating To Labour LawsTharun pranavÎncă nu există evaluări
- SMX Modular Units: CBGS-0Document84 paginiSMX Modular Units: CBGS-0Hary Benja ANDRIAMASINOROÎncă nu există evaluări
- 1.workye TsigeDocument83 pagini1.workye TsigeAlex WorkinaÎncă nu există evaluări
- Handayani 2018 J. Phys. Conf. Ser. 1013 012168Document9 paginiHandayani 2018 J. Phys. Conf. Ser. 1013 012168Nezuko CutieeeÎncă nu există evaluări
- The Optic NerveDocument7 paginiThe Optic NerveEdwin DarmawanÎncă nu există evaluări
- New Text DocumentDocument2 paginiNew Text Documentszabomark00Încă nu există evaluări