Documente Academic
Documente Profesional
Documente Cultură
Intralesional Bleomycin Sclerotherapy in Childhood Lymphangioma
Încărcat de
Hendrik0 evaluări0% au considerat acest document util (0 voturi)
36 vizualizări5 paginiThis clinical trial was conducted to evaluate the efficacy of intralesional bleomycin sclerotherapy in children with lymphangioma. Good response was seen in 50% of the lesions, complete resolution in 35.7%, and poor response in 14.3%. No serious complications or side effects were observed after IBS.
Descriere originală:
Titlu original
pdf_TJP_1225
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentThis clinical trial was conducted to evaluate the efficacy of intralesional bleomycin sclerotherapy in children with lymphangioma. Good response was seen in 50% of the lesions, complete resolution in 35.7%, and poor response in 14.3%. No serious complications or side effects were observed after IBS.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
36 vizualizări5 paginiIntralesional Bleomycin Sclerotherapy in Childhood Lymphangioma
Încărcat de
HendrikThis clinical trial was conducted to evaluate the efficacy of intralesional bleomycin sclerotherapy in children with lymphangioma. Good response was seen in 50% of the lesions, complete resolution in 35.7%, and poor response in 14.3%. No serious complications or side effects were observed after IBS.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 5
The Turkish Journal of Pediatrics 2013; 55: 396-400
Original
Intralesional bleomycin sclerotherapy in childhood
lymphangioma
Volkan Eriki, Mnevver Hogr, Melih Yldz, Ylmazcan rnek, Nail Aksoy,
zkan Okur, Yusuf Demircan, ncinur Geniol
Department of Pediatric Surgery, Dr. Behet Uz Children's Hospital, zmir, Turkey. E-mail: verikci@yahoo.com
SUMMARY: Eriki V, Hogr M, Yldz M, rnek Y, Aksoy N, Okur ,
Demircan Y, Geniol . Intralesional bleomycin sclerotherapy in childhood
lymphangioma. Turk J Pediatr 2013; 55: 396-400.
This clinical trial was conducted to evaluate the efficacy of intralesional
bleomycin sclerotherapy (IBS) in children with lymphangioma and to determine
the incidence of complications in the treatment. Seventeen lymphangioma cases
were treated with IBS from 2004 to 2012. Age, mode of presentation, locations
and types of lesions, and results of treatment were studied. Lymphangioma
was diagnosed by physical examination and imaging studies. Most of the
lesions were located in the cervical region (n=8) and of macrocystic type
(n=13). After the first injection, three patients were lost to follow-up. Good
response was seen in 50% of the lesions, complete resolution in 35.7%,
and poor response in 14.3%. No serious complications or side effects were
observed after IBS. The average follow-up was 18.5 months. IBS is effective
in the treatment of lymphangioma. Although no major adverse effects have
been encountered, complications should be kept in mind and in the event
of their occurrence be treated immediately.
Key words: lymphangioma, sclerotherapy, bleomycin.
Lymphangioma is a benign tumor of lymphatic
vessels and consists of cystic spaces of varying
size. The most frequent occurrence is in the
head and neck, accounting for 75% of all
cases1,2. It is typically detected at birth in
up to 65% and presents by the age of two
years in 90% of cases1,3,4. The incidence of
lymphangioma is reported to be from 1.5-2.8
per 1000 with no predilection for either sex5.
Swelling and cosmetic deformity are the most
common symptoms.
method of treatment for lymphangiomas
in children. Various sclerosing agents have
been used in the treatment of childhood
lymphangiomas.
Surgical excision is the traditional management,
the goal of which is the removal of the involved
tissue without sacrificing vital structures,
but this may not be achieved in most cases.
Due to the nature of the lesion, which has a
propensity to infiltrate tissue planes and encircle
important neurovascular structures, complete
excision is frequently impossible3. Multiple
nonsurgical therapies have been proposed,
including diathermy, cryotherapy, radiotherapy,
fibrin glue, and percutaneous sclerotherapy.
Intralesional sclerotherapy has become a
Material and Methods
A retrospective clinical trial was conducted to
evaluate the efficacy of intralesional bleomycin
sclerotherapy (IBS) in the treatment of
lymphangiomas in children and to determine
the incidence of complications during the
treatment.
A retrospective study of 17 children diagnosed
with lymphangioma and treated with IBS from
2004 to 2012 was conducted. Age, mode of
presentation, locations and types of lesions,
and results of treatment were studied. The
total number of lymphangioma patients in this
period was 23.
Lymphangioma was diagnosed by means of
physical examination and information from
imaging studies such as ultrasonography,
Presented at the XXXth National Annual Meeting of the Turkish Association of Pediatric Surgeons, 17-20 October 2012,
Ankara, Turkey
Volume 55 Number 4
Intralesional Bleomycin Sclerotherapy in Childhood Lymphangioma397
computed tomography and/or magnetic
resonance imaging (Figs. 1-3). These imaging
studies were also obtained to classify the type
of lymphangioma and extensions of the lesion.
As described by Ogita6, type was classified as
macrocystic (>1 cm), microcystic (<1 cm)
or mixed, indicating lesions containing both
macrocystic and microcystic components.
Fig. 1. Ultrasonographic view of a patient with macrocystictype lymphangioma.
Fig. 2. Ultrasonographic view of a patient with mixedtype lymphangioma.
Fig.3. Magnetic resonance image of a cervical lymphangioma.
As a primary mode of treatment, surgical
excision was performed in six patients with
lymphangiomas located in the abdominal or
thoracic cavity both to confirm the diagnosis
and to prevent complications that may
arise from their compressive effect on vital
structures. These patients were excluded in
this study. In the present study, none of the
patients was managed with embolization or
with microbiological treatment as a first-line
treatment modality, and no child was followed
without any treatment. All the patients with
lymphangioma received either IBS or surgical
excision as the first-line therapy, and patients
treated with IBS constituted the study group.
The patients were hospitalized before treatment.
Injections were performed to patients under
general anesthesia. The cystic components of
the tumor were aspirated. While keeping the
tip of the aspiration needle within the cyst
lumen, 1 mg per kg body weight of bleomycin
aqueous solution (1 mg/ml), described by
Yura 7, was injected. When more than one
cyst was aspirated, the calculated dose was
divided by the number of cysts aspirated and
the divided dose was injected into each cyst.
The maximum volume for replacement was 20
ml regardless of the volume of aspirated fluid.
This procedure was repeated after 4-6 weeks
if the cysts persisted and measured at least 1
cm in diameter.
All the patients were kept under close
observation for at least 24 hours following
Table I. Sites of Involvement in Lymphangiomas
Site of lesion
Cervical region
Thoracic wall
Shoulder
Gluteus
Lumbar region
Abdominal wall
398
Eriki V, et al
The Turkish Journal of Pediatrics July-August 2013
Table II. Response to Bleomycin Injection According to Lesion Type
Lesion type
n
(Complete resolution)
n
(Good response)
n
(Poor response)
Macrocystic
Mixed
the procedure. They were monitored for
possible immediate and late complications of
the treatment. The response was evaluated as
complete resolution (total disappearance), good
response (showing >50% reduction in size)
and poor response (showing <50% reduction
in size).
Results
Patients mostly presented with swelling in the
affected body regions. Most of the lesions were
located in the cervical region (n=8). Other
sites of involvement are depicted in Table I.
With the exception of five newborn patients,
the average age was 3.8 years (2 months-9
years). Most of the lesions were macrocystic
type (n=13), followed by mixed type (n=4);
there were no microcystic lesions in this series.
The number of procedures per patient varied
from one to five. After the first injection of
bleomycin, three patients were lost to followup. Good response was seen in 50% (7/14)
of the lesions, complete resolution in 35.7%
(5/14), and poor response in 14.3% (2/14)
(Table II). Two patients had undergone prior
surgery and these patients had good response
to IBS. No serious complications, side effects
or recurrences were observed after the injection
therapy. The average follow-up period was 18.5
months (2-46 months).
Discussion
Lymphangioma is one of the most common
benign lesions of childhood, which manifests
frequently before two years of age. Surgical
excision has been considered the treatment of
choice. Due to the property of the lymphangioma
of infiltrating adjacent structures, incomplete
resection or inadvertent nerve injury may
result. Recurrence rates after macroscopic total
excision were reported to be 15-40%8.
The first case of lymphangioma treated
by sclerotherapy with sodium morrhuate
was reported in 19339. Since then, various
sclerosing agents have been used. Bleomycin
is an antitumor agent discovered in 1965.
Besides its antineoplastic effect, it has an
irritant effect on endothelial cells of the
cyst wall of lymphangiomas. It seems that
bleomycin causes non-specific inflammatory
reaction leading to fibrosis of the cysts. The
first report of intralesional bleomycin therapy
was reported in 1977 with good results9. Since
then, various studies have produced promising
results9-13. Our study indicated that bleomycin
was effective in our patients (12 of 14 with
complete resolution or good response), and
the results were comparable to the published
series. Success rates of 36-63% for complete
tumor regression, of up to 88% for significant
lesion regression, and poor response of 12-18%
using bleomycin were reported5,9-13.
In this study, the procedure was performed
in children under general anesthesia in the
operating room. Bleomycin aqueous solution
at a dosage of 1 mg/kg described by Yura7 was
injected directly into a cyst after aspiration of
the cyst content. The total dose never exceeded
15 mg. There has been no uniformity about
the dose of this drug in the reported series.
Doses of 0.3-0.6 mg/kg for each injection, with
a total amount of bleomycin injected of up to
50 mg or 5 mg/kg, and up to 16 injections at
intervals of 2 weeks to 2 months have been
reported4,9,12,14,15.
Most of the patients with lymphangioma in
this series presented with macrocysts (10/14).
It may be that the high rate of satisfactory
response to IBS in our patients was related to
the fact that lesions consisting of macrocysts
respond well to local injection therapy, as
reported in the literature, while response
is lower in microcystic lymphangioma16-18.
There was good response in 3 of 4 patients
with mixed lymphangioma; no patients with
microcystic type were observed in this series.
Poor response to IBS was observed in two
patients. Sites of involvement in these patients
were the cervical and gluteal regions. In the
patient with cervical involvement, lesions
extended into the anterior mediastinum, and
Volume 55 Number 4
Intralesional Bleomycin Sclerotherapy in Childhood Lymphangioma399
only the neck components were aspirated and
injected with bleomycin. With this approach, it
was hoped that as the neck component reduced
in size, the mediastinal component would retract
into the neck. The mediastinal component
was not directly aspirated and injected for
the fear that it could cause mediastinitis and
compromise adjacent vital structures. The other
non-responder to bleomycin was a 2.5-year-old
boy with a macrocystic lesion in the gluteal
region. Repeat injection is to be planned for
future resolution in this patient.
No serious complications were seen after IBS.
Most patients complained of skin erythema,
local swelling, and induration, but these
symptoms did not prolong their stay in the
hospital. Although fever was reported as the
most common side effect, with an incidence
of 30%19, it was not observed in any of our
patients. The primary concern of bleomycin
therapy is the risk of pulmonary toxicity. The
risk is dose-related, and at total doses of below
150 mg or 450 U, life-threatening pulmonary
toxicity is rare20,21. It is reported that two
children who underwent bleomycin therapy died
posttreatment from pulmonary complications,
although a definitive link to the bleomycin
therapy was not established3. Clinical studies
from different centers, in which a total of 104
patients were treated with IBS, did not report
pulmonary fibrosis as a complication9-13.
difficulty. However, literature regarding
sclerotherapy for lymphangiomas has a low
level of evidence. With randomized clinical
trials focused on local bleomycin therapy,
standardized dosing protocols and reliable
outcome reporting, it is anticipated that the
heterogeneity of the literature will be decreased
and future development of treatment guidelines
will be possible.
REFERENCES
1. Ravitch MM, Rush Jr BF. Cystic hygroma. In: Welch
KJ, Randolph MM, Ravitch MM, et al. (eds). Pediatric
Surgery. Chicago, IL: Year Book Medical; 1986: 533539.
2. Emery PJ, Bailey CM, Evans JN. Cystic hygroma of
the head and neck: a review of 37 cases. J Laryngol
Otol 1984; 98: 613-619.
3. Acevedo JL, Shah RK, Brietzke SE. Nonsurgical
therapies for lymphangiomas: a systematic review.
Otolaryngol Head Neck Surg 2008; 138: 418-424.
4. Sanlialp I, Karnak I, Tanyel FC, et al. Sclerotherapy for
lymphangioma in children. Int J Pediatr Otorhinolaryngol
2003; 67: 795-800.
5. Rozman Z, Thambidorai CR, Zaleha AM, et al.
Lymphangioma: is intralesional bleomycin sclerotherapy
effective? Biomed Imaging Interv J 2011; 7: 1-8.
6. Ogita S, Tsuno T, Deguchi F, et al. OK-432 therapy
for unresectable lymphangioma in children. J Pediatr
Surg 1991; 26: 263-270.
7. Yura J, Hashimoto T, Tsuruga N, et al. Bleomycin
treatment for cystic hygroma in children. Arch Jpn
Chir 1977; 46: 607-614.
Nevertheless, there are limitations to the
present study. The retrospective design and
relative short follow-up period in the treatment
group may undermine the strength of this
study. Although there was no recurrence
in the children treated with IBS during the
follow-up period, the relative short duration of
surveillance in this series (18.5 months) should
prevent us from drawing clear conclusions
concerning the recurrence. Further prospective
studies involving more patients with longer
follow-up periods may provide fundamental
information on this matter.
8. Alqahtani A, Nguyen LT, Flagoele H, et al. 25 years
experience with lymphangiomas in children. J Pediatr
Surg 1999; 34: 1164-1168.
The majority of our patients (12/14) who
underwent sclerotherapy with bleomycin
achieved satisfactory response. No serious
side effects from IBS were observed. Though
the sample size in this series was limited,
local injection of bleomycin is recommended
as a first-line therapy, and surgical excision
should be reserved for refractory cases or
lymphangiomas that present a diagnostic
13. Mahajan JK, Bharathi V, Chowdhary SK, et al. Bleomycin
as intralesional sclerosant for cystic hygromas. J Indian
Assoc Pediatr Surg 2004; 9: 3-7.
9. Okada A, Kubota A, Fukuzawa M, et al. Injection of
bleomycin as a primary therapy of cystic lymphangioma.
J Pediatr Surg 1992; 27: 440-443.
10. Zulfiqar MA, Zaleha AM, Zakaria Z, et al. The treatment
of neck lymphangioma with intralesional injection of
bleomycin. Med J Malaysia 1999; 54: 478-480.
11. Tanigawa N, Shimomatsuya T, Takahashi K, et al.
Treatment of cystic lymphangioma with the use of
bleomycin fat emulsion. Cancer 1987; 60: 741-749.
12. Orford J, Baker S, Thonell S, et al. Bleomycin therapy
for cystic hygroma. J Pediatr Surg 1995; 30: 1282-1287.
14. Jiang JP. Local injection of bleomycin in lymphangioma
in children: analysis of 100 cases. Chung Hua Wai Ko
Tsa Chih 1989; 27: 741-744.
15. Stringel G. Hemangiomas and lymphangiomas.
In: Ashcraft KW (ed). Pediatric Surgery (3rd ed).
Philadelphia, London, New York, St. Louis, Sydney,
Toronto: W.B. Saunders Company; 2000: 965-986.
400
Eriki V, et al
16. Ogita S, Tsuto T, Nakamura K, et al. OK-432 therapy
in 64 patients with lymphangioma. J Pediatr Surg
1994; 29: 784-785.
17. Luzzatto C, Midrio P, Tchaprassian Z, et al. Sclerosing
treatment of lymphangiomas with OK-432. Arch Dis
Child 2000; 82: 316-318.
18. Sichel JY, Udassin R, Gozal D, et al. OK-432 therapy
for cervical lymphangioma. Laryngoscope 2004; 114:
1805-1809.
19. Churchill P, Otal D, Pemberton J, et al. Sclerotherapy
for lymphatic malformations in children: a scoping
review. J Pediatr Surg 2011; 46: 912-922.
The Turkish Journal of Pediatrics July-August 2013
20. Balis FM, Holcenberg JS, Blaney SM. General principles
of chemotherapy. In: Pizzo PA, Poplack DG (eds).
Principles and Practice of Pediatric Oncology (4th ed).
Philadelphia, Baltimore, New York, London, Buenos
Aires, Hong Kong, Sydney, Tokyo: Lippincott Williams
& Wilkins; 2001: 274-275.
21. D o r r RT, v o n H o f f D D . D r u g m o n o g r a p h s ,
bleomycin sulfate. In: Dorr RT, von Hoff (eds).
Cancer Chemotherapy Handbook (2nd ed). Norwalk,
Connecticut: Appleton & Lange; 1994: 227-235.
S-ar putea să vă placă și
- Patient Centered Pharmacology Tindall William N Sedrak Mona M Boltri John M SRG PDFDocument575 paginiPatient Centered Pharmacology Tindall William N Sedrak Mona M Boltri John M SRG PDFTedpwer100% (3)
- The AMBOSS 100 Day Study Plan - Your No1 Study Guide For Final ExamsDocument30 paginiThe AMBOSS 100 Day Study Plan - Your No1 Study Guide For Final Examsovermega100% (1)
- Ridge Split Technique Using Piezosurgery - A Case ReportDocument6 paginiRidge Split Technique Using Piezosurgery - A Case ReportInternational Journal of Innovative Science and Research TechnologyÎncă nu există evaluări
- Colorectal Cancer NCPDocument6 paginiColorectal Cancer NCPAudrie Allyson GabalesÎncă nu există evaluări
- Stages of Wellness & Illness and Levels of PreventionDocument3 paginiStages of Wellness & Illness and Levels of PreventionKhalid Epping100% (5)
- Physiotherapy Guide for Respiratory & Cardiac Issues in Adults & ChildrenDocument1 paginăPhysiotherapy Guide for Respiratory & Cardiac Issues in Adults & ChildrenAndrei Briceag100% (1)
- 001 Hilton Dr. Directory 2Document20 pagini001 Hilton Dr. Directory 2Muhammad Siraj KhanÎncă nu există evaluări
- Bleomycin Injection in Lymphangioma JournalDocument5 paginiBleomycin Injection in Lymphangioma JournalMarikar ArsyÎncă nu există evaluări
- Clinical and Radiological Outcomes of Infantile Hemangioma Treated With Oral Propranolol: A Long-Term Follow-Up StudyDocument7 paginiClinical and Radiological Outcomes of Infantile Hemangioma Treated With Oral Propranolol: A Long-Term Follow-Up StudyS FznsÎncă nu există evaluări
- Comparing Surgical Methods for Ileus MeconiumDocument4 paginiComparing Surgical Methods for Ileus MeconiumCirugía Pediátrica León Gto.Încă nu există evaluări
- Pi Is 0022346814003935Document5 paginiPi Is 0022346814003935wibizzzÎncă nu există evaluări
- Bleomycin Therapy For Cystic HygromaDocument6 paginiBleomycin Therapy For Cystic HygromaMinh ChíÎncă nu există evaluări
- JurnalDocument9 paginiJurnalNoraine Zainal AbidinÎncă nu există evaluări
- Primary Operative Versus Nonoperative Therapy For Pediatric Empyema: A Meta-AnalysisDocument9 paginiPrimary Operative Versus Nonoperative Therapy For Pediatric Empyema: A Meta-AnalysisDr Nilesh NagdeveÎncă nu există evaluări
- Adolescent Endometriosis: EditorialDocument2 paginiAdolescent Endometriosis: EditorialsaryindrianyÎncă nu există evaluări
- Abdominal Inflammatory Myofibroblastic Tumor: Report On Four Cases and Review of LiteratureDocument6 paginiAbdominal Inflammatory Myofibroblastic Tumor: Report On Four Cases and Review of LiteratureLisma EkayantiÎncă nu există evaluări
- Multifocal Osteoarticular Tuberculosis in Children: Journal of Orthopaedic Surgery 2011 19 (3) :336-40Document5 paginiMultifocal Osteoarticular Tuberculosis in Children: Journal of Orthopaedic Surgery 2011 19 (3) :336-40Suci IryandaÎncă nu există evaluări
- Injeksi BleomisinDocument4 paginiInjeksi BleomisinnovanÎncă nu există evaluări
- Pediatric Orbital Rhabdomyosarcoma OutcomesDocument7 paginiPediatric Orbital Rhabdomyosarcoma OutcomesNurul RiskiÎncă nu există evaluări
- Feasibility of Eliminating Ocular Chlamydia Trachomatis With Repeat Mass Antibiotic TreatmentsDocument5 paginiFeasibility of Eliminating Ocular Chlamydia Trachomatis With Repeat Mass Antibiotic TreatmentsAmelia RoziantyÎncă nu există evaluări
- Varicocele Management - A Comparison of Palomo Versus Inguinal ApproachDocument4 paginiVaricocele Management - A Comparison of Palomo Versus Inguinal ApproachWahid Hilmy SulaimanÎncă nu există evaluări
- Effectiveness of antibiotic-coated shunts for preventing infections in myelomeningocele patientsDocument6 paginiEffectiveness of antibiotic-coated shunts for preventing infections in myelomeningocele patientsBilal ErtugrulÎncă nu există evaluări
- Childhood Vitiligo: Response To Methylprednisolone Oral Minipulse Therapy and Topical Fluticasone CombinationDocument5 paginiChildhood Vitiligo: Response To Methylprednisolone Oral Minipulse Therapy and Topical Fluticasone CombinationFebniÎncă nu există evaluări
- Effect of Foot Reflexology On Neonatal JaundiceDocument7 paginiEffect of Foot Reflexology On Neonatal JaundiceSarini Suryadi100% (1)
- Madu Colostomy PDFDocument6 paginiMadu Colostomy PDFdiahÎncă nu există evaluări
- Dec24 16 PDFDocument8 paginiDec24 16 PDFijasrjournalÎncă nu există evaluări
- Pi Is 0196064409002704Document7 paginiPi Is 0196064409002704adesamboraÎncă nu există evaluări
- ParotitisDocument6 paginiParotitisredityoÎncă nu există evaluări
- Treating Phimosis Safely with Topical SteroidsDocument5 paginiTreating Phimosis Safely with Topical Steroidsmaria alisyahÎncă nu există evaluări
- Uterine Fibroid EmbolizationDocument8 paginiUterine Fibroid EmbolizationAlexandre Campos Moraes AmatoÎncă nu există evaluări
- Simple Conization and Lymphadenectomy For The Conservative Treatment of Stage IB1 Cervical Cancer. An Italian ExperienceDocument4 paginiSimple Conization and Lymphadenectomy For The Conservative Treatment of Stage IB1 Cervical Cancer. An Italian ExperienceKaleb Rudy HartawanÎncă nu există evaluări
- Management of Perforated Appendicitis in Children: A Decade of Aggressive TreatmentDocument5 paginiManagement of Perforated Appendicitis in Children: A Decade of Aggressive Treatmentapi-308365861Încă nu există evaluări
- 10 1 1 575 3365 PDFDocument8 pagini10 1 1 575 3365 PDFYaraÎncă nu există evaluări
- 3Document8 pagini3Agung SentosaÎncă nu există evaluări
- Ovarian Cysts and Tumors in Infancy and Childhood: Riginal RticleDocument4 paginiOvarian Cysts and Tumors in Infancy and Childhood: Riginal RticleIwan Budianto HadiÎncă nu există evaluări
- Lich GregoirDocument5 paginiLich GregoirIoannis ValioulisÎncă nu există evaluări
- EndometritisDocument6 paginiEndometritisMuh Syarifullah AÎncă nu există evaluări
- Hysteroscopy as a Minimally Invasive Alternative for Gynecological ProceduresDocument6 paginiHysteroscopy as a Minimally Invasive Alternative for Gynecological ProceduresJonathan LucisÎncă nu există evaluări
- Hypnoparenting Effects Towards Fatigue As An Impact of Chemotherapy Among Pediatric Patients With Acute Lymphoblastic LeukemiaDocument7 paginiHypnoparenting Effects Towards Fatigue As An Impact of Chemotherapy Among Pediatric Patients With Acute Lymphoblastic LeukemiaChika FebrianiÎncă nu există evaluări
- Yeung TeenagersDocument5 paginiYeung TeenagersAnonymous YyLSRdÎncă nu există evaluări
- Post Hysteroscopic Progesterone Hormone Therapy in The Treatment of Endometrial PolypsDocument5 paginiPost Hysteroscopic Progesterone Hormone Therapy in The Treatment of Endometrial PolypsherryÎncă nu există evaluări
- Neuroblastoma PDFDocument5 paginiNeuroblastoma PDFHanna G. FauziaÎncă nu există evaluări
- Buesing 2011 Partial Splenectomy For Hereditary Spherocytosis A Multi-Institutional ReviewDocument6 paginiBuesing 2011 Partial Splenectomy For Hereditary Spherocytosis A Multi-Institutional ReviewBogdan TrandafirÎncă nu există evaluări
- Pi Is 0002939409007910Document5 paginiPi Is 0002939409007910darendraabimayuÎncă nu există evaluări
- Successful Treatment of Multiple Common Warts With Intralesional OzoneDocument6 paginiSuccessful Treatment of Multiple Common Warts With Intralesional OzoneRubensÎncă nu există evaluări
- Amniocentesis Results and Retrospective Analysis Performed in The University ClinicDocument6 paginiAmniocentesis Results and Retrospective Analysis Performed in The University ClinicCindy Denti PratikasariÎncă nu există evaluări
- Low-Dose Cyclosporine Treatment For Sight-Threatening Uveitis: Efficacy, Toxicity, and ToleranceDocument8 paginiLow-Dose Cyclosporine Treatment For Sight-Threatening Uveitis: Efficacy, Toxicity, and ToleranceMauliza Resky NisaÎncă nu există evaluări
- Objectives:: Ahmed H. Al-SalemDocument4 paginiObjectives:: Ahmed H. Al-SalemHarun NasutionÎncă nu există evaluări
- Agaoglu2005 2Document6 paginiAgaoglu2005 2Ngo Quang MinhÎncă nu există evaluări
- Faz Zio 2016Document9 paginiFaz Zio 2016seruniallisaaslimÎncă nu există evaluări
- Diagnosis and Management of Atypical Endometrial HyperplasiaDocument9 paginiDiagnosis and Management of Atypical Endometrial HyperplasiaagathapradanaÎncă nu există evaluări
- Catuogno Relationship Activity FatigueDocument8 paginiCatuogno Relationship Activity FatigueMirjana MandićÎncă nu există evaluări
- Laparoscopic Versus Open Inguinal Hernia Repair in Children 3: A Randomized Controlled TrialDocument10 paginiLaparoscopic Versus Open Inguinal Hernia Repair in Children 3: A Randomized Controlled TrialRhandy SeptiantoÎncă nu există evaluări
- Saravanan 10.41030971 9261.43800Document7 paginiSaravanan 10.41030971 9261.43800CherÎncă nu există evaluări
- A Rare Case of Ovarian Dysgerminoma in A 6Document5 paginiA Rare Case of Ovarian Dysgerminoma in A 6sarinovitapratiwiÎncă nu există evaluări
- 20 WaqarDocument4 pagini20 WaqarmelisaberlianÎncă nu există evaluări
- Aydin 2017Document7 paginiAydin 2017Berry BancinÎncă nu există evaluări
- MUCINOUS-ADENOCARCINOMA-FINALDocument16 paginiMUCINOUS-ADENOCARCINOMA-FINALritzy0997Încă nu există evaluări
- 872 2540 2 PBDocument4 pagini872 2540 2 PBSteve D'HamsÎncă nu există evaluări
- Visual and Systemic Outcomes in Pediatric Ocular Myasthenia GravisDocument11 paginiVisual and Systemic Outcomes in Pediatric Ocular Myasthenia GravisGina Sonia Fy EffendiÎncă nu există evaluări
- Journal of Pediatric Surgery: Indepent Review ArticlesDocument4 paginiJournal of Pediatric Surgery: Indepent Review ArticlesNur Ainatun NadrahÎncă nu există evaluări
- Clinical Profile of Empyema in Tertiary Health Care Center, HyderabadDocument4 paginiClinical Profile of Empyema in Tertiary Health Care Center, HyderabadririÎncă nu există evaluări
- 1 s2.0 S002234681100933X MainDocument8 pagini1 s2.0 S002234681100933X Mainzahoor ul haqÎncă nu există evaluări
- Jurnal 1Document4 paginiJurnal 1TheQueensafa90Încă nu există evaluări
- Benign Esophageal TumorsDocument24 paginiBenign Esophageal TumorsMikael Angeloo100% (1)
- Mastery of IBD SurgeryDe la EverandMastery of IBD SurgeryNeil HymanÎncă nu există evaluări
- Population Analysis of The Perfect Breast A.8Document12 paginiPopulation Analysis of The Perfect Breast A.8HendrikÎncă nu există evaluări
- EScholarship UC Item 0kv2243nDocument3 paginiEScholarship UC Item 0kv2243nHendrikÎncă nu există evaluări
- A Matched Cohort Study of Superomedial Pedicle.6Document9 paginiA Matched Cohort Study of Superomedial Pedicle.6HendrikÎncă nu există evaluări
- GrotteDocument30 paginiGrotteHendrikÎncă nu există evaluări
- Inferior Wall ST Elevation (STEMI)Document46 paginiInferior Wall ST Elevation (STEMI)Hendrik100% (1)
- Acute Rheumatic Fever Kuliah TK 4Document47 paginiAcute Rheumatic Fever Kuliah TK 4Marwah ChiwaÎncă nu există evaluări
- Afdalia Cardio - Pericarditis AkutDocument30 paginiAfdalia Cardio - Pericarditis AkutHendrikÎncă nu există evaluări
- Acute Liver FailureDocument9 paginiAcute Liver FailureHendrikÎncă nu există evaluări
- ST Sement Elevation Myocardial Infarction (Stemi) NewDocument32 paginiST Sement Elevation Myocardial Infarction (Stemi) NewHendrikÎncă nu există evaluări
- 9#kuliah CAD Sistem 2011Document96 pagini9#kuliah CAD Sistem 2011HendrikÎncă nu există evaluări
- CHD For WebDocument42 paginiCHD For WebRodica PascalÎncă nu există evaluări
- NMJ Special OncologyDocument24 paginiNMJ Special OncologyMalcolm LeeÎncă nu există evaluări
- Herpes Simplex Oral: EpidemiologyDocument5 paginiHerpes Simplex Oral: EpidemiologyFariz RamadhanÎncă nu există evaluări
- Mapeh 10 - QTR 1 - Mod 2Document12 paginiMapeh 10 - QTR 1 - Mod 2Karu GreyÎncă nu există evaluări
- Antiprotozoal and Antihelminthic Drugs - HandoutDocument21 paginiAntiprotozoal and Antihelminthic Drugs - HandoutdonzÎncă nu există evaluări
- My C.V.Document2 paginiMy C.V.Saty VeeanÎncă nu există evaluări
- 1.structure of The TeethDocument5 pagini1.structure of The TeethCălin PavelÎncă nu există evaluări
- Population Dynamics and Control of ContraceptionDocument16 paginiPopulation Dynamics and Control of Contraceptionjaish8904Încă nu există evaluări
- Registered Health Information Technician Rhit Exam Content OutlineDocument2 paginiRegistered Health Information Technician Rhit Exam Content Outlineapi-278092659Încă nu există evaluări
- The Health Bank (THB) Connected Care Program A Pilot Study of Remote Monitoring For The Management of Chronic Conditions Focusing On DiabetesDocument5 paginiThe Health Bank (THB) Connected Care Program A Pilot Study of Remote Monitoring For The Management of Chronic Conditions Focusing On DiabetesInternational Journal of Innovative Science and Research TechnologyÎncă nu există evaluări
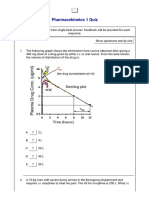
- Pharmacokinetcs 1 QuizDocument5 paginiPharmacokinetcs 1 QuizCarlos MaingeÎncă nu există evaluări
- AtulabhcvDocument13 paginiAtulabhcvTarun MathurÎncă nu există evaluări
- RCL Physical Exam Guidelines For Examining Physician 11-2014Document4 paginiRCL Physical Exam Guidelines For Examining Physician 11-2014George Mihalache100% (1)
- DLT211Document5 paginiDLT211Qudus SilmanÎncă nu există evaluări
- B-2211 Implementation of DCI Revised B.D.S. CourseDocument17 paginiB-2211 Implementation of DCI Revised B.D.S. CourseShital KiranÎncă nu există evaluări
- NCM 104-CHN1Document4 paginiNCM 104-CHN1Kyla VillamorÎncă nu există evaluări
- Comparative Study of The Topical Treatment With Etofenamato Combined With Iontoforesis Versus Fonoforesis, For Handling The Post Surgery PainDocument9 paginiComparative Study of The Topical Treatment With Etofenamato Combined With Iontoforesis Versus Fonoforesis, For Handling The Post Surgery PainucbrotherÎncă nu există evaluări
- Vet Diagnostix Products ListDocument4 paginiVet Diagnostix Products ListrcllanoscÎncă nu există evaluări
- Requirement in NCM 312: Submitted By: Jaylee C. Andaya Submitted To: Dr. Ma. Lynne C. ParambitaDocument8 paginiRequirement in NCM 312: Submitted By: Jaylee C. Andaya Submitted To: Dr. Ma. Lynne C. ParambitaChloie Marie RosalejosÎncă nu există evaluări
- Ards Prone PositionDocument9 paginiArds Prone PositionAnonymous XHK6FgHUÎncă nu există evaluări
- Hospital PharmacyDocument19 paginiHospital PharmacySUMIT MASKEÎncă nu există evaluări
- Hearing Loss-Decibels or PercentDocument75 paginiHearing Loss-Decibels or PercentGanti Santosh KumarÎncă nu există evaluări
- Obesity The Number 1 Problem in America Reading Comprehension Exercises 31128Document3 paginiObesity The Number 1 Problem in America Reading Comprehension Exercises 31128Dixon adolfo Tapia arayaÎncă nu există evaluări
- COVID-19 Staff Vaccine BriefingDocument5 paginiCOVID-19 Staff Vaccine BriefingtasialalalaÎncă nu există evaluări