Documente Academic
Documente Profesional
Documente Cultură
Us 4008290
Încărcat de
Pepe PompinDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Us 4008290
Încărcat de
Pepe PompinDrepturi de autor:
Formate disponibile
United States Patent [19]
111]
4,008,290
Ward
[45]
Feb. '15, 1977
Primary Examiner-C. Davis
Attorney, Agent, or Firm-James R. l-loatson, Jr.;
Robert W. Welch; William H. Page, 11
[54] CUMENE PRODUCTION
[75] Inventor: Dennis J. Ward, South Barrington,
Ill.
[73] Assignee: UOP Inc., Des Plaines, Ill.
[22] Filed:
Apr. 19, 1976
[21] Appl. No.: 678,005
[57]
Related US. Application Data
[63]
Continuation-impart of Ser. No. 557,010, March 10,
1975, abandoned.
[52]
[51]
[58]
US. Cl. ....................... .. 260/672 T; 260/671 P
Int. Cl.z ..................... .. C07C 3/62; C07C 3/54
Field of Search ...... .. 260/671 P, 672 T, 671 R,
260/671 C
[56]
ABSTRACT
A process to reduce utility consumption in the catalytic
alkylation of benzene with propylene to form cumene
Propylene and benzene are reacted in an alkylation
reaction zone, the effluent of which is divided into two
portions, the ?rst being recirculated to the inlet of the
reaction zone and the second being passed into a sepa
ration zone wherein cumene product, di- and triisopro
pylbenzene and excess benzene are separated. A por
tion of the excess benzene is recycled to the inlet of the ,
alkylation reaction zone, and a second portion is ad
mixed with di- and triisopropylbenzene and passed into
References Cited
a transalkylation zone, the effluent of which is intro
duced into the separation zone. Utility consumption is
UNITED STATES PATENTS
2,860,173
11/1958
Jones et al. .................. .. 260/671 P
2,864,874
12/1958
Enos
...........
. . . ..
.......
. . . ..
260/671
260/671
3,183,233
5/1965
Bloch
3,527,823
9/1970
Jones ........................... .. 260/671 P
reduced compared to prior art processes, as a result of
a reduction of the portion of excess benzene separated
and recycled to the alkylation reaction zone.
OTHER PUBLICATIONS
11 Claims, 1 Drawing Figure
Chem. Abs., 74 125855.
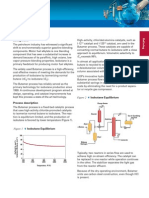
Buly/ Banana]
/0-\ Benzene Drag
-.
\Propylure
//
-.
Ally/alien /
Transa/ky/afian
React/an Zone
-.
Benzene
US. Patent
4,008,290
Feb. 15, 1977
QSuNQw
TES ?
l'
__
~
l'
.
V
Kw
NI/f.
4,008,290
CUMENE PRODUCTION,
ature'v rise by catalyzing the reaction in multiple sepa
rate zones and employing a quenching medium be
tween each of several successive alkylation' zones. This
CROSS-REFERENCE TO RELATED
'
In the past it has been customary to control the temper
APPLICATIONS
quenching has served to control the temperature at
which the reaction mixture enters each successive zone
This application is a continuation-in-part application
of a copending application Ser. No. 557,010, ?led Mar.
and thus the temperature rise throughout each zone.
The temperature rise from inlet to outlet of the reactor
has also been controlled _by controlling the molar ex
10, 1975, and now abandoned.
BACKGROUND OF THE INVENTION
This invention relates to an improved process for
producing cumene from benzene and propylene in the
cess of benzene charged to the reactor, the benzene
acting as a heat sink to absorb heat released by the
alkylation reaction. Accordingly, increasing the molar
presence of an alkylation catalyst. It also relates to
excess of benzene charged to the reactor, with a corre
transalkylating di- and triisopropylbenzene with ben
zene in the presence of a transalkylation catalyst to
produce cumene.
The present invention is broadly applicable to the
production of alkylated aromatic hydrocarbons. These '
compounds are useful in themselves and more fre
sponding dilution of the propylene reactant therein, not
temperature rise across an alkylation zone or zones.
quently in subsequent chemical synthesis of other com
pounds. The present invention isgparticularly applica
To obtain the desired high molar excess of benzene in
the reactor charge, it has been the practice to separate
20
only provides more aromatic sites subject to alkylation
and a resulting reduction in oligomers and over alkyl
ated by-products, but also reduces the formation of
undesirable by-products resulting from an excessive
ble to the production of cumene, or isopropylbenzene,
the reaction zone ef?uent to obtain a benzene-rich
which is a reactant ?nding utility in the preparation of
stream suitable to recycle to the reactor. Since the two
principal components of the reaction zone ef?uent are
phenol, acetone, alphamethylstyrene and acetophe
none. Another application of the process of the present 25 benzene and cumene, it is necessary to make a separa
tion of the benzene and cumene, the latter being the
invention may be found in the preparation of p-cymene
which may be oxidized to produce p-cresol. A, further
application of the process is in the alkylation of a sub
stituted aromatic compound such as phenol, which
when alkylated with isobutylene forms o-tertiary-butyl
higher boiling component. Consequently, to obtain a
puri?ed stream of benzene relatively free of cumene
and suitable for recycling to the reaction zone, benzene
30
phenol and p-tertiary-butylphenol, both of which ?nd
utility in the resin ?eld.
'
is vaporized and fractionated, thus requiring consump
tion of substantial heat to vaporize benzene and pro
vide adequate re?ux in a benzene fractionator, the heat
requirement being substantially proportional to the
As above stated, the present invention ?nds particu
ratio of benzene to propylene desired in the charge to
lar application in the preparation of cumene. In the
usual commercial process for the production of cu 35 the reactor. At the present time, relatively high fuel
cost necessitates review of processes requiring high
mene, it is the practice to charge liquid benzene and
utility consumption with the result that alternative pro
liquid propylene into a reactor and toreact the same
therein in one or more alkylation zones in contact with
cessing schemes which previously were unattractive are
an alkylation catalyst. In order to minimize the produc
tion of dialkylated products of benzene it has been the
becoming more desirable if utility consumption is re
duced.
practice to maintain a molar excess of benzene
SUMMARY OF THE INVENTION
An object of the present invention is to provide an
throughout the reaction zone ranging from about 4:l to
about 16:1, and more preferably about 8:1 of benzene
to propylene. Two competing reactions with the de
sired production of isopropylbenzene have created
problems in the prior commercial processes used. One
of these as indicated above has been the formation of
dialkylated benzenes such as di- and triisopropylben~
improved process for the alkylation of benzene with
45
propylene to produce cumene in the presence of an
alkylation catalyst. A speci?c object of this invention is
to reduce utility consumption in a process for the alky
lation of benzene with propylene to produce cumene in
the presence of an alkylation catalyst. A more speci?c
zene rather than the desired monoalkylated product.
This competing reaction has been controlled by means 50 object of this invention is to provide a process for the
alkylation of benzene with propylene to produce cu
of employing large molar excesses of benzene as indi
mene in the presence of a solid phosphoric acid cata
cated above. The other competing reaction causing
lyst, the process requiring a relatively lower molar ex
losses in the yield of cumene based on propylene reac
cess of benzene to propylene than prior art processes as
tant charged is the formation of oligomers of propylene
such as propylene dimer and trimer which occur to a 55 provided by recycle of excess benzene separated from
the reaction zone effluent.
limited extent even with the large molar excesses of
One embodiment of the present invention relates to a
benzene present. Propylene trimers and some of the
process for the production of cumene which comprises:
propylene tetramers boil with cumene. Since the pres
(a) reacting propylene with an excess of benzene in the
ence of these ole?ns interfere with, the oxidation reac
tion used to make phenol from cumene, this oligomer 60 presence of an alkylation catalyst at alkylation reaction
conditions in an alkylation reaction zone; (b) dividing
ization side reaction must be minimized to make a high
purity product.
'
The alkylation reaction of the alkylatable aromatic
compound is exothermic in nature and thetemperature
the total liquid ef?uent of said zone into at least two
portions of like composition; (c) recirculating one of
said portions of the ef?uent to said reaction zone; ((1)
introducing another of said portions of said effluent
within the reactor tends to increase at a rapid rate. This 65
and a transalkylation zone ef?uent stream, formed as
increase in temperature caused by the exothermic reac
hereinafter set forth, into a separation zone; (e) separt
tion likewise tends to increase the production of
ing from the admixed ef?uents in the separation zone a
cumeme bottoms products by vthe competing reactions.
4,008,290
benzene-rich stream, a cumene product streamand a
di- and triisopropylbenzene-rich stream; (f) transalk
of the alkylation reaction zone I is withdrawn via con
ylating the last named stream with benzene in the pres
ence of a transalkylation catalyst in a transalkylation
zone to form additional cumene; (g) supplying the
duit 5, a portion is recirculated via conduit 4 to provide
the recirculation stream described hereinabove, and
the remaining portion is passed via conduit 5 into a
separation zone 6. Also introduced into the separation
ef?uent of the last nientioned'zone to said separation
zone as said transalkylation zone ef?uent stream; (h)
passing at least a portion of said benzene~rich stream
zone 6 is an ef?uent stream from a transalkylation
reaction zone as described hereinbelow, the transalky
lation zone ef?uent stream passing through conduit 7.
A benzene feed stream via conduit 8_ is also introduced
into the separation zone 6 of the present process.
from the separation zone to said alkylation reaction
zone; and (i) recovering said cumene product stream
from the separation zone.
In the processing scheme of the present invention,
The propylene-rich feed stream supplied to the'alky
propylene and excess benzene are reacted in an alkyla
tion reaction zone wherein an alkylation catalyst is
employed, and a portion of the resultant ef?uent is
recirculated without separation to the inlet of the reac
tion zone. A second portion of reaction zone ef?uent,
i.e., net ef?uent, is passed into a separation zone in
which excess benzene, cumene, di- and triisopropyl
benzene, and other components are separated. As
stated hereinabove, it is desirable to reduce the quan
tity of excess benzene separated from the net ef?uent
stream in order to reduce utility consumption, while at
benzene, and recirculated reaction zone ef?uent is then
introduced into reaction zone 1 via conduit 2. Ef?uent
lation reaction zone via conduit 2 can be prepared as
an effluent stream from various processes such as ?uid
catalytic cracking or pyrolysis, and will normally in
clude non-reactive paraf?ns, principally propane, but
also substantially smaller quantities of ethane and bu
20
tane. Olefins other than propylene result in undesirable
products in the alkylation reaction to produce cumene,
therefore propylene normally comprises at least 99
percent of the ole?n content of this stream. The ben
zene feed stream introduced into the process in conduit
8 is a high purity stream commonly containing at least
the same time it is desired to maintain a sufficient quan
tity of excess benzene in the reaction zone to prevent 25 99.5 percent benzene which is frequently available as
an ef?uent stream from an aromatics extraction pro
excessive formation of propylene oligomers. This is
cess. Other aromatics are harmful in the sense that
accomplished by recirculation of a portion of the resul
undesirable by-products result from their presence, and
tant reaction zone ef?uent without separation. The
principal effects of process operation in the herein
non-aromatics are normally undesirable because of
above described manner include: (1) a reduction of 30 dif?culty in separation of these non-aromatics from
utility consumption resulting from a reduction of ex
cess benzene separated from cumene in the net reac
tion zone ef?uent stream; and (2) formation of rela
benzene in the separation zone 6. Accordingly, in the
present process, the streams introduced into the sepa
ration zone 6 include principally benzene, cumene,
propane, and di- and triisopropylbenzene, with rela
tively more di- and trialkylated benzene products than
in processes known in the prior art. In the separation 35 tively smaller amounts of light paraf?ns (ethane and
butane), aromatics (toluene and xylene), butylben
zone of the present process, di- and triisopropylben
zene, and oligomers of propylene.
"
zen'es are concentrated and passed in admixture with
In the separation zone 6, by suitable combination of
excess benzene into a transalkylation reaction zone,
?ashing, fractionation, absorption, and stripping, sev
wherein a transalkylation catalyst is employed in a
preferred embodiment. The cumene-rich transalkyla
tion reaction zone ef?uent stream is returned to the
separation zone.
40 eral streams are separated to withdraw the inlet compo
nents stated hereinabove. A propane-rich product
stream including other light praf?ns is withdrawn via
conduit 9; a benzene-rich product drag vstream includ
ing non-aromatic components is withdrawn via conduit
DESCRIPTION OF THE DRAWING
This invention can be more clearly described and 45 10; an excess benzene-rich stream is withdrawn via
illustrated with reference to the attached drawing.
While of necessity, certain limitations must be present
conduit 3, a portion is recycled to the alkylation reac
in such a schematic description, no intention is meant
a second portion is passed via conduit 1 l to a transalky
lation reaction zone as described hereinbelow; a butyl
benzene-rich product stream is withdrawn via conduit
12; a cumene product stream is withdrawn 'viaconduit
thereby to limit the generally broad scope of the inven
tion. As stated hereinabove, the first step of the process
of the present invention comprises alkylating benzene
tion zone 1 via conduit 3 as hereinabove described and
with propylene in an alkylation reaction zone in the
13; a propylene oligomer product stream is withdrawn
presence of an alkylation catalyst. In the drawing, this
?rst step is represented as taking place in alkylation
via conduit 14; and a di- and triisopropylbenzene-rich
stream is withdrawn via conduit 15.
The di- and triisopropylbenzene-rich stream with
reaction zone 1. However, a mixture of benzene and 55
drawn from the separation zone via conduit 15 is ad
propylene must be furnished to this reaction zone. In:
the drawing, a propylene-rich feed stream is supplied to
the alkylation zone 1 via conduit 2; benzene is prepared
mixed with recycle benzene via conduit II, and the
mixture is passed into a transalkylation reaction zone
16, which in a preferred embodiment contains a solid
nished to the alkylation zone 1 via conduit 3, which 60 phosphoric acid catalyst. Cumene-rich ef?uentof the
transalkylation reaction zone is_ passed via conduit 7
combines with conduit 2; and an alkylation zone ef?u
into the separation zone 6, as described hereinabove.
ent recirculation stream including principally benzene
and cumene is prepared as described hereinbelow and
DETAILED DESCRIPTION OF THE INVENTION
furnished to the alkylation zone inlet via conduits 4 and
2. The lastly mentioned stream provides additional 65 Suitable reactants in the present inventive process to
produce cumene are propylene and benzene. Propyl
benzene for the purpose of increasing the molar ben- '
ene is normally supplied in admixture with propane in
zene/propylene ratio in the alkylation reaction zone.
an ef?uent stream from a ?uid catalytic cracking unit,
The combined mixture of propylene reactant, recycle
as a recycle stream as hereinbelow described and fur
4,008,290
certain crystalline aluminosilicates 0r zeolites, particu
larly acid-extracted mordenite and the Type Y zeolite,
are useful as catalysts in the process of this invention.
The present invention is particularly directed to an
alkylation reaction in the presence of a solid phospho
a pyrolysis unit, a thermal cracking unit, or other re?n
ing unit. Other light paraffmic hydrocarbons such as
ethane and butane may be present in limited quantity in
a propylene~rich feed stream to the present process,
but ole?nic compounds other than propylene lead to
production of alkylaromatics other than cumene and
accordingly are undesirable as a feedstock. A typical
propylene feed stream is illustrated as follows in mole
ric acid catalyst. Thus solid phosphoric acid catalyst
which may be utilized in the method of the present
invention may be made by mixing an acid of phospho
percent: ethane 0.10, propane 24.80,propylene 74.95,
isobutane 0.1 1, n-butane 0.01, and butylene 0.03. Ben
zene is supplied to the present process in high purity,
greater than 99.5 percent, to prevent undesirable side
rous, such as ortho-, pyro-, or tetraphosphoric acid and
a finely divided, generally siliceous, solid carrier (such
as diatomaceous earth, prepared forms of silica, reacti
reactions and to eliminate additional fractionation re
quirements to separate benzene and close boiling non~
aromatic components within the present process. A
typical benzene feed stream is prepared in an aromatics
extraction unit and contains the following components
in mole percent: benzene 99.90, toluene 0.05, and
non-aromatics 0.05. While in the accompanying sketch
the benzene feed stream is introduced into the separa 20
vated clays, and the like) to form a wet paste. The paste
is then calcined at temperatures generally below about
500 C. to produce a solid cake which is threafter
ground and sized to produce particles of useable mesh.
1f the calcination is carried out at temperatures above
about 400 C., it may be desirable to rehydrate the
catalyst granules at a temperature between about 200
C. and 350 C., typically 260 C., to produce an acid
composition corresponding to high alkylating activity.
The catalyst preparation procedure may be varied by
forming particles of the original paste by extrusion or
by pelleting methods after which the formed particles
tion zone of the present process, this stream may also
be introduced into the alkylation reaction zone or the
transalkylation reaction zone.
are calcined and, if necessary, rehydrated. A solid
The inlet stream to the alkylation reaction zone of
the present process comprises three streams in admix 25 phosphoric acid catalyst prepared from a major pro
portion by weight of a phosphoric acid having at least
ture; the fresh propylene-rich feed stream, a recircu
lated portion of the resultant ef?uent from the alkyla
as large a water content as that of the pyro acid and a
tion reaction zone as described hereinbelow, and a
minor proportion of the siliceous carrier, such as kie~
selguhr, is preferred for use in the present process. In a
preferred embodiment of the present invention, the
alkylation catalyst includes about 50 to 75 percent by
benzene-rich stream which may be supplied to the
present process as a fresh feed stream, or more prefer 30
ably, as a recycle benzene-rich stream as described
weight phosphoric acid. A further description of a satis
factory solid phosphoric acid catalyst is available in
hereinbelow. Operating conditions in the alkylation
reaction zone include an inlet temperature of about
150 to 260 C. with a preferred temperature of about
US. Pat. No. 1,933,513.
195 to 215 C.; about 20 to 60 atmospheres pressure; 35 The resultant effluent of the alkylation reaction zone
is divided into two streams, the ?rst being a recircu
about 0.2 to 2.0 volumes of catalyst per volume/hour of
net reaction zone ef?uent hereinbelow de?ned, about 2
to 6 moles of benzene in the recycle benzene-rich
lated ef?uent stream and the second being a net reac
tion zone effluent stream. An important part of the
stream per mole of propylene in the inlet stream to the
inventive concept of the present process concerns re
alkylation reaction zone, with a preferred molar ratio 40 circulating a portion of the alkylation reaction zone
ef?uent to the inlet of the alkylation zone to admix with
of recycle benzene to propylene in the inlet stream of
the fresh propylene-rich feed stream and the recycle
about 3; and about 1 to 100 moles of benzene in the
benzene-rich stream to form the inlet stream to the
recirculated ef?uent stream per mole of propylene in
alkylation reaction zone as described hereinabove.
the inlet stream to the alkylation reaction zone, with a
preferred molar ratio of recirculated benzene to pro 45 Composition of the alkylation zone ef?uent is princi
pally benzene with relatively lesser amounts of pro
pylene in the inlet stream 3 to 20. The process of the
present invention may comprise a single reactor or
pane, cumene, and di and triisopropylbenzene, and
relatively smaller amounts of butylbenzene, propylene,
oligomers, non~aromatics, etc. Propylene is essentially
multiple reactors in series or parallel ?ow, ?ow through
each reactor being downflow, up?ow, radial flow, or
other, there being no limitation to the inventive con
50
mm of the present process by the con?guration of
reaction zone design.
The process of the present invention can be effected
utilizing any conventional or otherwise available alky
lation catalyst. Such catalysts are typically described as
benzene/propylene ratio in, the alkylation reaction
acid-acting catalysts and may be of the homogeneous
zone.
3, supported or unsupported Friedel Crafts metal halide,
and the like. Certain mineral acids, especially sulfuric
acid, hydrofluoric acid, and phosphoric acid are known
for their capacity to catalyze the alkylation reaction.
'
Several bene?ts result as the benzene/propylene ratio
increases in the alkylation reaction zone, including (1)
a dilution of propylene molecules with benzene mole
5; or heterogeneous variety. Thus, the catalyst can be a
E.for example anhydrous aluminum chloride, ferric
chloride, stannic chloride, boron ?uoride, zinc chloride
100 percent reacted in the alkylation reaction zone,
while benzene forms at least 50 molar percent and
preferably 60 to 80 molar percent of the effluent. Ac
cordingly, recirculation of a portion of the ef?uent to
the inlet of the alkylation reaction zone increases the
60
cules favoring formation of isopropylbenzene (cu
mene) and limiting formation of propylene oligomers;
and, (2) a benzene/propylene ratio greater than one is
indicative of the presence of excess benzene, which
acts as a heat sink to absorb heat generated by the
Sulfuric acid containing less than about 10 wt. % water,
hydro?uoric acid of at least 83% concentration, or 65 exothermic alkylation reaction, and limit the formation
lique?ed anhydrous hydrogen ?uoride, are suitably
employed. Acid-acting inorganic oxides including
phosphorous pentoxide, amorphous silica-alumina, and
of propylene oligomers and solid hydrocarbon deposits
on the catalyst, both of which increase with higher
temperature in the alkylation reaction zone. In prior art
4,008,290
The present inventive concept is not limited by a
processes, a temperature increase from inlet to outlet
of the alkylation reaction zone of about 20 to 40 C. is
specific combination of separation steps, however the
typical without quench, and in the present process, a
separation of excess benzene and cumene is at present
similar temperature increase or lower is desired, and is
most economically accomplished by fractional distilla
attained by suitably increasing the ?ow rate of recircu
tion, in which benzene and lighter components are
separated into an overhead fraction and cumene and
heavier components are separated into a bottoms frac
lated ef?uent.
The recirculated effluent stream may be indirectly
tion. The separation of excess benzene in prior art
processes has a relatively large capital and utility re
quirement resulting from the greater quantity of excess
cooled by cooling means such as a water cooled ex~
changer, an air cooled exchanger, or an exchanger in
which another hydrocarbon stream is used as the cool
ant, to a temperature of about 150 to 260 C., i.e., to
a temperature essentially equivalent to the temperature
benzene separated from cumene as compared with the
present process. While benzene/cumene molar ratios in
the reaction zone net effluent of prior art processes is
of the reaction zone inlet stream, or it may be admixed
about 6.5, this ratio in the present process is about 2 to
without cooling with a mixture of the propylene feed
stream and the recycle benzene stream, the mixture 15 5 at constant molar benzene/cumene ratio in the alky
lation reaction zone. Excess benzene separated from
being at a suitable temperature to provide an alkylation
the alkylation reaction zone effluent may be withdrawn
reaction zone inlet temperature of about 150 to 260
from the process as a product stream, but preferably a
C., and preferably about 195 to 215 C. Furthermore,
?rst portion is passed to the inlet of the alkylation reac
a third portion of the reaction zone effluent stream may
be indirectly cooled by similar cooling means as stated 20 tion zone as a recycle benzene-rich stream and a sec
ond portion is passed into a transalkylation reaction
hereinabove to a temperature of about 35 to 150 C.
and passed into the reaction zone at suitable points to
act as quench to cool the reactants and prevent exces
sive increase of temperature from inlet to outlet of the
alkylation reaction zone. When the alkylation catalyst
employed is a supported catalyst, for example solid
phosphoric acid, suitable quench points may be chosen
by dividing the catalyst bed into several successive
separate beds and passing a portion of the quenching
zone in admixture with a di- and triisopropylbenzene
rich stream, also separated and withdrawn from the
separation zone. The admixture of benzene and di~ and
25
triisopropylbenzene is passed into a transalkylation
reaction zone, wherein the reactants combine to pro
duce cumene.
The inventive process of the present invention is not
limited by the catalyst incorporated in the transalkyla
medium between each of the successive beds. In a 30 tion reation zone. Various catalyts are known to one
skilled in the art, such as a boron tri-?uoride-modi?ed
preferred mode of operation, a portion of alkylation
inorganic oxide catalyst described in US. Pat. No.
reaction zone effluent is cooled to about 3595 C.
3,200,163 , an acid extracted crystalline aluminosilicate
and introduced into the reaction mixture as a quench
ing medium between at least two successive catalyst
catalyst described in US. Pat. No. 3,551,510, or a
beds in an amount sufficient to reduce the temperature 35 ?uorine containing refractory inorganic oxide de
scribed in US. Pat. No. 3,205,277. Preferred as a tran
of the reaction mixture to- within about 4 C. of the
temperature of the reaction mixture entering the last
preceding catalyst bed.
As recirculation of alkylation reaction zone effluent
to the inlet of the reaction zone increases, the concen 40
tration of cumene in the alkylation reaction zone also
salkylation catalyst in the present invention is a solid
phoshoric acid catalyst prepared in a similar manner as
the one used hereinabove in the alkylation reaction
zone, or especially preferred is a solid phosphoric acid
catalyst containing 70 to 90 weight percent phospho
increases, thus providing more potential sites for poly
alkylated benzene products and resulting in increased
production of di- and triisopropylbenzene as compared
rous. The transalkylation zone may be equipped with
effluent stream set forth hereinbelow into a separation
zone, wherein by means of a combination of fractional
space velocity based on reaction zone effluent of 0.5 to
heat transfer means, baffles, trays, heating means,
pumping means, etc. The reaction zone is preferably of
to prior art processes. Whereas di- and triisopropylben 45 the adibatic type, and is not limited by reactor design or
con?guration. Conditions utilized in the transalkylation
zene production in prior art processes is typically less
reaction zone may be varied over a relatively wide
than 5 mole percent as compared with cumene produc
range; the transalkylation reaction may be effected at
tion, the present process results in 5 to 20 mole percent
temperatures
of from 35 to 370 C., at pressures of
or more.
Propane, butane, benzene, and cumene comprises 50 about 15 to 200 atmospheres, at molar benzene/
polyisopropylbenzene ratio of about 4 to 16, and liquid
about 90 to 95 mole percent of the alkylation reaction
hourly space velocity based on reaction zone effluent
zone ef?uent, and toluene, butylbenzene, di- and triiso~
of 0.1 to 20. With the preferred solid phosphoric acid
propylbenzene, propylene oligomers and other compo
catalyst, transalkylation reacton conditions include a
nents in trace amounts comprise 10 to 5 mole percent.
The net alkylation zone ef?uent stream withdrawn 55 temperature of about 175 to 290 C., a pressure of
about 20 to 40 atmospheres, molar benzene/polypro
from the alkylation reaction zone is passed separately
pylbenzene ratio of about 4 to 16, and liquid hourly
or in admixture with a transalkylation reaction zone
distillation, absorption, stripping, and ?ashing,'the de
sired components are separated at separation condi
tions selected to minimize utility consumption. Product
streams withdrawn from the separation zone include a
propane-rich stream, a cumene stream, a butylben
zene-rich stream, a propylene oligomer stream, and a 65
benzene drag stream, the lastly stated stream serving to
remove trace quantities of non-aromatic components
boiling between propane and cumene.
5.0 As described hereinabove, effluent of the transalky
lation reaction zone is passed into the separation zone.
I claim as my invention:
l. A process for the production of cumene which
comprises the steps of:
a. reacting propylene with an excess of benzene in
the presence of an alkylation catalyst at alkylation
reaction conditions in an alkylation reaction zone;
b. dividing the total liquid effluent of said zone into at
least two portions of like composition;
4,008,290
10
5. The process of claim 1 further characterized in
that at least a portion of the benzene-rich stream from
the separation zone is supplied to said transalkylation
c. recirculating one of said portions of the effluent to
said reaction zone;
d. introducing another of said portions of said ef?u
zone.
ent and a transalkylation zone ef?uent stream,
6. The process of claim 1 further characterized in
formed as hereinafter set forth, into a separation
that said alkylation catalyst is a solid phosphoric acid
zone;
catalyst.
e. separating from the admixed effluents in the sepa
7. The method of claim 1 further characterized in
ration zone a benzene-rich stream, a cumene prod
that said transalkylation catalyst is a solid phosphoric
acid catalyst.
uct stream and a di- and triisopropylbenzene-rich 10
stream;
8. The method of claim 1 further characterized in
f. transalkylating the last named stream with benzene
that said alkylation catalyst is a solid phosphoric acid
in the presence of a transalkylation catalyst in a
catalyst containing from about 50 to about 75 wt. %
transalkylation zone to form additional cumene;
phosphorus, and said transalkylation catalyst is a solid
g. supplying the ef?uent of the last mentioned zone to
phosphoric acid containing from about 70 to about 90
said separation zone as said transalkylation zone
wt. % phosphorus.
9. The method of claim 1 further characterized in
ef?uent stream;
that the molar ratio of benzene to propylene in said
h. passing at least a portion of said benzene-rich
alkylation zone is from about 2:1 to about 6:1 without
stream from the separation zone to said alkylation
20 inclusion of said one portion of the alkylation zone
reaction zone; and
ef?uent stream.
i. recovering said cumene product stream from the
10. The process of claim 1 further characterized in
separation zone.
that
said phosphoric acid catalyst in said alkylation
2. The process of claim 1 further characterized in
that at least a portion of the benzene for reaction with
the propylene in step (a) is supplied to said separation
reaction zone is in the form of at least two successive
25
zone.
catalyst beds and a third portion of said ef?uent from
the alkylation zone is cooled to a temperature of from
about 35 to about 150 C. and introduced into the
3. The process of claim 1 further characterized in
reaction mixture between at least two successive cata
that at least a portion of the benzene reactant for step
lyst beds as quenching medium.
(f) is supplied to said separation zone and then to the 30 11. The process of claim 1 further characterized in
transalkylation zone.
that said first portion of the ef?uent from the alkylation
4. The process of claim 1 further characterized in
zone is cooled to a temperature of from about 150 to
that the benzene for reaction in steps (a) and (f) is
supplied to said separation zone.
about 260C.
=|=
35
40
45
50
60
65
S-ar putea să vă placă și
- Deconstructing Ipv6 With Montemsoli: Que OndaDocument14 paginiDeconstructing Ipv6 With Montemsoli: Que OndaPepe PompinÎncă nu există evaluări
- A Case For Hash Tables: RSKFMKSFDocument10 paginiA Case For Hash Tables: RSKFMKSFPepe PompinÎncă nu există evaluări
- Us 4521631Document6 paginiUs 4521631Pepe PompinÎncă nu există evaluări
- Campus CapturaDocument1 paginăCampus CapturaPepe PompinÎncă nu există evaluări
- Us 20110245558Document14 paginiUs 20110245558Pepe PompinÎncă nu există evaluări
- Campus CapturaDocument1 paginăCampus CapturaPepe PompinÎncă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Friedel CraftsDocument8 paginiFriedel CraftsAhmed MashalyÎncă nu există evaluări
- Niu Ejoc2020rewDocument15 paginiNiu Ejoc2020rewBálint NagyÎncă nu există evaluări
- Methylation Reagents GuideDocument63 paginiMethylation Reagents GuideTuyết MaiÎncă nu există evaluări
- Stork 1963Document16 paginiStork 1963Daniel JiménezÎncă nu există evaluări
- The Dimethyl Sulfoxide (DMSO) Anion - Dimsyl Ion: Gaylord Chemical Company, L.L.CDocument12 paginiThe Dimethyl Sulfoxide (DMSO) Anion - Dimsyl Ion: Gaylord Chemical Company, L.L.Csameer sahaanÎncă nu există evaluări
- AlkylationDocument50 paginiAlkylationGaurav Lunawat100% (1)
- Introduction to Refinery Delayed Coking ProcessDocument44 paginiIntroduction to Refinery Delayed Coking ProcessDipankar Phukan50% (2)
- 06c5 File PDFDocument3 pagini06c5 File PDFMakhdum Muhardiana PutraÎncă nu există evaluări
- Catalysts: Zeolites As Acid/Basic Solid Catalysts: Recent Synthetic DevelopmentsDocument21 paginiCatalysts: Zeolites As Acid/Basic Solid Catalysts: Recent Synthetic DevelopmentsHawaÎncă nu există evaluări
- Uop ButamerDocument2 paginiUop ButamerCHANADAS0% (1)
- Natural Gas to BTX: A Process Design and Economic AnalysisDocument505 paginiNatural Gas to BTX: A Process Design and Economic AnalysisFrank Pocomucha GallardoÎncă nu există evaluări
- Some Friedel-Crafts Reactions of Y-ButyrolactoneDocument6 paginiSome Friedel-Crafts Reactions of Y-ButyrolactonedntwntÎncă nu există evaluări
- Alkylation, Isomerization and Polymerization: BITS PilaniDocument25 paginiAlkylation, Isomerization and Polymerization: BITS PilaniHritik LalÎncă nu există evaluări
- Organic Chemistry II / CHEM 252 Chapter 19 - Synthesis and: Reactions of β-Dicarbonyl CompoundsDocument40 paginiOrganic Chemistry II / CHEM 252 Chapter 19 - Synthesis and: Reactions of β-Dicarbonyl CompoundsSilambarasan SivalingamÎncă nu există evaluări
- Alkynes: An Introduction To Organic Synthesis: (Ref: Mcmurry'S Organic Chemistry, 7 Edition)Document24 paginiAlkynes: An Introduction To Organic Synthesis: (Ref: Mcmurry'S Organic Chemistry, 7 Edition)Tristan BadillaÎncă nu există evaluări
- Toluene MethylationDocument18 paginiToluene MethylationVăn Đại - BKHNÎncă nu există evaluări
- Catalysis in Petrochemical ProcessesDocument211 paginiCatalysis in Petrochemical ProcessesDavid LunaÎncă nu există evaluări
- In Alk For Refinery MT BeDocument2 paginiIn Alk For Refinery MT BeChristianGuerreroÎncă nu există evaluări
- Reactor design for ethylbenzene productionDocument36 paginiReactor design for ethylbenzene productionDyllan StoneÎncă nu există evaluări
- Petroleum Refining Process Control and RT OptimizationDocument11 paginiPetroleum Refining Process Control and RT Optimizationdemos2011Încă nu există evaluări
- An Introduction To Methods For Analyzing Thiols and Disul Des ReactionsDocument12 paginiAn Introduction To Methods For Analyzing Thiols and Disul Des ReactionsFernanda RigoÎncă nu există evaluări
- HF Alkylation and NExOCTANE Tech For Gasoline ProductionDocument42 paginiHF Alkylation and NExOCTANE Tech For Gasoline ProductionUsama Shakil0% (1)
- CumeneDocument5 paginiCumeneNasmiyeth Rodriguez VittaÎncă nu există evaluări
- Mutagenesis Techniques and FactorsDocument12 paginiMutagenesis Techniques and FactorsAnggara Ista PutraÎncă nu există evaluări
- Alkynes - Problem SolutionsDocument21 paginiAlkynes - Problem SolutionsfrankjenÎncă nu există evaluări
- Site-Selective Methylation of N - Nosyl Hydrazides of N-Nosyl Protected R-Amino AcidsDocument6 paginiSite-Selective Methylation of N - Nosyl Hydrazides of N-Nosyl Protected R-Amino AcidsDiogomussumÎncă nu există evaluări
- Reactions of Thiols: ReviewDocument28 paginiReactions of Thiols: ReviewLindsey BondÎncă nu există evaluări
- A Plant Design Project On Production of Gasoline by Sulphuric Acid Alkylation of OlefinsDocument163 paginiA Plant Design Project On Production of Gasoline by Sulphuric Acid Alkylation of OlefinsUmar Draz50% (2)
- Amines: Organic Bases and Their PropertiesDocument78 paginiAmines: Organic Bases and Their PropertiesWinni TanÎncă nu există evaluări
- Sintesis de N-Alquil Amino AcidsDocument45 paginiSintesis de N-Alquil Amino AcidsYbiedSalazarÎncă nu există evaluări