Documente Academic
Documente Profesional
Documente Cultură
11
Încărcat de
jinny1_0Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
11
Încărcat de
jinny1_0Drepturi de autor:
Formate disponibile
Napa Suansuwan, BSc, DDS, Grad Dip Clin Se,
MSc'
Michael Vincent Swain, BSc (Hons), PhD''
New Approach for Evaluating
Metal-Porcelain Interfacial Bonding
Purpose: The purpose of this study was to evaluate the honding characteristics of
porcelain-fused-to-metal (PFM) systems hy determining the strain energy release rate
associated with interface fracture of porcelain and metals. Materials and Methods:
Porcelain-veneered metal plates cast from commercially pure titanium and 3 metal alloys
(gold, palladium, and nickel-chromium alloys) were made to dimensions of 25 mm x 8
mm X 2,5 mm with comparable thicknesses of porcelain and melal. The porcelain side
of the specimens was notched to the interface with a thin diamond saw, and a small
precrack was initiated at the metal-porcelain interface. The samples were subjected to a
limited number (typically less than 4) of load-unload cycles under 4-point bending at a
crosshead speed of 0,1 mm/min. The loading and unloading force displacements
associated with stable crack extension were recorded. The strain energy release rate was
calculated. The interfacial area was also examined under scanning electron microscope
(SEMI after the test. Results: The mean strain energy release rates were 72.7 10,0 |/m-,
58,5 1 3 , 5 l/m-, 39,4 4,3 J/m^ and I 6 , 6 2,5 |/m^ tor the samples of gold, palladium,
nickel-chromium alloys, and titanium, respectively. The SEM photographs showed that
the crack occurred in the porcelain layer close to the interface. Conclusion: The bonding
characteristics of PFM systems were determined with .^i types of metal alloys and
commercially pure titanium by a fracture mechanics approach. The gold alloy and
titanium are considered to obtain the greatest and least adhesion, respectively. The test
system has proven to be a simple and reliable approach lo determine the bonding in
bimaterial systems. Int j Prosthodont 1999:12:547-552.
old alloys have been used in dentistry as both
cast and porcelain-fused-to-metal (PFM) restorations for decades because of their accuracy in the
casting process and durability and corrosion resis-
^Assistant Professor, Department of Prosthodontics, Facuity of
Dentistry, Khan Kaen University, Thailand.
"Professor, Department of Mechanics and Mechatronics, Facuity
of Engineering: and Department of Dentai Materials Science,
Faculty of Dentistry, The University of Sydney, Austraiia.
Reprint requests: Prof Dr Michaei Vincent Swam. Biomateriais
Science Research Unit. Faculty of Dentistry, The University of
Sydney, Suite Cli, National Innovation Centre, Australian
Technology Park, Eveleigh, NSW 1430, Australia. Fax: + 61-29351 !8IS. e-maii mswain<Smail.usyd edu.ou
12, Number 6,
547
tance in the aggressive conditions of the mouth,'
However, their disadvantages, ie, high density, low
elastic modulus, poor sag resistance at porcelain firing temperature, and relatively high cost, especially
of high-gold alloys used in PFM restorations, stimulated a search for alternatives.^'- Many types of dental aiioys, such as Pd, Co-Cr, and Ni-Cr alloys, have
been developed as economical alternatives that also
have a lower density and higher elastic modulus,'-^
Recently, commercially pure titanium and titanium
alloys have been introduced to replace these metallic alloys because of their mechanical and corrosion
resistance properties compared to those of noblemetal alloys. They are also lightweight, inexpensive,
and biocompatible."'
The InternaiionsI lournal of Proslhodontk
New Approach for Evalualing Metl-PorcelLiin Bonding
Su snsu wan/Swain
Apart from these mechanical properties of Ihe metal
alloys required for PFM restorations, the alloys must
form a strong bond with the compatible porcelain.
Shear tests, which are simple to perform, are the most
popular, and several metal-ceramic bond tests have
been done in this mode with different test configuration designs.^"-' The planar shear test is claimed to be
a well-suited design for evaluation of metal-ceramic.'Other studies use a flexural mode, ie, 3- and 4-point
bending tests. In this mode, porcelain was bonded to
the tension surface of a metal bar only in the middle
part.--'--" However, the result in all of these studies is
quoted as the critical stress at bond failure. Some researchers have calculated a "bond strength ratio" by
dividing the maximum stress required to break the
bond by the elastic modulus ofthe metal substrate to
minimize the influence of elastic modulus on the fracture strength.^"*'^^ However, neither bond strength nor
bond strength ratio represent the energy or work required to separate porcelain from metal. Rather, they
measure only the comparative stresses to initiate catastrophic breaking ofthe specimen.
As a result of the inherent limitations of shear tests,
and also in an attempt to compare the actual characteristics ofthe stress distribution in different bond tests,
finite element stress analysis has been reported.^^-^"^
These stress distribution characteristics have been compared with the experimental observations of fracture location and type of failure.^''' In the 3- and 4-point
bending tests there is a severe stress concentration at the
porcelain endpoints.'^'^ However, the stress distribution
in shear tests shown in the finite element stress analysis resulted from a force ideally applied at the metalporcelain interface, which does not occur experimentally. Eor this reason, the failure of the bonded system
may possibly be initiated within the porcelain, close to
the interface but not on it, in which case the failure is
caused by tensile stresses.^^'^^ The studies also showed
that the bond strength could change with specimen
geometry, loading configurations, or material stiffness
as these give rise to different stress distributions at the
bonded interface.^" It is also important to remember that
alloy with an elevated elastic modulus will resist bending to a greater extent, thus creating the impression of
ahigherbond.^^Therefore, the traditional bond strength
data based on static load-to-failure tests should not be
used to characterize the bond. Another approach,
which is proposed as an alternative to the Intemational
Standards Organization 9693 test, considers the 3point flexure of a metallic strip partly coafed with ceramic. Although in this test Young's modulus and the
thickness ofthe metal substrate are taken into account,
this is also a bond strength measurement test.^^
Recently, there has been an upsurge of interest in
the characterization of interfacial fracture toughness
The International loumal of Proi^tliod
548
of dissimilar materials.^* There have also been some
studies determining the fracture resistance of bimaterial interfaces, including metal-ceramu" inierfaces,
based on the concepts of fracture mech.mics.-' " A
4-point bending test has been used to demonstrate the
characteristics of crack propagation and u show the
sensitivity ofthe fracture resistance to moisture through
a stress corrosion mechanism,^^'*" The test method has
been recently applied to evaluate the interfacial fracture toughness of a titanium-porcelain bonding system.^^ From these studies, it has been suggested that
the bimaterial bend test geometry combined with interfacial fracture toughness measurement is an appropriate alternative method for studying the bonding
characteristics in a PFM system. In the present study
the strain energy release rate at the metal-porcelain interface of 3 types of dental alloys and commercially
pure titanium bonded to compatible porcelain was determined using a 4-point bending test.
Materials and Methods
Twelve rectangular plates (8 mm x 25 mm x 1.4 mm)
were cast for each group of gold, palladium, and
nickel-chromium alloys and for titanium using a centrifugal technique, except for titanium. An argonpressure, one-chamber casting machine (Autocast
HC III, CC) was used to cast the titanium. The gold
alloy (KIK, Ishifugu Metal) is classified as Au-Pd-Pt^
and the palladium alloys (KIK Wing, Ishifugu) as PdSb-ln. The nickel-chromium alloy (UniMetai, Shofu)
contains 77.0% nickel, 14.9% chromium, and 8.1%
other elements. The titanium used in this study is
commercially pure titanium (ASTM Ti Grade 2, TAlloy M, CC).
The cast metal plates were ground with silicon carbide paper to achieve flat and smooth surfaces. One
of the 8 mm x 25 mm surfaces was grit blasted with
50-|jm AUO^. The specimens were cleaned in an ultrasonic bath before bonding to porcelain. Vintage
Halo porcelain (Shofu) was used for the gold, palladium, and nickel-chromium alloys and Vita
Titankeramik porcelain (Vita) was used with titanium.
The porcelain was applied onto the grit blasted surface
ofthe metal plates and baked layer by layer atthe temperatures recommended by the manufacturers. The
paste bonder of Vita Titankeramik was applied to titanium plates as the first layer, followed by the normal
steps of opaque-and body-porcelain application.
After firing, both 8 mm x 25 mm surfaces of the
specimens were ground flat and smooth but still retained an even layer of each material. The edges of
the specimens were also ground at right angles to the
previous surfaces. Notching across the width and
entirely through the depth ofthe porcelain layer at the
Volume 12, Number 5
Suansuwan/Swairi
New Approach br Ev^lualing Metdi-Pomeiain Bonding
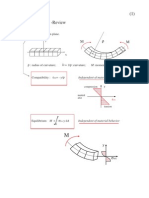
Fig 1 (left)
fhe notch.
Initial inlerfaciai crack (precrack) af the bottom of
Fig 2 (beiowj
Tesfing configuration and relative dimensions of
system. P= ioad necessary lo sfably propagate crack; = specimen width, h = tolal specinnen fhicitness; = moment arm or disfance between inner and outer load iines (rollers) on the same side.
middle of fhe specimen was performed using a rotary
diamond cutting blade with kerosene lubricant,
which gave an accurate notch width of 0.4 mm. The
notch was filled with kerosene when a precrack was
created in a bending jig in which the applied load is
controlled by a screw knob and the specimen is supported completely on rubber. The precrack started
from the base of the notch and extended along the interface with a total length of 2.0 mm (Fig 1). Without
the presence of a small precrack, it was found that the
specimen had to be overloaded to initiate crack
growth, often resulting in complete debonding of the
porcelain from the metal substrate.
The precracked specimen was then placed on a 4point bending jig mounted in a universal testing machine (Autograph AG-50kNE, Shimadzu). The specimen was subjected to a limited number of load and
partial unload cycles (typically less than 4) at a
crossheadspeedofO.i mm/min until the crack reached
the inner rollers. During crack extension, the crack
length was estimated using a measuring gauge with a
traveling light microscope located in front of the jig. For
aclear view ofcrack length, one side of the specimen
was thinly painted with white correction liquid
(Coverup, Marbig Rexel), which readily revealed the
crack beneath during loading. The load and crosshead
displacement data were collected for calculation.
The testing configuration shown in Fig 2 illustrates
the relationship between the sample and the rollers
ofthe4-point bending jig. The strain energy release
rate, G, is given by^^
width and total thickness, respectively. The nondimensional parameter T| is calculated by
+*
+ 3*
\tl
/here
J1-V)
V and E are the Poisson ratio and elastic modulus
or porcelain, respectively, and h and b^ are the
thickness of porcelain and metal layers, respectively.
As T) increases with increase in relative specimen
thickness,-*^ ''p-Ai/ ='" specimens in this study have a
ralio of thickness of approximately 1 ;1.
A feature of the expression relating the strain energy release rate, C, (equation 1 ) to measured parameters is that it is independent of interface crack
iength, provided that the crack lies within the inner
rollers. In a previous study it was shown that the
value of C calculated from equation 1 was in good
agreement with a simple estimation of the fracture energy per unit area of crack surface generated, as determined from the area under the force displacement
curve and fracture surface created.-"
Results
A typical load-displacement curve demonstrating the
load-unload cycles is shown in Fig 3. The specimen
stiffness (F/) is initially constant up to a critical load
forthe onset of stable crack extension, whereupon, as
anticipated from equation 1, the load remains almost
constant, although the stiffness decreases with crack extension. The mean load at the plateau area of each
cuive, which is associated with stable crack extension,
was used to calculate fhe value of C; this is presented
with the Poisson ratio and elastic modulus values of tbe
where Pis the load necessary to stably propagate the
crack, / is the moment arm or distance between inner
and outer load lines (rollers) on the same side, v^ and
E arethe Poisson ratio and elastic modulus of metal
substrate, respectively, and band /i are the specimen
Volume12, Number 6, 1999
),
1
/]
549
The lnlernalion.il lournai of Prosthodortics
New Approach lor Evaiuating Metsi-Porcelain Bonding
Suanu wan/Swam
Table 1 Characteristics of 4 Metal Aiioys Bonded to
Porcelain
t3{J/m')
Specimen
Gold-porceiain
Paliadium-porceiain
Nicitel-chromium-porcelain
Titanium-porcelain
72,7
58,5
39,4
16,6
10.0
13.5
4.3
2.5
E(GPa)
93,0
115.2
203,6
107.3
0.39
0.38
0.30
0.31
G= resultant strain energy release rate^ E= elastic modulus, w = Poisscn
0.02
0.04
0 Oe
0.08
0,10
0.12
Dispiacemant (mm)
Fig 3 Load-dis placement curve illustrates the plateau during
crack extension.
.*
Fig 4 Intertaciai area ot goid (Au) bonded to porceiain (P)
shows ttie reaction zone (R) and the crack Cj.
Fig 5 Intertaciai zone ot paliadium (Pd) bonded to porcelain (Pj
shows the reaction zone (R) and the crack (C).
metal substrates in Table 1. The difference between the
means of the C values of each group was significant
{P<0.05).
Scanning electron microscope (5EM) photographs of
cross-sectioned samples showed the location of the
crack and the unique characteristics of the reaction
zone at the interfacial area of the gold and palladium
specimens (Figs 4 and 5). Similar images ofthe interface region and cracking for nickel-chromium alloy and
titanium are shown in Figs 6 and 7. The crack in all
groups was located at the interfacial area and was
more likely to be in the porcelain. From observation,
the crack in the palladium and gold sampies was more
irregular than in the nickel-chromium and titanium
samples. There were some small cracks extending from
the interface into the inner layer of porcelain in all
groupsexcepttitanium. A reaction zone was noticeable
in the gold and palladium samples but was hardly
seen in the nickel-chromium and titanium groups.
Discussion
Tlie Inlernational lournal of Pro5tliodontf<
The present technique has a number of advantages
over previous estimations of bond strength (both
tensile and shear). Recent finite element analysis of
shear bond strength tests indicated that a very complex stress situation exists about the interface, which
may result in crack initiation within or propagating
through the porcelain rather than along the metalporcelain interface.^"'^^ In the present study, the
specimen geometry and the precrack made before
testing contributed to the observed stability of crack
extension along the Interface. It prevented high stress
concentration and specimen overload from occurring at the initiation of the crack growth. Such features could be misleading because the force employed for breaking the bond would be higher than
it should be, and tbat fracture tended to be very unstable.
550
Volume 12, Number 6, t
Suaiisuwan/Swain
New Approach for Evaiuating Melal-Porcclain Bonding
Fig 6 Interfaciai area of nickel-chromium (Nij bonded to porcelain IP) shows the crack (C) located ciose to the interface.
Fig 7 Interfaciai area of tuanium (Til bonded to porceiam fP)
shows tiie crack (C) iocated at the interface.
One ofthe advantages of using this 4-point bending technique is that it is simple to perform experimentally. The interfacial strain energy release rate, C,
is also a simple function of the critical load observed
during crack propagation; other parameters remain
constant for a given specimen dimension and elastic
properties, Tbe test has also been found to be highly
reproducible, even with a 20% to 30% variation in
the ratio of h :h^ in the test geometry, provided that
allowance for the r\ dependence of this ratio is incorporated into the analysis. Therefore, this test can
be used for almost any bimaterial system.
It has been suggested that aqueous environments can
decrease the interfacial fracture resistance or increase
the rate of slow crack growth by the mechanism of
stress corrosion,^^'^ The present study minimized tbe
influence of moisture by using kerosene to fill the notch
and crack during testing. This was considered to be simpler and more economical than putting the entire system in an H^O-free environment, even though it may
not entirely prevent such reactions from occurring.
The gold-porcelain system in this study gave fhe
highest value of strain energy release rate, whereas
palladium-, nickel-chromium-, and titanium-porcelain systems presented weaker bonds, respectively.
The previously reported bond strength data vary
widely. Some studies on shear mode^''^ and flexural
m o d e " have suggested that the bond strength of
nonprecious alloy-porcelain is higher than that of
noble alloy-porcelain, while in others the opposite
has been reported,^!^ It has been reported that the
banding of titanium to porcelain is much weaker
than that of palladium-copper alloy to porcelain
wben the load at tensile bond failure from the 3point bending test was measured,"" However, another
5tudy using a shear test based on a torsional load
showed that the bonding of titanium to porcelain
was better than that of the gold-porcelain system.-'
These results obviously vary and are noncomparable.
The SEM photographs showed a reaction zone in
the gold and palladium specimens, which occurred
dur ing firing of the porcelain. The reaction zone contained meta I oxides from the bonded metal alloy and
porcelain. This zone appeared to be brittle and allowed the crack to pass through it in some areas.
Although the reaction zone in nickel-chromium and
titanium samples is not clearly seen in the SEM photographs, their weak bond allowed the crack to propagate mostly along the interface.
12, Number 6, 1
Conclusion
The bonding characteristics of 4 dental alloys bonded
to porcelain were determined in terms of the strain energy release rate, G. The gold-porcelain samples gave
the highest value of C, while palladium, nickelchromium, and titanium samples exhibited lower values, respectively. The SEM photographs ofthe crosssectioned interfacial area revealed that the crack was
located mostly in porcelain, close to the interface, and
also showed the wide reaction zone between metal
and porcelain in the gold and palladium samples.
This interfacial 4-point bending test provides a more
intrinsic estimation of interface properties resulting
from the stable crack extension along the interface.
The present measurements show a high degree of repeatability of the value determined for the interface
toughrtess when allowing for the specimen dimensions. The test has also proven to be a simple approach
to determine the bonding in bimaterial systems.
551
The International lournal of Proithodontii
New Approath for
ng Metal-Porcelain Bonding
Suansu wan/Swain
Acknowledgments
20,
Theauthoiswish to iicknovvleciiie ihe support provided by Ishifugu
Metai industry, Tokyo, |apan, tor the loan of the gold and palladium alloys. We also appreciate the generosity of Shofu, Kyoto,
Japan, tor providing the nickei-chromium alloy and dental porcelain; and Vita Zahnfabrik, Bad Sckingen, Germany, for the titanium porcelain used in ihis study.
21,
22,
23,
References
1,
2,
3,
4,
5,
(i.
7,
8,
9,
10.
11.
12,
13.
14.
15,
16,
17,
18,
19.
24,
Brown D, Curtis RV. Alternatives to gold. Dent Update
1992;19:325-326, 328-330,
Anusavice KJ, Noble metal alloys for metal-ceramic restorations, DentClin Norlh Am 19S5;29:789-e0J.
Craig RC, Restorative Dental Materials, ed 9, St l.ouisi Mosby,
1993:491-501,
Lautenschiager EP, Monaglian P. Titanium and titanium alloys
as dental materials, int DentJ 1993;43:245-253,
Mofla ]P, Lugassy AA, Cuckes AD, Gettleman L, An evaluation
of nonprecious alloys for use with porcelain veneers. Part 1,
Piiysicai properties, J Prosthet Dent 1973;30:424-431.
Wight TA, Bauman JC, Pelleu GB. An evaluation of four variables
affecting the bond strengtii of porcelain to nonprecious alloy. I
Prosthet Dentl977;37:57-577,
25,
Lubovich RP, Coodfcind RJ. Bond strength studies of precious,
semiprecious, and nonprecious ceramic-metal alloys with hvo
porcelains. J Prosthet Dent l977;37;38B-299.
Dent RJ, Preilon JD, Moffa |P, CaputoAA. Effect of oxidation on
ceramometal bund strength, J Prosthet Dent ]9B2;47:59.-62,
Drummond JL, Randolph RC, lekkals VJ, LenkeJW. Sheartesting
of the porcelain-metal bond, J Dent Res 1984;63:1,400-1,401,
BullardIT, Dill RE, Marker VA, Payne EV, Effects ot sputtered
metai oxide films on the ceramic-to-metal bond. J Prostliet Dent
198.');54:776-778,
Quiones EE, Verniilyea SG, Griswold W H , Apparent bond
strength of nonnoble alloy-porcelain combinations. J Prosthet
Dentl9B5 54:359-361,
tdammad lA, Cocdkind R|, Gerberich WV\' A shear test fot the
bond strength of ceramometals. I Prosthet Dent 1987:56:431^37.
30,
26,
W u , Moser JB, lameson LM, Malone WFP, The effect of oxidation heat treatment on porcelain bond strength in selected base
metal alloys, ) Prosthel Dent 1991 ; 6 6 : 4 3 9 ^ 4 4 .
28,
Rake PC, Coodacre CJ, Moore K, Munoz CA. Effect of two
opaquing techniques and two metal surface conditions on metalceramic bond strength, ) Prosthet Dent 1995:74:8-17,
Anusavice K|, DeiHoff PH, Eairburst CW. Comparative evaluation of ceramic-metal bond tests using finite element stress
analysis. J Dent Res 1960:59:608-613.
31,
32,
33,
34,
35,
Hammad lA, Stein RS. A qualitative study for the bond and
colorofceramometals. Parti.J Prosthet Dent 1990;63:643-653,
Lund PS, Davis PW, Shear bond strength of textured opaque
portelain, Int | Proslhodont 1992;5:505-509,
Inoue K, Murakami T, Terada Y. The bond strength of porcelain
to Ni-Cr alloyThe influence of tin or chromium plaling, int |
Prosthodont 1992;5:262-260,
552
Van Noort R, Noroozi S, Howard IC, Cardew C, A critique of
bond strengtb measurements, J Dent 1989;17:61-67,
DeHoffPH, Anusavice KJ,HathcockPW. An evaluation of tbe
four-point flexural test foi metal-ceramic bond strengtb. | Dent
Res 1982:61:1,066-1,069.
DeiHff PH, Anusavice K|, Wang Z, Three-dimensional finite element analysis of the shear bond test. Dent Mater 1995;11:
126-131,
Len; J, Schwarz S, Schvvickeratb I I , Sperner E, Schafpr A, Bond
strength of metal-ceramic systems in three-point flexure bond
test, J Appl Bromater 1995:6:55-64.
Mencik J. Mechanics of Components witb Treated or Coated
Surfaces. Dordrecbl, The Netberlands: Kluwer Academic, 1996:
92-176.
Charaldmbides PG, Lund J, Evans AG, McMeeking RM, A test
specimen for determining the fractLjre resistance ot bimaterial interfaces, J Appl Mech 1989:56:77-82,
36,
Cao l i e , Evans AG, An experimental study of tbe fracture resistance of bimaterial interfaces, Mecb Mater 1989;7:295-304,
37
Reimanis E, Dalgleisb B|, Evans AG, The fracture resistance ot a
model metai/ceramif interface. Acta Metali Mater 1991:39:
3,133-3,141,
Cazzato A, Eaber KT, Fracture eneigy of glass-alumina interfaces via tbe himaterial bend test, J Am Ceram Soc 1997:
80:181-188,
38.
Wagner w e , Asgar K, BigelowWC, Elinn RA. Effect of intcriaciai variabies on metal-porceiain bonding. J Biompd Mater Res
1993;27:531-537.
Hurzeier MB, Kohal R, EiscberC, Eischer], Strub |R. in vitro and
in vivo evaluation of a new opaque system for metal-ceramic
restorations, int | Prosthodont 1995:8:142-149,
The International journal of Prosthodontics
27,
29,
Uusalo EK, Lassila VP, Yli-Urpo A U , Bonding of dental porcelain to ceranijc-metal aiioys. J Prosthet Dent 19B7;57:26-29.
Murai<ami I, Vaidyanathan J, Vaidyanatban TK, Schjiman A,
interactive effeas of etching and pre-oxidation on porcelain
adherence (o non-precious alloys: A guided planar shear test
study. DentMater1990;6:217-222.
Persson M, Bergman M, M eta I .-ceramic bond strength. Acta
Odontol Scand 1996;54:16O-165.
Deger S, Canikliogiu MB, Effects of lin pialin.- '!> base metal
alloy.-ceramic bond strengtb. int| ProsthodonL I'l ^ ' ' . i ' ;l 65-172,
Hammad [A, Taiic VF, Designs of bond strengli i ^sts for metalceramic complexes: Review of tbe lileraturr. I Prosthet Dent
1996:75:602-608,
CaputoAA, Dunn B, RetsbickMH, A flpxural mctbod for evaluation of metal-ceramic bond strengths, i Dent Res 1 977;56:
1,501-1,506.
Scbaffer SP, An approacb to determining the bond strengtb of
ceramometal systems. J Prosthet Dent 1982;4e:282-284.
|ocbenDG,CaputoAA,MatyasJ. Effect of metal surface treatment
on ceramic bond strength, | Prosthet Dent 19B6;55:186-188.
Jochen DC, CaputoAA, Matyas], Eftect of opaque porcelain application on strength of bond to silver-paiiadium alloys. J Prostbet
Dent1990;63:414.-4l8,
39.
Chung HG, Swain MV, Mori T. Evaluation ofthe strain energy
release rate for the fraclure of titanium-porcelain interfacial
bonding, Biomaterials 1997;18:1,553-1,557.
40.
Pang I, Gilbert |L, Chai J, Lautcnschlager EP. Bonding characteristicsof low-fusing porcelain bonded to pure titanium and palladium-copper alloy, J Prosthet Dent 1995:73:17-25,
Volume 12, Number 6,
S-ar putea să vă placă și
- Pulp Therapy-Guideline AapdDocument8 paginiPulp Therapy-Guideline Aapdjinny1_0Încă nu există evaluări
- Groper's ApplianceDocument4 paginiGroper's Appliancejinny1_0Încă nu există evaluări
- G - TMD-pedo MNGMT PDFDocument6 paginiG - TMD-pedo MNGMT PDFjinny1_0Încă nu există evaluări
- A Systematic Review of Impression Technique For ConventionalDocument7 paginiA Systematic Review of Impression Technique For ConventionalAmar BhochhibhoyaÎncă nu există evaluări
- Afpam47 103v1Document593 paginiAfpam47 103v1warmil100% (1)
- E Adaproceedings-Child AbuseDocument64 paginiE Adaproceedings-Child Abusejinny1_0Încă nu există evaluări
- AAPD Policy On - FluorideUseDocument2 paginiAAPD Policy On - FluorideUsejinny1_0Încă nu există evaluări
- Article-PDF-Amit Kalra Manish Kinra Rafey Fahim-25Document5 paginiArticle-PDF-Amit Kalra Manish Kinra Rafey Fahim-25jinny1_0Încă nu există evaluări
- GicDocument5 paginiGicmrnitin82Încă nu există evaluări
- Assertmodule 1Document9 paginiAssertmodule 1Olivera Mihajlovic TzanetouÎncă nu există evaluări
- EEL 7 SpeakingDocument20 paginiEEL 7 SpeakingSathish KumarÎncă nu există evaluări
- Mucosa in Complete DenturesDocument4 paginiMucosa in Complete Denturesspu123Încă nu există evaluări
- Implant AngulationDocument17 paginiImplant Angulationjinny1_0Încă nu există evaluări
- GoldsDocument11 paginiGoldsjinny1_0Încă nu există evaluări
- Spacer Design Jips 2005 PDFDocument5 paginiSpacer Design Jips 2005 PDFjinny1_0Încă nu există evaluări
- Avg Vs NormalDocument1 paginăAvg Vs Normaljinny1_0Încă nu există evaluări
- KurthDocument18 paginiKurthjinny1_0Încă nu există evaluări
- Oral Mucosal Lesions Associated With The Wearing of Removable DenturesDocument17 paginiOral Mucosal Lesions Associated With The Wearing of Removable DenturesnybabyÎncă nu există evaluări
- Denture Base Resin CytotoxityDocument7 paginiDenture Base Resin Cytotoxityjinny1_0Încă nu există evaluări
- ResinDocument8 paginiResinjinny1_0Încă nu există evaluări
- Die HardnerDocument4 paginiDie Hardnerjinny1_0Încă nu există evaluări
- DisinfectionDocument5 paginiDisinfectionjinny1_0Încă nu există evaluări
- AlginateDocument5 paginiAlginatejinny1_0Încă nu există evaluări
- 7Document8 pagini7jinny1_0Încă nu există evaluări
- 11Document7 pagini11jinny1_0Încă nu există evaluări
- Patient-Assessed Security Changes When Replacing Mandibular Complete DenturesDocument7 paginiPatient-Assessed Security Changes When Replacing Mandibular Complete Denturesjinny1_0Încă nu există evaluări
- 3Document7 pagini3jinny1_0Încă nu există evaluări
- Stress Fatigue PrinciplesDocument12 paginiStress Fatigue Principlesjinny1_0Încă nu există evaluări
- Long-Term Cytotoxicity of Dental Casting AlloysDocument8 paginiLong-Term Cytotoxicity of Dental Casting Alloysjinny1_0Încă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- All SubjectsDocument473 paginiAll SubjectsAnonymous R8KAp2100% (4)
- Plane Sections Remain Plane. Y: CompressionDocument5 paginiPlane Sections Remain Plane. Y: CompressionakanyilmazÎncă nu există evaluări
- Me-331 Design of Machine Elements Unit Iv Design of Basic ElementsDocument25 paginiMe-331 Design of Machine Elements Unit Iv Design of Basic ElementsMuthuvel MÎncă nu există evaluări
- ADAPT T901 Effective Width PT BeamsrDocument2 paginiADAPT T901 Effective Width PT BeamsrJesus Jimenez JuarezÎncă nu există evaluări
- Som Assignment - 1,2,3Document6 paginiSom Assignment - 1,2,3manmohansinghloteyÎncă nu există evaluări
- The EST Method Structural AnalysisDocument262 paginiThe EST Method Structural Analysispoop100% (1)
- Structural Timber - Strength Classes: British Standard BS EN 338:2003Document14 paginiStructural Timber - Strength Classes: British Standard BS EN 338:2003Patricia Gabriela SavuÎncă nu există evaluări
- Wind Pressure Calculation For 3 Sec Gust Extreme StormDocument5 paginiWind Pressure Calculation For 3 Sec Gust Extreme StormChinnaraja GandhiÎncă nu există evaluări
- Standard Test Methods For Bend Testing of Material For DuctilityDocument10 paginiStandard Test Methods For Bend Testing of Material For DuctilitysahidÎncă nu există evaluări
- Analysis of Raft Foundation Using Finite Element ApproachDocument15 paginiAnalysis of Raft Foundation Using Finite Element ApproachShadin Asari ArabaniÎncă nu există evaluări
- Design Of Superstructure Box GirderDocument51 paginiDesign Of Superstructure Box Girderankit100% (3)
- Flexural Design of Singly Reinforced Beam Sections by LSMDocument12 paginiFlexural Design of Singly Reinforced Beam Sections by LSMKallem KiranmayiÎncă nu există evaluări
- Perma Lite Aluminium Purl in SolutionsDocument44 paginiPerma Lite Aluminium Purl in SolutionsArif RusyanaÎncă nu există evaluări
- 1996 Bookmatter ReinforcedConcreteDesignToEuro PDFDocument20 pagini1996 Bookmatter ReinforcedConcreteDesignToEuro PDFJy YeshiberÎncă nu există evaluări
- Big NJODocument6 paginiBig NJOwalterÎncă nu există evaluări
- BENDING LAB 3..latestDocument28 paginiBENDING LAB 3..latestKabuki Mask100% (2)
- Aisc Clean Columns WorksheetDocument5 paginiAisc Clean Columns Worksheetscrbdgharavi100% (1)
- Structures in Architecture, 1990-2006 EditionDocument300 paginiStructures in Architecture, 1990-2006 EditionNitu GabrielÎncă nu există evaluări
- Torsion PDFDocument21 paginiTorsion PDFm liovicÎncă nu există evaluări
- BEeM 101.01Document34 paginiBEeM 101.01Anonymous I8nZsDqlmh100% (1)
- High-Strength Concrete Deep Beams With Effective Span and Shear Span VariationsDocument9 paginiHigh-Strength Concrete Deep Beams With Effective Span and Shear Span VariationspicottÎncă nu există evaluări
- Parameters:: 32.0 Mpa 460.0 Mpa 3000.0 MM 300.0 MM 502.0 MM 2700.0 KN-M 450.0 KN 30 MMDocument6 paginiParameters:: 32.0 Mpa 460.0 Mpa 3000.0 MM 300.0 MM 502.0 MM 2700.0 KN-M 450.0 KN 30 MMErnest NavarroÎncă nu există evaluări
- Stresses in Rigid PavementsDocument84 paginiStresses in Rigid PavementsCharl de Reuck100% (1)
- Sump Wall Design - From StaadDocument10 paginiSump Wall Design - From StaadOuseppachan AmbookenÎncă nu există evaluări
- Structural Drawing Information SheetDocument40 paginiStructural Drawing Information SheetfikremaryamÎncă nu există evaluări
- Projek Solid Mechanics 1Document3 paginiProjek Solid Mechanics 1Shaktivell Letchumanan0% (1)
- DESIGN of STEEL STRUCTURES Multiple Choice QuestionsDocument31 paginiDESIGN of STEEL STRUCTURES Multiple Choice QuestionsEni VinoÎncă nu există evaluări
- PT Slab Bridge Deck 20 MDocument8 paginiPT Slab Bridge Deck 20 Msamirbendre1Încă nu există evaluări
- Purlin and Girth Design for Roof StructureDocument11 paginiPurlin and Girth Design for Roof StructureRajveer SinghÎncă nu există evaluări
- LSDSS - TM & CM MCQSDocument26 paginiLSDSS - TM & CM MCQSravi maske100% (1)