Documente Academic
Documente Profesional
Documente Cultură
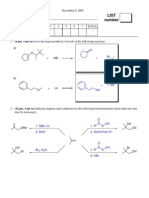
(15 Points) Predict The Products of The Following Reactions. Show Relative Stereochemistry (Only One Stereoisomer) Where Appropriate
Încărcat de
parnaTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
(15 Points) Predict The Products of The Following Reactions. Show Relative Stereochemistry (Only One Stereoisomer) Where Appropriate
Încărcat de
parnaDrepturi de autor:
Formate disponibile
242a Honors Organic Chemistry
Ghosh
October 4th 2007
Exam II
Name_____________________________
1. (15 points) Predict the products of the following reactions. Show relative stereochemistry (only one
stereoisomer) where appropriate.
CH2I2, Zn(Cu)
Ether
1. Hg(OAc)2, H2O
2. NaBH4
1. BH3, THF
2. H2O2, OH-
1. OsO4
1. O3
2. Zn, H3O+
2. NaHSO3 , H2O
Bonus: (3 points) Provide a name for any three reactions shown above, such as hydroboration (write the names
above the appropriate reaction)
242a Honors Organic Chemistry
Ghosh
October 4th 2007
Exam II
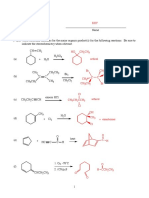
2. (18 points) Provide the starting material, products or reagents for the following reactions.
1. NaNH2, NH3
Br
2.
H2, Pd/C
Reagent:
1. Hg(OAc)2, H2O/THF
2. NaBH4
H C C
1. NaNH2, NH3
2.
Br
H2, Lindlar Catalyst
Bonus: (6 points) Draw the enol that leads to the ketone (A) shown above. What is this process called? Provide a
mechanism for the reaction.
242a Honors Organic Chemistry
Ghosh
October 4th 2007
Exam II
3. (10 points) a) Provide a step-by-step mechanism that explains the formation of the product shown and
clearly indicate stereochemistry at each step.
Br2, CH3CH2OH
Br
H O
b) Is the product shown a result of Markovnikov or anti-Markovnikov addition? Which would be favored
and why?
c) What factors influence carbocation stability?
242a Honors Organic Chemistry
Ghosh
October 4th 2007
Exam II
4. (24 points) Provide a reasonable synthetic scheme for making the following compounds with the
correct stereochemistry from the indicated starting materials. Each synthesis requires several steps.
Please show the structures of all the intermediate compounds.
H
C C
Multiple Steps
Br
Multiple Steps
OH
Multiple Steps
H
C C
H
HO
242a Honors Organic Chemistry
Ghosh
Exam II
October 4th 2007
5. (8 points) Identify all (a-c) of the following compounds as E or Z isomers. Provide the standard IUPAC
name for (c) and also, draw the structure corresponding to (d).
b)
a)
d) Draw (4E)-2,4-dimethyl-1,4-hexadiene
c)
Name:
6. (20 points) Provide a plausible (you are trying to explain how the product is formed) curved-arrow
mechanism for the following reactions as shown below. Show every mechanistic step clearly and number
the carbon atoms in both starting material and product.
a)
OH
HO
H3O+
b)
HO
242a Honors Organic Chemistry
Ghosh
Exam II
October 4th 2007
7. (5 points) Provide compounds (draw the compounds and circle the acid H) with pKa values of 4, 10, 16,
25, and 60
Bonus (8 points) Please try this only after you have finished the exam.
Compound A (C9H12) absorbs 3 equivalents of H2 on catalytic hydrogenation to give compound B (C9H18).
Upon ozonolysis, compound A gave several different products, one of which was identified as
cyclopentanone. Compound A reacted with NaNH2 in NH3, followed by addition of 1-bromoethane, to
give a new hydrocarbon, C (C11H16). Pleased provide structures for A, B, and C.
A.
B.
C.
S-ar putea să vă placă și
- B2: Module 3: Lesson 3 Assignment Chem 30: (?/44 Marks)Document4 paginiB2: Module 3: Lesson 3 Assignment Chem 30: (?/44 Marks)Jeannie XecÎncă nu există evaluări
- Business Plan For Water Nature Refilling StationDocument7 paginiBusiness Plan For Water Nature Refilling StationJonah JonahÎncă nu există evaluări
- Beng (Hons) Petroleum Engineering: Course: Introduction To Petroleum Engineering Instructor Dr. Tarek DarwichDocument33 paginiBeng (Hons) Petroleum Engineering: Course: Introduction To Petroleum Engineering Instructor Dr. Tarek Darwichshanecarl50% (4)
- CHEM2700 Exam Answer - 2007W 2nd MidtermDocument11 paginiCHEM2700 Exam Answer - 2007W 2nd MidtermBruce CourtinÎncă nu există evaluări
- Although This Process Is No Longer in Common UseDocument15 paginiAlthough This Process Is No Longer in Common Usedia_aldy100% (1)
- Assignment 1 - Aldehyde and Ketone Mac-Jul 2013Document2 paginiAssignment 1 - Aldehyde and Ketone Mac-Jul 2013anessismanisÎncă nu există evaluări
- Organic Chemistry Additional Problems Final Exam Part2Document6 paginiOrganic Chemistry Additional Problems Final Exam Part2John SmithÎncă nu există evaluări
- Test 01 Spring 07Document8 paginiTest 01 Spring 07amiraliiiÎncă nu există evaluări
- Additional Problems Final Exam Part 2 AnswersDocument10 paginiAdditional Problems Final Exam Part 2 AnswersJohn SmithÎncă nu există evaluări
- Write Your Answers On This Sheet: N O O O O O PHDocument2 paginiWrite Your Answers On This Sheet: N O O O O O PHGhadeer M HassanÎncă nu există evaluări
- 231 F 2010 Practice MT4 - Pp1to12Document12 pagini231 F 2010 Practice MT4 - Pp1to12Chemist MeÎncă nu există evaluări
- Tutorial 3Document8 paginiTutorial 3Ahmad WahideeÎncă nu există evaluări
- List Number: 1 2 3 4 5 6 7 TotalDocument5 paginiList Number: 1 2 3 4 5 6 7 Totaltcrupi1Încă nu există evaluări
- Carboxylic Acids and Acid Derivatives Carbonyl PDFDocument124 paginiCarboxylic Acids and Acid Derivatives Carbonyl PDFrvignesh2809Încă nu există evaluări
- 2004 OrgSeminarTestAnswerDocument2 pagini2004 OrgSeminarTestAnswerGhadeer M HassanÎncă nu există evaluări
- 2423 e 2Document24 pagini2423 e 2Agustin KurniatiÎncă nu există evaluări
- Assessment Acids BasesDocument3 paginiAssessment Acids BasesJuju ZenemijÎncă nu există evaluări
- Organic Chemistry Questions 3Document12 paginiOrganic Chemistry Questions 3Ram KrishnaÎncă nu există evaluări
- CHEM 135 Exam 2 F15 KeyDocument7 paginiCHEM 135 Exam 2 F15 KeyMikeÎncă nu există evaluări
- SP 2007 Final Examination Organic II 200pts (Weighted As 300)Document21 paginiSP 2007 Final Examination Organic II 200pts (Weighted As 300)Ummi Khairani UrfaÎncă nu există evaluări
- SP 2006 Final Examination Organic II 200pts (Weighted As 300)Document22 paginiSP 2006 Final Examination Organic II 200pts (Weighted As 300)Ummi Khairani UrfaÎncă nu există evaluări
- Organic Exam Answer.Document11 paginiOrganic Exam Answer.S JÎncă nu există evaluări
- Organic Chemistry 3A Additional Problems Final Exam Part 1Document7 paginiOrganic Chemistry 3A Additional Problems Final Exam Part 1John SmithÎncă nu există evaluări
- Final Exam Review - 2011Document5 paginiFinal Exam Review - 2011Elissa BakerÎncă nu există evaluări
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 paginiCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- O O H O Product Starting Material: CH O O O O O ODocument4 paginiO O H O Product Starting Material: CH O O O O O ODerick CheruyotÎncă nu există evaluări
- CHM 241-Practice Exam FinalDocument12 paginiCHM 241-Practice Exam FinalPreeti SharmaÎncă nu există evaluări
- Revision A Level 2022 QPDocument3 paginiRevision A Level 2022 QPJulianÎncă nu există evaluări
- Sample Test One Chemistry 353: PAVIA 4-17-2000Document9 paginiSample Test One Chemistry 353: PAVIA 4-17-2000Phi NguyenÎncă nu există evaluări
- Spring 2002 Org II Exam #2 CH 18-19 (100 Points)Document14 paginiSpring 2002 Org II Exam #2 CH 18-19 (100 Points)Erica Alviyanti BastiandÎncă nu există evaluări
- Q4 06710-12-14 Qs and AsDocument13 paginiQ4 06710-12-14 Qs and AsGhadeer M HassanÎncă nu există evaluări
- CHM556 Assignment1Document4 paginiCHM556 Assignment1Saidin AhmadÎncă nu există evaluări
- JP XII Organic ChemistryDocument7 paginiJP XII Organic ChemistryNibha PandeyÎncă nu există evaluări
- Applied Science ICA 3 2010Document7 paginiApplied Science ICA 3 2010Lee HollidayÎncă nu există evaluări
- CH CH CCH C CHDocument15 paginiCH CH CCH C CHVirgilio Ebajo Jr.Încă nu există evaluări
- Alcohol & EtherDocument217 paginiAlcohol & EtherAmitÎncă nu există evaluări
- Practice 3ADocument12 paginiPractice 3ACamha NguyenÎncă nu există evaluări
- Final Exam For Organic II 200pts (Weighted As 300) : ROH ROR RCNDocument23 paginiFinal Exam For Organic II 200pts (Weighted As 300) : ROH ROR RCNUmmi Khairani UrfaÎncă nu există evaluări
- QuestionDocument12 paginiQuestionKiranSinglaÎncă nu există evaluări
- Assignment 2 CHEM 215Document6 paginiAssignment 2 CHEM 215Abdullah AlteneijiÎncă nu există evaluări
- DQ of Alcohol, Phenol and EtherDocument15 paginiDQ of Alcohol, Phenol and Etherjeeaspirant2024jaiÎncă nu există evaluări
- Test1 342 PracticeV1Document5 paginiTest1 342 PracticeV1Camha NguyenÎncă nu există evaluări
- SP 2004 Final Organic II 200pts (Weighted As 300) : RNH C N H O RN +Document25 paginiSP 2004 Final Organic II 200pts (Weighted As 300) : RNH C N H O RN +Ummi Khairani UrfaÎncă nu există evaluări
- Chapter 7Document30 paginiChapter 7Apichat Junsod100% (4)
- Practice Exam 2.3-1Document6 paginiPractice Exam 2.3-1jamalÎncă nu există evaluări
- Organic Chem Exam 3Document4 paginiOrganic Chem Exam 3Alyssa McCallÎncă nu există evaluări
- Practice Exam 4Document6 paginiPractice Exam 4api-197124028Încă nu există evaluări
- Quiz 1 Sko3033 PDFDocument4 paginiQuiz 1 Sko3033 PDFFiona Tiwon100% (1)
- Organic Chemistry: Chem 210 Practice Exam 3BDocument13 paginiOrganic Chemistry: Chem 210 Practice Exam 3Bemmanferrer482Încă nu există evaluări
- SCH 2358 - Organic Synthesis - Print ReadyDocument4 paginiSCH 2358 - Organic Synthesis - Print ReadyDerick CheruyotÎncă nu există evaluări
- HW 1 KeyDocument6 paginiHW 1 Keywinadoo87789697Încă nu există evaluări
- Resonance Chemistry DPP 6 (Advanced)Document11 paginiResonance Chemistry DPP 6 (Advanced)Anurag1210701067% (6)
- 2013 HSC ChemistryDocument46 pagini2013 HSC ChemistrylillianaÎncă nu există evaluări
- OCHEM Practice FinalsDocument13 paginiOCHEM Practice FinalsNoleÎncă nu există evaluări
- SP 2000 Final Organic II 200pts (Weighted As 300) : ROH ROR O RoorDocument25 paginiSP 2000 Final Organic II 200pts (Weighted As 300) : ROH ROR O RoorUmmi Khairani UrfaÎncă nu există evaluări
- College Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsDe la EverandCollege Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsÎncă nu există evaluări
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsDe la EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsÎncă nu există evaluări
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsDe la EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsÎncă nu există evaluări
- Handbook of Coordination Catalysis in Organic ChemistryDe la EverandHandbook of Coordination Catalysis in Organic ChemistryÎncă nu există evaluări
- Polyatomic Molecules: Results of ab Initio CalculationsDe la EverandPolyatomic Molecules: Results of ab Initio CalculationsÎncă nu există evaluări
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersDe la EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersEvaluare: 3 din 5 stele3/5 (2)
- Lewis MathesonDocument5 paginiLewis Mathesonneft100% (1)
- GTFC Exit Exam Model Q and A - 2 ADocument53 paginiGTFC Exit Exam Model Q and A - 2 AAjal ShajahanÎncă nu există evaluări
- Diagram Alir Proses Pengolahan Gula Pabrik Gula Cinta Manis: Cane LifterDocument1 paginăDiagram Alir Proses Pengolahan Gula Pabrik Gula Cinta Manis: Cane LifterTika SeptiaÎncă nu există evaluări
- Combol PDFDocument3 paginiCombol PDFJay Em Kristel MengulloÎncă nu există evaluări
- Fischer Tropsch SynthesisDocument18 paginiFischer Tropsch Synthesisdeion29Încă nu există evaluări
- Fuels and CombustionDocument24 paginiFuels and Combustionmukirir47Încă nu există evaluări
- Mu Et Al 2024 Shape Selectivity of Ael Channels For Anomalously Facilitating Biojet Fuel Production From Long Chain NDocument11 paginiMu Et Al 2024 Shape Selectivity of Ael Channels For Anomalously Facilitating Biojet Fuel Production From Long Chain Nrozsor2100% (1)
- AIChE 2011 NSDC Problem StatementDocument16 paginiAIChE 2011 NSDC Problem Statementcmm4671Încă nu există evaluări
- PET 521 A Natural Gas by Engr DR AnyadiegwuDocument60 paginiPET 521 A Natural Gas by Engr DR Anyadiegwudavidchinedu008Încă nu există evaluări
- SN1 and SN2 ReactionDocument10 paginiSN1 and SN2 Reactionarizza_mendozaÎncă nu există evaluări
- Changes in Vapour Pressure. (Vapour Pressure Lowering) : V - P Depends Only On The SolventDocument5 paginiChanges in Vapour Pressure. (Vapour Pressure Lowering) : V - P Depends Only On The SolventMarthy DayagÎncă nu există evaluări
- Tugas 2 SINPRODocument5 paginiTugas 2 SINPROcitra maharaniÎncă nu există evaluări
- Project Department Kuwait National Petroleum Company: Daily Welding Inspection ReportDocument1 paginăProject Department Kuwait National Petroleum Company: Daily Welding Inspection ReportMuthazhagan SaravananÎncă nu există evaluări
- Module 2 - Solutions and Their PropertiesDocument10 paginiModule 2 - Solutions and Their PropertiesRuth Aquino50% (2)
- Rev1-Part III - Iec 60567Document10 paginiRev1-Part III - Iec 60567သူ ရိန်Încă nu există evaluări
- ETBE Synthesis Via Reactive Distillation. 1. Steady-State Simulation and Design AspectsDocument15 paginiETBE Synthesis Via Reactive Distillation. 1. Steady-State Simulation and Design AspectsDeepshikhaSinghÎncă nu există evaluări
- CVD PVD: CVD - Chemical Vapor Deposition Technique PVD - Physical Vapor Deposition TechniqueDocument13 paginiCVD PVD: CVD - Chemical Vapor Deposition Technique PVD - Physical Vapor Deposition Techniquerahat s.haiderÎncă nu există evaluări
- Beverage Refrigeration System: Refrigerant: Ammonia (NHDocument31 paginiBeverage Refrigeration System: Refrigerant: Ammonia (NHDionis DagumanÎncă nu există evaluări
- Propane de Hydrogen at I On PD HDocument17 paginiPropane de Hydrogen at I On PD Hahmadreza azadiÎncă nu există evaluări
- Uop FCCDocument23 paginiUop FCCHarish Kotharu100% (1)
- 97 03 EDocument28 pagini97 03 EAgustin CesanÎncă nu există evaluări
- ELCAS5 Exergoceonomics Methanol ProductionDocument26 paginiELCAS5 Exergoceonomics Methanol ProductionAnsinath BarathiÎncă nu există evaluări
- BPCLDocument17 paginiBPCLTausif KhanÎncă nu există evaluări
- Air PollutionDocument22 paginiAir PollutionSaba waseemÎncă nu există evaluări
- Foam ConcentratesDocument5 paginiFoam ConcentratesSureshKumar DevulapallyÎncă nu există evaluări