Documente Academic
Documente Profesional
Documente Cultură
Acs Jnatprod 5b00337 PDF
Încărcat de
Thieres M. Costa PereiraDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Acs Jnatprod 5b00337 PDF
Încărcat de
Thieres M. Costa PereiraDrepturi de autor:
Formate disponibile
Article
pubs.acs.org/jnp
Transferring Fungi to a Deuterium-Enriched Medium Results in
Assorted, Conditional Changes in Secondary Metabolite Production
Bin Wang,, Elizabeth M. Park,, Jarrod B. King,, Allison O. Mattes,, Susan L. Nimmo,
Chaevien Clendinen, Arthur S. Edison, Clemens Anklin, and Robert H. Cichewicz*,,
Natural Product Discovery Group, Institute for Natural Products Applications and Research Technologies, and Department of
Chemistry & Biochemistry, Stephenson Life Science Research Center, University of Oklahoma, 101 Stephenson Parkway, Norman,
Oklahoma 73019, United States
Department of Biochemistry & Molecular Biology, College of Medicine, University of Florida, Gainesville, Florida 32610, United
States
NMR Applications Support, Bruker Biospin Corporation, 15 Fortune Drive, Billerica, Massachusetts 01821, United States
S Supporting Information
*
ABSTRACT: Deuterium is one of the few stable isotopes that
have the capacity to signicantly alter a compounds chemical
and biological properties. The addition of a single neutron to a
protium atom results in the near doubling of its mass, which
gives rise to deuteriums characteristic isotope eects. Since the
incorporation of deuterium into organic substrates is known to
alter enzyme/proteinsubstrate interactions, we tested the
extent to which deuterium enrichment would modify fungal
secondary metabolite production. Several fungal cultures were
tested, and in all cases their secondary metabolomes were
marked by changes in natural product production. Workup of
one Aspergillus sp. grown under deuterium-enrichment conditions resulted in the production of several secondary metabolites not
previously detected from the fungus. Bioassay testing revealed that in comparison to the inactive crude fungal extract derived
from growing the fungus under non-deuterium-enriched conditions, an extract derived from the same isolate cultured in a
deuterium-enriched medium inhibited methicillin-resistant Staphylococcus aureus. Using an assortment of NMR and mass
spectrometry experiments, we were able to identify the bacterial inhibitor as an isotope-labeled version of pigmentosin A (6).
Five additional isotopically labeled metabolites were also obtained from the fungus including brevianamide F (1), stephacidin A
(2), notoamide D (3), notoamide L (4), and notoamide C (5). Given the assorted changes observed in the secondary metabolite
proles of this and other fungi grown in deuterium-enriched environments, as well as the fact that 1 and 36 had not been
previously observed from the Aspergillus sp. isolate used in this study, we propose that deuterium enrichment might oer an
eective method for further expanding a funguss chemical diversity potential.
in their physical and chemical properties compared to CH
bonds.
Although both deuterium and tritium have the potential to
signicantly alter the chemical and biological properties of
compounds, both isotopes occur in relatively low abundances
on Earth and, therefore, have limited practical eects on lifes
chemical and biochemical processes. In the case of tritium, the
amount present in the biosphere uctuates at very low levels,3
while deuteriums abundance has remained small on a geologic
time scale relative to the proportion of available protium (D/H
= (149 3) 106).4,5 Consequently, the considerably more
abundant protium atoms have had a far greater inuence on the
evolutionary development of lifes chemical processes compared to its isotopic counterparts.
table isotopes occur throughout the natural world and are
woven into the chemical fabric of life. In most cases, their
assimilation into biomolecules aords negligible eects on
chemical substances since the addition of one or more neutrons
in an atom typically imparts trivial deviations in a compounds
physical and chemical properties. However, deuterium (2H or
D) and tritium (3H or T) represent exceptions to this rule since
the gain of one and two neutrons, respectively, results in the
near doubling (2H) and tripling (3H) of protiums (1H or H)
atomic mass. The chemical consequences of replacing protium
with deuterium are considerable, resulting in marked isotope
eects. Specically, the lower vibrational frequency of
deuterium (lower zero-point energy of vibration) increases
the bond dissociation energy of CD bonds by 5 kJmol1
compared to their CH counterparts,1 and these eects are
even further amplied for CT bonds.2 Thus, the shorter and
stronger CD and CT bonds exhibit appreciable dierences
XXXX American Chemical Society and
American Society of Pharmacognosy
Received: April 17, 2015
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
Journal of Natural Products
Article
acclimated and nonacclimated control fungal cultures were used
to seed asks containing modied versions of Czapek broth
prepared with deuterium-enriched (treatment) and nondeuterium-enriched (control) components. After 4 weeks of
static incubation, fungal cultures were partitioned against
EtOAc, and their secondary metabolites were recovered from
the organic layers. We observed that the acclimation process
had little eect on the appearances or relative amounts of
mycelial biomass produced by the fungi (Figure S1), which
indicated that either acclimation was unnecessary or our
acclimation process was ineective at priming fungi for the
switch to the deuterium-enriched Czapek broth. Comparisons
of the metabolomes by LC-ESIMS of fungi grown in
deuterium-enriched versus non-deuterium-enriched environments revealed that in many cases deuterium enrichment was
eective at altering their metabolite proles (Figures S2S9).
These changes appeared to be nonpermanent since switching
the fungi back from a deuterium-enriched medium to a nondeuterium-enriched medium was sucient for restoring each
funguss original metabolite prole. On the basis of this
screening, we deduced that deuterium enrichment induces an
assortment of quantitative and qualitative changes in a funguss
natural product output.
Preparation and Structure Determination of Deuterium-Enriched Metabolites. To explore in detail how
deuterium enrichment specically impacted the secondary
metabolome of a fungus, we picked one of the fungi from
our screening experiments for further investigation. The
selected isolate was a soil-derived Aspergillus sp. that had
been obtained from Waikiki Beach (Honolulu, HI, USA).12
This isolate had been subjected to extensive prior chemical
investigation, and our new observations were highly suggestive
that the fungus was now producing a qualitatively dierent
assortment of secondary metabolites that had not been
previously encountered in our lab.
Small (125 mm3) pieces of deuterium-acclimated fungal
mycelium and its attached agar were used to inoculate 1 L
Erlenmeyer asks containing 25 mL of the modied,
deuterium-enriched Czapek broth. The asks were capped
with foil and incubated at room temperature for 8 weeks with a
12 h light and 12 h dark cycle. Although the modied Czapek
medium was prepared using only D2O and deuterium-enriched
organic and inorganic components (>99% deuterium), the
autoclaving process and maintenance of the foil-sealed asks
under atmospheric conditions for 8 weeks resulted in a
substantial amount of protium in the form of water entering the
asks. Since we expected limited yields of metabolites from the
fungus grown in the deuterium-enriched culture broth, we
utilized glucose that was fully labeled with 2H and 13C as the
major carbon source; however, the mediums deuterated acetate
was not augmented by 13C labeling. Our initial experiments
showed that the 13C labeling of the glucose was sucient to
provide the enhanced carbon sensitivity needed for 2D NMR
experiments.
The treated (deuterium-enriched modied Czapek broth)
and control (nonenriched modied Czapek broth) Aspergillus
sp. cultures were pooled separately and partitioned three times
against EtOAc (1:1, vol/vol). The organic residue obtained
from the EtOAc layer from the treated cultures exhibited
inhibition (>85%) of methicillin-resistant Staphylococcus aureus
cell proliferation at 25 g/mL, whereas the sample derived
from the control culture did not. Subsequent purication
performed on the EtOAc-soluble components (0.87 g)
Earths natural ecosystems do not aord conditions in which
the relative abundance of hydrogen isotopes is vastly skewed.
However, that has not dissuaded researchers from testing the
consequences of how organisms might respond to synthetic
environments containing elevated quantities of deuterium or
tritium. Many studies have evaluated the eects of tritiumcontaining substances on cells and whole organisms due mainly
to concerns associated with the isotopes radiological decay.6,7
In comparison, deuterium is nonradioactive, which has enabled
researchers to obtain a better understanding of the isotopes
bond-strength-related toxicity. A majority of these studies have
focused on the toxicity of D2O, which generally causes death or
the arrest of cell growth/function at elevated concentrations.8,9
Intriguingly, some microorganisms can be acclimated to
environments containing very high and otherwise inhibitory
levels of D2O.10,11 On the basis of these observations, we were
curious about how a deuterium-enriched environment might
impact secondary metabolism in fungi. We hypothesized that
secondary metabolism would be altered as a result of both the
direct (e.g., eciency of biosynthetic enzymes to utilize
deuterated substrates) and indirect (e.g., modulation of primary
metabolic and intracellular cell signaling pathways) eects of
deuteriums presence. In this investigation, we tested the impact
of a deuterium-enriched environment on a panel of fungi whose
natural product compositions had been previously examined in
our lab. Herein, we describe the eects of deuteriumenrichment on secondary metabolite production by these fungi.
RESULTS AND DISCUSSION
Impact of Deuterium Enrichment on the Fungal
Secondary Metabolome. Eight fungi that had been subject
to previous chemical studies in our lab were selected for a
screening experiment.1216 Published work had indicated that
acclimation is an important consideration for enabling
microorganisms to grow on a deuterium-enriched medium.10,11
Accordingly, each isolate was cultured on a medium containing
malt extract agar made with D2O to create deuteriumacclimated fungal strains. In all cases, fungi transferred to
plates containing the D2O-based medium initially exhibited
slowed growth, but recovered to near normal rates of mycelium
expansion after one or more weeks of incubation. Both D2OB
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
Journal of Natural Products
Article
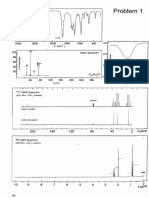
Figure 1. HRESIMS and NMR data for compound 1 illustrating the eects of deuterium enrichment and 13C incorporation: (A) expansion of an
HRESIMS ion cluster showing the Gaussian distribution resulting from isotope enrichment; (B) 1H NMR spectrum showing the relatively low
intensity nonexchangeable 1H signals with large 1H13C couplings; (C) 13C13C INADEQUATE NMR spectrum revealing a combination of onebond and multiple-bond correlations.
provided the putative bioactive substance and ve additional
secondary metabolites. Since all chromatography procedures
were performed using nondeuterated solvents, the exchangeable deuterium atoms were lost during the Sephadex LH-20,
preparative C18 HPLC, and semipreparative C18 HPLC
purication steps.
While the use of dual-labeled glucose did provide increased
capacity for NMR-based carbon detection, it also added certain
challenges for the structure determination experiments. The
mixed incorporation of hydrogen (1H and 2H) isotopes and the
high degree of 13C labeling achieved under our experimental
conditions meant that routine dereplication and structure
determination eorts become challenging processes. For
example, the HRESIMS data for all products exhibited Gaussian
distributions (Figure 1A) that hindered the straightforward
deduction of secondary metabolite molecular weights. We also
noted that the presence of signicant quantities of three (1H,
2
H, and 13C) NMR-active nuclei in the metabolites resulted in
problematic multinuclear couplings that were not easily
suppressed. This resulted in extensive signal overlap (Figure
1B), as well as spurious long-range couplings that manifested in
most spectral data (Figure 1C).
Compound 1 was obtained as a white, amorphous solid.
HRESIMS displayed clusters of isotopic ions exhibiting
Gaussian distributions in a range of m/z 324.2189 to
337.2688 under positive mode, as well as m/z 300.2215 to
313.2720 under negative mode (Figures S10S13), which were
informative for establishing a range of putative molecular
weights for the metabolite. The 1H NMR spectrum of 1 (Figure
S14) exhibited two amide protons (H 7.69, 10.85) that
integrated as singlets, which were many times more intense
than the compounds other proton signals. This enabled us to
estimate that roughly 8595% of the nonexchangeable
hydrogens in 1 were deuterium atoms, which was further
conrmed by BilevelDec 1H NMR. While 2H NMR spectra
were collected (data not shown), we found that for practical
reasons (e.g., 2H signal broadening and relatively weak 3JDD
couplings) the residual protium in 1 made 1H NMR and 1Hbased multinuclear detection strategies more feasible for most
experiments. All of the 1H signals displayed large couplings
(1JCH 140160 Hz), and the HSQC NMR (Figure S17)
exhibited the expected 13C satellite signals. Upon closer
inspection, the 13C NMR signals (1H-decoupled 13C NMR,
Figure S16) were found to be composed of clusters of peaks in
Gaussian distributions, which conrmed the metabolites
substantial 2H and 13C isotope labeling. The 13C and HSQC
NMR results (Table S1) suggested the presence of four
methylene, seven methine (including ve olenic), and ve
quaternary (including three olenic and two carbonyl) carbons.
Taking these NMR data into consideration, we returned to the
MS results and concluded that the [M + Na]+ ion at m/z
337.2688 indicated a molecular formula of 13C16H2D15N3O2Na
(calcd 337.2697, 2.67 ppm) for compound 1. INADEQUATE (Figure S18) correlations supported the presence of
phenylalanine and proline residues. These observations enabled
us to reengage in a more informed dereplication search, which,
when used in combination with the UV, IR, and optical rotation
values obtained for 1, led us to identify the metabolite as
isotope-labeled brevianamide F.17,18
Similarly, compound 2 was obtained as a white, amorphous
solid. Like 1, the HRESIMS data for compound 2 exhibited a
cluster of ions with a Gaussian distribution in the range of m/z
483.3558 to 506.4615. 1H, 13C, and HSQC NMR (Figures S21,
S23, S25; Table S1) conrmed that a majority of the carbons
and hydrogens, except for two exchangeable amide protons (H
8.62, 10.39), were isotopically labeled. The 13C NMR spectrum
was recognized as identical to that of stephacidin A (Figure
S24), which had been previously puried by our group. On the
basis of INADEQUATE correlation data (Figure S26) and
comparisons of the metabolites UV, IR, and optical rotation
values with published values, compound 2 was conrmed to be
an isotope-labeled version of stephacidin A.19
Compound 3 was puried as a white, amorphous solid with
HRESIMS data that appeared as a cluster of ions in a Gaussian
distribution over the range of m/z 507.4074 to 525.4807. 1H,
13
C, and HSQC NMR results (Figures S29, S31, and S33)
suggested the presence of one amide proton (H 5.42). The 13C
and HSQC NMR data (Table S1) indicated the presence of
four methyl, ve methylene (including one olenic carbon),
seven methine (including ve olenic carbons), and 10
quaternary (including two carbons attached to oxygen, four
olenic carbons, and two carbonyl carbons) carbons. A
comparison of the 13C NMR signals for C-11C-18, C-4C9, and C-25C-29 in 3 revealed they were comparable to data
obtained for compound 2 (Figure S32). This pointed toward
the presence of a 2,5-diketopiperazine incorporating a proline
residue and a 5,6-substituted-2,2-dimethyl-2H-chromene moiety. INADEQUATE correlations between C-22C-21, C-21
C-20, and C-20C-23/C-24/C-2 (Figure S34) were informative for revealing the presence of an isoprenyl group. In contrast
C
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
Journal of Natural Products
Article
presence of two fused aromatic rings. HMBC correlations
(Figure S56) from H2-8 to C-9, as well as 13C13C TOCSY
correlations from C-5 to C-4, C-8, and C9 and from C-8 to C7, indicated the presence of a lactone-containing ring adjacent
to the fused aromatic ring system. Further observation derived
from HMBC correlations, as well as a comparison of its 1D
NMR data with published values, conrmed that the planar
structure of 6 was identical to pigmentosin A.22 Since 6 started
to degrade when stored in DMSO and its yield was limited (0.5
mg), the absolute conguration of 6 was not determined.
Determination of Deuterium Content in Aspergillus
sp. Metabolites. Given that all the metabolites contained one
or more exchangeable protons, we surmised that these could be
used as internal references to determine the relative proportion
of protium/deuterium in each compound. This approach, while
simple, was not entirely satisfactory since the 13C satellites
(one-bond coupling of 13C1H) resulted in the overlap of
many proton signals. Although 13C decoupling of the 1H NMR
was attempted, we were not able to completely remove all
couplings, especially with the added contributions from 2H
couplings. To address this problem directly and eectively
eliminate the 13C satellites, a bilevel adiabatic decoupling 1H
NMR pulse sequence was used.23,24 Although this diminished
the signal-to-noise capacity of our experiments, we were able to
identify spurious peaks and eliminate them by comparing each
metabolites 1H NMR data with its corresponding 1H13C
HSQC spectrum. Using this method, we determined that the
level of deuterium incorporation was relatively even across all
positions in each compound with about 90% deuterium
incorporation in 15 (Figures S15, S22, S30, S38, S46).
Methicillin-Resistant Staphylococcus aureus Activity
of Compound 6. Compounds 16 were evaluated for
anticancer activity against MIA PaCa-2 pancreatic cells;25
antimicrobial activities against methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecium, Acinetobacter
baumannii, and Klebsiella pneumonia; as well as antifungal
activity against Candida albicans and Aspergillus f umigatus.26
Only compound 6 displayed anti-MRSA activity at 25 g/mL
(Figure S58). Unfortunately, the MIC value of 6 was not
determined due to its limited yield and propensity for
decomposition. The anti-MRSA activity of nonisotopically
enriched pigmentosin A has not been reported; however, the
anti-MRSA activity of 6 is consistent with published bioactivity
reports for its analogue, lichenicolin A (MIC of 16 g/mL).27
to compound 2, quaternary carbons C-11 and C-17 were
replaced by methine carbons in 3, which indicated that the
bicyclo[2.2.2]diazaoctane system formed by the cyclization of
the isoprenyl unit in 2 was open in 3. Further observations
derived from the INADEQUATE data for 3, along with
comparisons of its 1D NMR, UV, IR, and optical rotation
results to published values, indicated that the metabolite was an
isotopically isotope-labeled version of notoamide D.20
Compound 4, which appeared as a white, amorphous solid,
was analyzed by HRESIMS, leading to the production of a
cluster of ions with a Gaussian distribution ranging from m/z
489.3724 to 508.4468. The 1H, 13C, and HSQC NMR data
(Figures S37, S39, and S41) suggested the presence of amide
(H 8.21, 1H) and amine (H 7.30, 2H) protons. The 13C and
HSQC NMR data (Table S1) revealed that compound 4
contained three methyl, six methylene (including an olenic
carbon), ve methine (including four olenic carbons), 11
quaternary (including one attached to an oxygen, ve olenic,
and three carbonyl carbons) carbons. A comparison of the 13C
NMR spectra for compounds 4 and 2 indicated the presence of
bicyclo[2.2.2]diazaoctane and 5,6-substituted-2,2-dimethyl-2Hchromene moieties, as well as an additional ketone in the
compound (Figure S40). INADEQUATE correlations from C22 to C-21, C-23, and C-24 indicated the presence of an
isopropenyl unit at C-21. The correlations from C-3 to C-9 and
C-10 were used to assign the position of the ketone between C9 and C-10 (Figure S42). Further observations from the
INADEQUATE correlation data, as well as comparisons of its
1D NMR, UV, IR, and optical rotation values with published
data, conrmed that 4 was an isotope-labeled form of
notoamide L.21
Compound 5 appeared as a white, amorphous solid. The
HRESIMS data exhibited a cluster of ions with a Gaussian
distribution from m/z 506.4045 to 527.4128. The 1H, 13C, and
HSQC NMR data (Figures S45, S47, and S49) suggested the
presence of two amide protons (H 6.20, 10.64). The 1H,
HSQC, and 13C NMR data (Table S1) for compound 5 were
similar to those of compound 3, except that C-2 in 3 showed a
downeld shift from C 91.3 to 180.6, while C-3 moved upeld
from C 87.4 to 55.3 (Table S1, Figure S48). Additionally, the
presence of an amide proton on N-19 indicated that the ring
system to which it belonged in 3 was now open. The HMBC
correlations (Figure S50) from H2-22, H3-23, and H3-24 to C20 and from H3-23 and H3-24 to C-21 provided evidence for an
isoprenyl unit. HMBC correlations from H3-23 and H3-24 to C9 supported the connection between the isoprenyl unit and C9. Additional observations derived from HMBC correlation
results, as well as comparisons of its 1D NMR, UV, IR, and
optical rotation data with published values, conrmed 5 was
isotope-labeled notoamide C.20
Compound 6 was obtained as a yellow, amorphous solid.
HRESIMS analysis provided a cluster of ions with a Gaussian
distribution in the range of m/z 572.2839 to 591.3526. On the
basis of the 1H, 13C, and HSQC NMR data for 6 (Figures S53
S55), two exchangeable protons were detected along with two
methyl (including a methoxy carbon), one methylene, three
methine (including two olenic carbons and one attached to an
oxygen atom), and nine quaternary (including eight olenic
and a carbonyl carbon) carbons. This accounted for less than
half of the compounds presumed mass, which suggested that 6
was a dimer. 13C13C TOCSY correlations (Figure S57) from
C-2 to C-1, C-3, C-4, C-11, C-13, and C-14, as well as from C10 to C-1, C-2, C-4, C-11, C-12, and C-13, supported the
CONCLUSIONS
Deuterium enrichment of the fungal growth medium appears to
have a range of eects on an organisms secondary metabolic
production. In this study, it was notable that we observed a
compound (pigmentosin A, 6) that represented a biosynthetic
product that was previously undetected by LC-ESIMS
(selected-ion trace) from the fungus, although this isolate has
been cultured under many dierent conditions by our group. In
addition, deuterium enrichment unleashed the production of
numerous indole alkaloid analogues (1, 35) previously not
detected from the fungus.12 These results suggest that
deuterium enrichment might oer a simple and helpful method
for further expanding a funguss chemical diversity potential.
As a corollary to these studies, it is noteworthy that
deuterium-labeled compounds have shown signicant promise
in the clinic due to their altered CD bond strength properties,
which changes their susceptibility to in vivo metabolic
transformations. For example, Auspex Pharmaceuticals
D
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
Journal of Natural Products
Article
extract, 15 g/L agar, 0.05 g/L chloramphenicol, and 1 L of distilled
deionized water. A portion of the fungus was transferred to an MEA
plate made with 25% D2O. Over the course of 3-week increments, the
Aspergillus sp. isolate was subcultured onto plates containing MEA
with 50%, 75%, and 100% D2O. Similarly, the other fungal isolates
were sequentially acclimated on an MEA plate containing 33%, 66%,
and 100% D2O. Fungi exhibiting active hyphal expansion on 100%
D2O MEA plates were considered acclimated strains.
For the screening experiment, 125 mm3 pieces of mycelium and
agar were taken from fungal cultures grown under control (MEA with
no deuterium enrichment) and deuterium acclimation (MEA prepared
with D2O) conditions and placed into separate 1 L Erlenmeyer asks
containing 25 mL of non-deuterium-enriched (2.0 g of NaNO3, 1.0 g
of NaH2PO42H2O, 0.5 g of MgSO47H2O, 0.5 g of KCl, 0.2 g of
CaCO3, 0.01 g of FeSO47H2O, 0.005 g of ZnSO4, 4.0 g of glucose,
and 0.25 g of sodium acetate per 1 L of H2O) or deuterium-enriched
(2.0 g of NaNO3, 1.0 g of NaD2PO42D2O, 0.5 g of MgSO47D2O, 0.5
g of KCl, 0.2 g of CaCO3, 0.01 g of FeSO47D2O, 0.005 g of ZnSO4,
4.0 g of D-glucose (U13C6, 1,2,3,4,5,6,6-d7), and 0.25 g of sodium
acetate-d3 per 1 L of D2O) modied Czapek broth. The deuteriumenriched inorganic salts solution was made by taking nonenriched,
preweighed portions of each salt and dissolving it in D2O. The excess
D2O was then removed under vacuum at approximately 45 C. Two or
more replicates of each fungus/condition were incubated for 4 weeks
at room temperature, at which point the cultures were subjected to
partitioning with 1:1 (vol/vol) EtOAc (3). The organic layers were
recovered, the solvent was removed under vacuum, and the residue
was analyzed by LC-ESIMS.
For the scale-up production of deuterated Aspergillus sp.
metabolites, 45 Erlenmeyer asks (1 L vol) containing 25 mL of the
deuterium-enriched modied Czapek broth were prepared and
sterilized by autoclaving. Each ask was inoculated with a 125 mm3
piece of mycelium taken from a culture that had been maintained on
MEA agar made with 100% D2O. The asks were covered with
aluminum foil and incubated at room temperature for 8 weeks under a
lighting schedule of 12 h light/12 h dark.
Natural Product Purication. Secondary metabolites were
obtained by partitioning the pooled fungal culture broth against
EtOAc (3 , 1:1 vol/vol). The organic layers were retained and the
solvent was removed under vacuum to provide 0.87 g of residue. The
solids were applied to a column of Sephadex LH-20 and eluted with
MeOHDCM (1:1) to yield 22 fractions. The fractions were
recombined into ve fractions based on their LC-ESIMS proles.
The fractions were tested for anti-MRSA activity, and the two active
samples were combined to give 0.19 g of material. Further purication
was carried out by C18 HPLC (250 mm 20.2 mm, 5 m) with a
MeOHH2O gradient (from 30:70 to 100:0 over 30 min), followed
by isocratic C18 HPLC (250 mm 10 mm, 5 m) with MeCNH2O
(22:78, 54:46, 48:52, or 50:50) to yield 1 (2.5 mg), 2 (2.0 mg), 3 (1.0
mg), 4 (1.5 mg), 5 (1.2 g), and 6 (0.5 mg).
Bilevel Adiabatic Decoupling (BilevelDec) 1H NMR and
Calculation of Deuterium Content in Aspergillus sp. Metabolites. The major parameters considered in the design of the
experiment were decoupling bandwidth and decoupler position.
Bearing in mind stephacidin A and its analogues represented the
most complicated structures in our study, we used compound 2 as a
model substrate to test the various experimental parameters. We
adjusted the decoupling bandwidth from 150 to 220 ppm and the
decoupler position from 75 to 110 ppm. For the experiment,
compounds were analyzed by BilevelDec 1H NMR (CDCl3 and/or
DMSO-d6) with a relaxation delay of 1 s, observe pulse at 3.3 s,
decoupling bandwidth at 220 ppm, decoupler position at 110 ppm,
and the maximum J to be decoupled at 200 Hz. Due to the relatively
small amount of protium in the samples, it was dicult to completely
remove artifacts, some of which might cause incorrect recognition of
real decoupled 1H signals. Therefore, we conrmed each 1H NMR
datum by comparison with its corresponding 1H13C HSQC NMR
cross-peak. In both regular and BilevelDec 1H NMR spectra, the
amide/hydroxy protons were assigned an integration value of 1. The
integration values for the other nonexchangeable protons were
launched SD-809, a deuterated analogue of the drug
tetrabenazine, in a phase III clinical trial to treat Huntingtons
disease.28,29 In another case, Concert Pharmaceuticals has
developed CTP-499, a deuterated analogue of 1-(5-hydroxyhexyl)-3,7-dimethylxanthine, and initiated its phase II clinical
trials for treating diabetic kidney disease.28,30 Likewise, Avanir
Pharmaceuticals is conducting phase II clinical studies of AVP786, deuterated dextromethorphan, for depressive disorders.31
The growing appeal of developing pharmaceutical candidates
with strategically incorporated deuterium atoms attests to the
power that this approach has for the control of a drugs
susceptibility to in vivo metabolic processes. The current
experiments provide a direction for exploring additional
deuterium-labeling strategies that could be applied to situations
requiring improvements in the metabolic stability of drugs and
related clinical candidates.
Figure 2. Secondary metabolome proles (PDA detection at 190400
nm) of the Aspergillus sp. grown in non-deuterium-enriched (A) and
deuterium-enriched (B) media showing the substantial dierences in
metabolite composition.
EXPERIMENTAL SECTION
General Experimental Procedures. UV data were recorded on a
Hewlett-Packard 8452A diode array spectrophotometer. Optical
rotation measurements were made on an AUTOPOL III automatic
polarimeter. IR spectra were recorded on a Shimadzu IRAnity-1 FTIR spectrometer. The LC-ESIMS analyses were performed on a
Shimadzu UFLC system with a quadrupole mass spectrometer using a
Phenomenex Kinetex C18 column (3.0 mm 75 mm, 2.6 m) and
MeCNH2O (with 0.1% HCOOH) gradient solvent system.
HRESIMS spectra were measured using an Agilent 6538 Ultra High
Denition (UHD) Accurate-Mass Q-TOF system. NMR spectra were
obtained on Varian spectrometers (500 MHz for 1H and 125 MHz for
13
C at the University of Oklahoma; 600 MHz for 1H and 150 MHz for
13
C at the University of Florida) and Bruker Avance III spectrometer
equipped with a nitrogen-cooled cryoprobe (600 MHz for 1H and 150
MHz for 13C at Bruker Biospin Corp.) using DMSO-d6 and CDCl3
(Aldrich) as solvents. Column chromatography was conducted using
Sephadex LH-20. HPLC was carried out using a Waters System
equipped with a 1525 binary HPLC pump coupled to a 2998 PDA
detector with a Phenomenex C18 column (21.2 250 mm or 10 250
mm, 5 m particle size). Glucose-13C6,d7, sodium acetate-d3, and D2O
were purchased from Cambridge Isotope Laboratories, Inc. All other
materials were purchased from Sigma-Aldrich.
Fungal Isolates, Deuterium Acclimation, and Culture
Conditions. The Aspergillus sp. isolate was obtained from a soil
sample collected from Waikiki Beach (Honolulu, HI, USA) as
previously described.12 The other fungal isolates, Fusarium solani,
Alternaria longissima, Emericellopsis terricola, two Tolypocladium spp.,
Aspergillus terreus, and Bionectria ochroleuca, were acquired from our
cryopreserved fungal collection. The Aspergillus sp. isolate was grown
initially on malt extract agar (MEA): 10 g/L malt extract, 1 g/L yeast
E
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
Journal of Natural Products
Article
determined (Hn), and the percent deuterium was calculated as (1
Hn) 100%.
Antibacterial Activity. The antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) was determined as
described.32 Chloramphenicol was used as a positive control. The
MRSA strain (ATCC 700787) was maintained on tryptic soy blood
agar plates (30 g/L Bacto Tryptic Soy Broth (TSB), 15 g/L Amresco
high-purity agar, 5% debrinated sheeps blood). An overnight stock
culture of S. aureus was diluted in brain-heart infusion (BHI) broth (37
g/L Bacto BHI), 100 L of which was placed in each well of a 96-well
plate. Aliquots (1 L) of each compound stock solution (in DMSO)
were added to the wells of a microtiter plate. An OD600 value was
obtained for each well immediately after it was prepared, and the plate
was placed into a 37 C incubator for 1618 h. The resulting OD600
values for all test and control wells were recorded again, and the data
analyzed.
Brevianamide F (U-13C16, 2,5,6,7,8,10,10,11,14,14,15,15,16,16,17d15, 1): white, amorphous solid; UV (MeOH) max (log ) 222 (4.36),
280 (3.71) nm; []25
D 148 (c 0.11, CHCl3); IR (lm) max 3260,
1620, 1420 cm1; 1H and 13C NMR (see Table S1).
Stephacidin A (U-13C26, 4,5,10,10,14,14,15,15,16,16,20,20,21,23,23,23,24,24,24,25,26,28, 28,28,29,29,29-d27, 2): white, amorphous
solid; UV (MeOHCHCl3) max (log ) 208 (4.39), 242 (4.31), 308
(3.83), 328 (3.65, sh) nm; []25
D +41 (c 0.13, MeOHCHCl3, 1:1); IR
(lm) max 3338, 1700, 1666, 1622, 1396 cm1; 1H and 13C NMR (see
Table S1).
Notoamide D (U-13C26, 4,5,10,10,11,14,14,15,15,16,16,17,21,22,22,23,23,23,24,24,24,25,26,28,28,28,29,29,29-d29, 3): white, amorphous solid; UV (MeOH) max (log ) 208 (4.28), 236 (4.28), 286
(3.88), 330 (3.74) nm; []25
D 150 (c 0.08, MeOH); IR (lm) max
3356, 1710, 1670, 1622, 1362 cm1; 1H and 13C NMR (see Table S1).
Notoamide L (U-13C25, 4,5,10,10,14,14,15,15,16,16,20,20,21,23,23,24,24,24,25,26,28,28, 28,29,29,29-d26, 4): white, amorphous
solid; UV (MeOH) max (log ) 206 (4.20), 236 (4.15), 270 (4.13),
368 (3.78) nm; []25
D 52 (c 0.07, MeOH); IR (lm) max 3435, 3315,
1700, 1650, 1575, 1380, 1220 cm1; 1H and 13C NMR (see Table S1).
Notoamide C (U-13C26, 4,5,10,10,11,14,14,15,15,16,16,17,21,22,22,23,23,23,24,24,24,25, 26,28,28,28,29,29,29-d29, 5): white, amorphous solid; UV (MeOH) max (log ) 208 (4.27), 248 (4.43), 266
(4.12, sh), 304 (3.70, sh) nm; []25
D +24 (c 0.20, MeOH); IR (lm)
max 3200, 1700, 1650, 1420, 1290 cm1; 1H and 13C NMR (see Table
S1).
Pigmentosin A (U- 13 C30 , 7,7,8,8,8,8,10,10,14,14,15,15,15,15,15,15,16,16,16,16,16,16-d22, 6): yellow solid; 1H and 13C
NMR (see Table S1).
supported by the NIH (R01EB009772) and the National High
Magnetic Field Laboratory (NSF cooperative agreement DMR1157490 and the State of Florida). The LC-ESIMS instrument
used for this project was provided in part by a Challenge Grant
from the Oce of the Vice President for Research, University
of Oklahoma, Norman Campus, and an award through the
Shimadzu Equipment Grant Program.
ASSOCIATED CONTENT
S Supporting Information
*
Secondary metabolome proles for 16 fungal strains grown in
deuterium-enriched and nonenriched media, 1D, 2D NMR
spectra and HRESIMS data for compounds 16, as well as
primary anti-MRSA activity of isolated compounds. The
Supporting Information is available free of charge on the
ACS Publications website at DOI: 10.1021/acs.jnatprod.5b00337.
REFERENCES
(1) Jones, R. A. Y. Physical and Mechanistic Organic Chemistry;
Cambridge University Press: New York, 1979.
(2) Yakushin, F. S. Russ. Chem. Rev. 1962, 31, 123.
(3) Boyer, C.; Vichot, L.; Fromm, M.; Losset, Y.; Tatin-Froux, F.;
Guetat, P.; Badot, P. M. Environ. Exp. Bot. 2009, 67, 34.
(4) Lecuyer, C.; Gillet, P.; Robert, F. Chem. Geol. 1998, 145, 249.
(5) Robert, F. In Meteorites and the Early Solar System II; Lauretta, D.
S., Mcsween, H. Y., Eds.; University of Arizona Press: Tuscon, 2006.
(6) Hofer, K. G.; Hughes, W. L. Radiat. Res. 1971, 47, 94.
(7) Geselowitz, D. A.; McManaway, M. E.; Hofer, K. G.; Neumann,
R. D. Radiat. Res. 1995, 142, 321.
(8) Thomson, J. F. Biological Eects of Deuterium; Pergamon Press,
Macmillan: New York, 1963.
(9) Adams, W. H.; Adams, D. G. J. Pharmacol. Exp. Ther. 1988, 244,
633.
(10) Barber, J. WO9707216A1, 1997.
(11) Paliy, O.; Bloor, D.; Brockwell, D.; Gilbert, P.; Barber, J. J. Appl.
Microbiol. 2003, 94, 580.
(12) Wang, X.; You, J.; King, J. B.; Powell, D. R.; Cichewicz, R. H. J.
Nat. Prod. 2012, 75, 707.
(13) Cai, S.; Du, L.; Gerea, A. L.; King, J. B.; You, J.; Cichewicz, R. H.
Org. Lett. 2013, 15, 4186.
(14) Wang, B.; You, J.; King, J. B.; Cai, S.; Park, E.; Powell, D. R.;
Cichewicz, R. H. J. Nat. Prod. 2014, 77, 2273.
(15) Du, L.; Robles, A. J.; King, J. B.; Powell, D. R.; Miller, A. N.;
Mooberry, S. L.; Cichewicz, R. H. Angew. Chem., Int. Ed. 2014, 53, 804.
(16) Cai, S.; King, J. B.; Du, L.; Powell, D. R.; Cichewicz, R. H. J. Nat.
Prod. 2014, 77, 2280.
(17) Birch, A. J.; Russell, R. A. Tetrahedron 1972, 28, 2999.
(18) Grundmann, A.; Li, S. M. Microbiology 2005, 151, 2199.
(19) Qian-Cutrone, J.; Huang, S.; Shu, Y.-Z.; Vyas, D.; Fairchild, C.;
Menendez, A.; Krampitz, K.; Dalterio, R.; Klohr, S. E.; Gao, Q. J. Am.
Chem. Soc. 2002, 124, 14556.
(20) Kato, H.; Yoshida, T.; Tokue, T.; Nojiri, Y.; Hirota, H.; Ohta,
T.; Williams, R. M.; Tsukamoto, S. Angew. Chem., Int. Ed. 2007, 46,
2254.
(21) Tsukamoto, S.; Kawabata, T.; Kato, H.; Greshock, T. J.; Hirota,
H.; Ohta, T.; Williams, R. M. Org. Lett. 2009, 11, 1297.
(22) Elix, J. A.; Hardlaw, J. H. Aust. J. Chem. 2004, 57, 681.
(23) Kupce, E.; Freeman, R.; Wider, G.; Wuthrich, K. J. Magn. Reson.
A 1996, 122, 81.
(24) Agilent Technologies. Accessed Feb 17, 2014; available from
http://www.chem.agilent.com/Library/applications/5990-9896EN.
pdf.
(25) Du, L.; Risinger, A. L.; King, J. B.; Powell, D. R.; Cichewicz, R.
H. J. Nat. Prod. 2014, 77, 1753.
(26) Henrikson, J. C.; Ellis, T. K.; King, J. B.; Cichewicz, R. H. J. Nat.
Prod. 2011, 74, 1959.
(27) He, H.; Bigelis, R.; Yang, H. Y.; Chang, L.-P.; Singh, M. P. J.
Antibiot. 2005, 58, 731.
(28) Katsnelson, A. Nat. Med. 2013, 19, 656.
(29) Auspex Pharmaceuticals. Accessed Aug 28, 2014 2014; available
from http://www.auspexpharma.com/pipeline/.
(30) Concert Pharmaceuticals. Accessed Sept 10, 2014; available
from
http://www.concertpharma.com/
CTP499WindhoverTop10Project.htm.
(31) Avanir Pharmaceuticals. Accessed Sept 10, 2014; available from
http://ir.avanir.com/phoenix.zhtml?c=61699&p=irolnewsArticle&ID=1960980.
AUTHOR INFORMATION
Corresponding Author
*E-mail: rhcichewicz@ou.edu. Tel: 405-325-6969. Fax: 405325-6111.
Notes
The authors declare no competing nancial interest.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the
National Institutes of Health (R01AI085161). The 13Coptimized NMR probe at the University of Florida was
F
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
Journal of Natural Products
Article
(32) Cai, S.; King, J. B.; Du, L.; Powell, D. R.; Cichewicz, R. H. J. Nat.
Prod. 2014, 77, 2280.
DOI: 10.1021/acs.jnatprod.5b00337
J. Nat. Prod. XXXX, XXX, XXXXXX
S-ar putea să vă placă și
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Isolation and Characterization of B-SitosterolDocument6 paginiIsolation and Characterization of B-Sitosterolnirajvyas4meÎncă nu există evaluări
- Kinetics and Mechanism of Aqueous Hydrolysis and CondensationDocument14 paginiKinetics and Mechanism of Aqueous Hydrolysis and CondensationMiguel Angel Ramos RuizÎncă nu există evaluări
- crf312143 PDF Jsessionid f04t01Document18 paginicrf312143 PDF Jsessionid f04t01Saiful HaffiziÎncă nu există evaluări
- Cleistopholis PatensDocument5 paginiCleistopholis PatensamensetÎncă nu există evaluări
- Petrotech 2019: Guidelines & Instructions To Authors For Full Manuscript GuidelinesDocument4 paginiPetrotech 2019: Guidelines & Instructions To Authors For Full Manuscript GuidelinesfelujiluÎncă nu există evaluări
- 2425 Chapt 13Document10 pagini2425 Chapt 13Ivan Alberto NinaÎncă nu există evaluări
- 11 - Chapter 2 PDFDocument40 pagini11 - Chapter 2 PDFماجد عليÎncă nu există evaluări
- NMR HandoutDocument23 paginiNMR HandoutVirendra Singh RajputÎncă nu există evaluări
- The Basics of NMRDocument59 paginiThe Basics of NMRalexpharmÎncă nu există evaluări
- 9701 w16 QP 42Document16 pagini9701 w16 QP 42Abdelmoneim Elmansy IgcseÎncă nu există evaluări
- Ppcatalogue 2 PDFDocument527 paginiPpcatalogue 2 PDFrutwickÎncă nu există evaluări
- Spectroscopy & ChromatographyDocument23 paginiSpectroscopy & ChromatographyKamrul Alam MasumÎncă nu există evaluări
- CH 41b Midterm: Please Write Your Name On All PagesDocument16 paginiCH 41b Midterm: Please Write Your Name On All PagesMinh TieuÎncă nu există evaluări
- Soal Elsus 2Document10 paginiSoal Elsus 2Istinganatun KhoeriyahÎncă nu există evaluări
- Analysis of Neem Oils by LC-MS and Degradation KineticsDocument11 paginiAnalysis of Neem Oils by LC-MS and Degradation KineticstramiÎncă nu există evaluări
- Plastic Additives PDFDocument824 paginiPlastic Additives PDFNguyễn Ngọc Phước VươngÎncă nu există evaluări
- J Jpba 2019 112939Document33 paginiJ Jpba 2019 112939Sonia Ayu AndiniÎncă nu există evaluări
- SKL1043 Lab 2Document4 paginiSKL1043 Lab 2Lingesswari NagarajanÎncă nu există evaluări
- Organic Structure Elucidation WorkbookDocument498 paginiOrganic Structure Elucidation WorkbookKajan Muneeswaran75% (4)
- Structural Analysis For Lignin Characteristics in Biomass StrawDocument17 paginiStructural Analysis For Lignin Characteristics in Biomass StrawSo RoÎncă nu există evaluări
- C-Glucosidic Ellagitannin Oligomers From Melaleuca Squarrosa Donn Ex SM., MyrtaceaeDocument10 paginiC-Glucosidic Ellagitannin Oligomers From Melaleuca Squarrosa Donn Ex SM., MyrtaceaeDheva NiaÎncă nu există evaluări
- IChO32 Prep ProbDocument68 paginiIChO32 Prep ProbMuhammad GhifariÎncă nu există evaluări
- Detection of Hydroxyl and Perhydroxyl Radical Generation From Bleaching Agents With Nuclear Magnetic Resonance SpectrosDocument10 paginiDetection of Hydroxyl and Perhydroxyl Radical Generation From Bleaching Agents With Nuclear Magnetic Resonance SpectrosDivya SharmaÎncă nu există evaluări
- Anna Zurek Chem 231 Lab Report - Portfolio VersionDocument31 paginiAnna Zurek Chem 231 Lab Report - Portfolio VersionAnna ZurekÎncă nu există evaluări
- Chem 425 395 DCDocument3 paginiChem 425 395 DCKasun RatnayakeÎncă nu există evaluări
- B SCDocument3 paginiB SCAnuj semwalÎncă nu există evaluări
- B.SC Organic Chemistry (Paper-5) - Q and ADocument59 paginiB.SC Organic Chemistry (Paper-5) - Q and ASyed furkhanÎncă nu există evaluări
- (2002) The Whole Palette of Hydrogen Bonds.Document29 pagini(2002) The Whole Palette of Hydrogen Bonds.Patrick Rodrigues Batista100% (1)
- NMR Spectroscopy-1Document63 paginiNMR Spectroscopy-1Abhishek Kumar SinghÎncă nu există evaluări
- Saponification of Ester ReportDocument11 paginiSaponification of Ester Reportapi-397833575100% (3)