Documente Academic
Documente Profesional
Documente Cultură
FAC of Palm Oil Palm Olein and Palm Stearin
Încărcat de
Anonymous DJrec2Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
FAC of Palm Oil Palm Olein and Palm Stearin
Încărcat de
Anonymous DJrec2Drepturi de autor:
Formate disponibile
Journal of Oleo Science
Copyright 2008 by Japan Oil Chemists Society
J. Oleo Sci. 57, (5) 275-285 (2008)
Development of Palm-Based Reference Materials
for the Quantification of Fatty Acids Composition
Azmil Haizam Ahmad Tarmizi, Siew Wai Lin and Ainie Kuntom
Analytical and Quality Development Unit, Malaysian Palm Oil Board (6, Persiaran Institusi, Bandar Baru Bangi, 43000 Kajang, Selangor,
MALAYSIA)
Abstract: Characterisation of fatty acids composition of three palm-based reference materials was carried
out through inter-laboratory proficiency tests. Twelve laboratories collaborated in these tests and the
fatty acids compositions of palm oil, palm olein and palm stearin were determined by applying the MPOB
Test Methods p3.4:2004 and p3.5:2004. Determination of consensus values and their uncertainties were
based on the acceptable statistical agreement of results obtained from the collaborating laboratories. The
consensus values and uncertainties (%) for each palm oil reference material produced are listed as follows :
0.20% (C12:0), 1.660.05% (C14:0), 43.39 0.39% (C16:0), 0.140.06% (C16:1), 3.90 0.11% (C18:0),
40.95 0.23% (C18:1), 9.68 0.21% (C18:2), 0.16 0.07% (C18:3) and 0.31 0.08% (C20:0) for fatty
acids composition of palm oil; 0.23 0.04% (C12:0), 1.020.04% (C14:0), 39.66 0.19% (C16:0), 0.18
0.07% (C16:1), 3.81 0.04% (C18:0), 44.01 0.08% (C18:1), 10.73 0.08% (C18:2), 0.20 0.06%
(C18:3) and 0.34 0.04% (C20:0) for fatty acids composition of palm olein; and 0.20% (C12:0), 1.14
0.05% (C14:0), 49.42 0.25% (C16:0), 0.16 0.08% (C16:1), 4.15 0.10% (C18:0), 36.14 0.77%
(C18:1), 7.95 0.29% (C18:2), 0.110.07% (C18:3) and 0.300.08% (C20:0) for fatty acids composition
of palm stearin.
Key words: characterisation, palm-based reference materials, fatty acids composition, MPOB Test Methods p3.4:2004 and
p3.5:2004, consensus values, uncertainties
1 INTRODUCTION
Oils and fats consist mainly of fatty acid triesters and
glycerol or well-known as triglycerides. They are recognised as essential nutrients and source of energy in both
human and animal diets1). Oils and fats also enhanced the
functionality of food products, as they act as heating medium, tenderising agents, flavour and colour carriers, facilitate aeration and others.
Functionality of food products depends on physicochemical properties such as fatty acids composition (FAC),
which is often used to characterise oils and fats2). FAC is
defined as weight percentage of individual fatty acids in
their methyl ester forms after esterification of triglyceride
molecules. Boyaci and colleagues3) emphasised that the
physical properties of oils and fats are dependent on the
distribution of fatty acids on the glycerol backbones, chain
length and degree of unsaturation. FAC is said to be the
most useful chemical feature as many of the chemical tests
for oil identification can be related to that property4). Thus,
FAC is widely used in establishing authenticity of oils and
fats. FAC provides information on the total content of saturated and unsaturated fatty acids, which is often used as
health indicators. FAC can be also used as one of the indicator to determine the oxidative stability of oils and fats1).
In 1987, the Community Bureau of Reference (BCR),
Commission of the European Communities produced two
lipid reference materials of soya-maize oil blend (CRM No.
162) and beef-pig fat blend (CRM No. 163) and the certification of their FAC5). The certification programmes, which
involved 13 European laboratories, were required to determine principal (major) fatty acids of C16:0, C18:0, C18:1,
C18:2 and C18:3 in both CRM No. 162 and CRM No. 163 and
additional fatty acids of C14:0 and C16:1 in CRM No. 163.
Fatty acid levels of less than 1 % (mass fraction) were
defined as minor fatty acids.
Some research works that correlate FAC with other
Correspondence to: Azmil Haizam Ahmad Tarmizi, Analytical and Quality Development Unit, Malaysian Palm Oil Board, 6, Persiaran
Institusi, Bandar Baru Bangi, 43000 Kajang, Selangor, MALAYSIA
E-mail: azmil_haizam@mpob.gov.my
Accepted February 22, 2008 (received for review January 25, 2008)
Journal of Oleo Science ISSN 1345-8957 print / ISSN 1347-3352 online
http://www.jstage.jst.go.jp/browse/jos/
275
A.H. Ahmad Tarmizi, W.L. Siew and A. Kuntom
properties of oils and fats have been reported. Boyaci et
al. 3) investigated the relationship between slip melting
point (SMP) and FAC of forty-four vegetable oil blends
before and after interesterification. Boyaci and colleagues6)
developed an equation for simple and rapid viscosity estimation based on the FAC of seven vegetable oils. This
study represented a model for viscosity predictions of vegetable oils (primary form) and their binary mixtures
(blends) that have different FAC. Furthermore, Rabelo et
al.7) related viscosities of oleic acid, canola oil and model
fatty systems based on their FAC. Apart from that, the liquid specific heat capacity of fatty acids can be estimated
through correlation with FAC8). The researchers applied
the Rowlinson-Bondi equation to determine the specific
heat capacity for pure fatty acid components. Halvorsen, et
al.9) estimated the density of fatty acids and vegetable oils
based on their FAC. The model was developed through
modification of Rackett equation, which was applied over a
wide range of temperature.
The importance of FAC measurement of oils and fats has
initiated the Malaysian Palm Oil Board (MPOB) to develop
and characterise palm-based reference materials through
inter-laboratory proficiency tests. The unavailability of reference materials from palm oil products was also taken
into account to cater to the needs of oils and fats industry,
especially the palm oil sector. Reference materials provide
convenient means of calibrating and validating analytical
measurements and to assess the analysts capability in performing the measurements.
This paper presents the results of an inter-laboratory
proficiency test performed prior to the characterisation of
the reference materials from palm oil, palm olein and palm
stearin for FAC quantification. These reference materials
were evaluated based on nine fatty acids. Stability test of
the reference materials produced was also conducted and
reported in this paper.
2 EXPERIMENTAL
2.1 Preparation of the reference materials
Refined palm oil, palm olein and palm stearin were purchased from Golden Jomalina Food Industries Sdn. Bhd.
The oils were heated gently until completely liquid before
being dissolved with 0.02 % tert-butylhydroquinone (97 %
purity, Sigma-Aldrich, Steinheim, Germany) and stirred to
ensure homogeneity. Portions of 5 mL of the homogenised
oil were dispersed in 5-mL dark amber glass ampoules
(Scherf Praezision, Meiningen-Dreissigacker, Germany),
flushed with nitrogen and flame-sealed at the ampoule
sealing workbench. The utilisation of such glass ampoules
will help to prevent photo-oxidation and colour changes
during storage. The oil standards were labelled, packed in
fabricated boxes and stored at -20 until dispatch.
2.2 Inter-iaboratory proficiency tests
Twelve laboratories collaborated in the inter-laboratory
proficiency tests which include the Analytical and Quality
Development Unit, Malaysian Palm Oil Board, Selangor,
Malaysia; Advance Oleochemicals Technology Division,
Malaysian Palm Oil Board, Selangor, Malaysia; Golden
Hope Research Sdn. Bhd., Selangor, Malaysia; PGEO Edible Oils Sdn. Bhd., Johor, Malaysia; Edtech Associates Sdn.
Bhd., Penang, Malaysia; Southern Edible Oil Industry (M)
Sdn. Bhd., Selangor, Malaysia; Pan-Century Edible Oils
Sdn. Bhd., Johor, Malaysia; Biochem Laboratories Sdn.
Bhd., Penang, Malaysia; IOI Edible Oils Sdn. Bhd., Sabah,
Malaysia; Chemara Lab Sdn. Bhd., Negeri Sembilan,
Malaysia; Lotus Laboratory Services Sdn. Bhd., Johor,
Malaysia; ITS Testing Services Sdn. Bhd., Selangor,
Malaysia.
Each collaborator was provided with four ampoules of
each oil standard representing four replications. The measurement (FAC) should be carried out in accordance to
MPOB Test Methods p3.4:2004 and p.3.5:2004 10) . These
methods are based on the procedure of ISO 5508:1990 Animal and vegetable fats and oils - Analysis by gas chromatography (GC) of the methyl esters of fatty acids11).
The oil sample was firstly dissolved in n-hexane (Merck,
Darmstadt, Germany) prior to methylation using sodium
methoxide (Merck, Darmstadt, Germany). The mixture was
then diluted with distilled water and allowed to settle and
separate into two distinct layers. The upper clear supernatant, fatty acids methyl ester (FAME) layer was decanted
and injected into a GC, which was fitted with a fused silica
capillary column (DB-23, 60 m 0.25 m, i.d. 0.25 mm film) (J
and W Scientific, Folsom, USA). The flame ionisation
detector (FID) and injector temperatures were set at 240.
The carrier gas (helium) was positioned at 0.8 mL/min as
the column temperature was isothermal at 185. FAME
was quantified via the retention time and peak areas. The
FAC is represented as a percentage of each fatty acid in
terms of mass fractions. FAC is expressed as grams of
individual FAME per 100 grams of total FAME.
The FAC analysis had to be carried out within two
months upon receiving the oil standards. Reporting cards
were provided to the collaborators to compute their analysis results. In addition, each collaborator was supplemented with detailed instruction and study protocol of the proficiency tests.
2.3 Data evaluation
Data from the inter-laboratory tests were evaluated
using the SoftCRM 1.2.0 software, which was developed by
the European Commission of Standards, Measurements
and Testing Programmes12). The software is specifically
meant for statistical evaluation of the reference materials
data and documentation of the quality of the reference
276
J. Oleo Sci. 57, (5) 275-285 (2008)
Palm-Based Reference Materials for Fatty Acids Composition
materials produced. Consensus values (percentage of individual fatty acids) of each oil standard were generated with
95 % confidence interval (CI). Outlying data (extreme values) were detected by Grubb and Cochran tests. Grubb test
identifies outlying mean values (variability) among laboratories whereas Cochran test determines data variability
within laboratories5,13).
Repeatability relative standard deviation, RSDr (relative
standard deviation within laboratory) and reproducibility
relative standard deviation, RSDR (relative standard deviation between laboratories) were determined for each fatty
acid measured according to ISO 5725-213). RSDr is determined from data produced under repeatability conditions
of the same method on identical test items in the same laboratory by the same operator using the same instrumentation within short intervals of time whereas RSDR identified
from data generated under reproducibility conditions,
which the data are produced using the same method on
identical test items in different laboratories, operators and
instrumentations14).
2.4 Stability test
Stability test of the palm-based reference materials produced were carried out for 12 months at four storage temperatures of -20, 0, 4 and 24 (ambient temperature). Periodically, the oil samples were randomly selected
and analysed for their FAC as well as other general stabili-
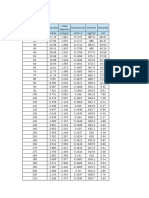
Table 1
ty markers such as free fatty acid (FFA) and totox value
(TV).
FFA was analysed through titration according to the
AOCS Official Method Ca 5a-40 and expressed as a percentage of palmitic acids15). FFA is used to determine the
hydrolytic behaviour of oil standards upon storage.
Totox value is defined as TV = 2PV + AnV. Peroxide
value (PV) was determined based on iodometric titration
according to the AOCS Official Method Cd 8b-9015). Anisidine value (AnV) was measured according to the IUPAC
2.50416). PV and AnV measured the primary oxidation and
secondary decomposition products, respectively and were
determined throughout the storage period.
3 RESULTS AND DISCUSSION
3.1 Assessment of statistical data
Statistical evaluations for palm oil, palm olein and palm
stearin are summarised in Table 1, Table 2 and Table 3,
respectively. The consensus values (percentage of individual fatty acids) were calculated at 95 % CI using the SoftCRM 1.2.0 software. Repeatability relative standard deviation (RSDr) of the quantified FAC in palm oil ranged from 0 %
to 9.24 % while reproducibility relative standard deviation
(RSDR) ranged from 0 % to 60.38 % (Table 1). Within-laboratory variation (RSDr) of less than 10 % for all fatty acids
Statistical Evaluation of FAC in Palm Oil.
FAa
Pb
Nc
Consensus Value
(Uncertainty)d
sre
RSDrf
rg
SRh
RSDRi
Rj
C12:0
C14:0
C16:0
C16:1
C18:0
C18:1
C18:2
C18:3
C20:0
7
11
9
10
11
8
9
10
12
28
44
36
40
44
32
36
40
48
0.20 ( )
1.66 (0.05)
43.39 (0.39)
0.14 (0.06)
3.90 (0.11)
40.95 (0.23)
9.68 (0.21)
0.16 (0.07)
0.31 (0.08)
0
0.04
0.19
0
0.08
0.12
0.09
0
0.03
0
3.64
0.43
0
2.09
0.30
0.89
0
9.24
0
0.11
0.52
0
0.23
0.35
0.24
0
0.08
0
0.07
0.53
0.08
0.18
0.29
0.29
0.10
0.13
0
7.17
1.23
60.23
4.58
0.71
2.97
60.38
42.33
0
0.21
1.49
0.24
0.50
0.82
0.81
0.27
0.37
Fatty acid (FA) measurement using capillary column GC
Number of laboratories retained after eliminating outliers
c
Number of accepted test results (replicates)
d
Consensus value and uncertainty (%) generated as 95% CI
e
Repeatability standard deviation
f
Repeatability relative standard deviation
g
Repeatability limit
h
Reproducibility standard deviation
i
Reproducibility relative standard deviation
j
Reproducibility limit
b
277
J. Oleo Sci. 57, (5) 275-285 (2008)
A.H. Ahmad Tarmizi, W.L. Siew and A. Kuntom
Table 2
Statistical Evaluation of FAC in Palm Olein.
FAa
Pb
Nc
Consensus Value
(Uncertainty)d
sre
RSDrf
rg
SRh
RSDRi
Rj
C12:0
C14:0
C16:0
C16:1
C18:0
C18:1
C18:2
C18:3
C20:0
12
10
9
12
10
10
8
12
9
48
40
36
48
40
40
32
48
36
0.23 (0.04)
1.02 (0.04)
39.66 (0.19)
0.18 (0.07)
3.81 (0.04)
44.01 (0.08)
10.73 (0.08)
0.20 (0.06)
0.34 (0.04)
0.03
0.03
0.22
0
0.11
0.16
0.08
0.03
0
14.48
3.37
0.55
0
2.86
0.37
0.73
12.63
0
0.09
0.10
0.61
0
0.31
0.46
0.22
0.07
0
0.06
0.06
0.31
0.11
0.11
0.53
0.12
0.11
0.05
28.82
5.86
0.79
60.80
2.86
1.21
1.08
53.21
15.27
0.18
0.17
0.87
0.31
0.31
1.49
0.32
0.29
0.15
Fatty acid (FA) measurement using capillary column GC
Number of laboratories retained after eliminating outliers
c
Number of accepted test results (replicates)
d
Consensus value and uncertainty (%) generated as 95% CI
e
Repeatability standard deviation
f
Repeatability relative standard deviation
g
Repeatability limit
h
Reproducibility standard deviation
i
Reproducibility relative standard deviation
j
Reproducibility limit
b
Table 3 Statistical Evaluation of FAC in Palm Stearin.
FAa
Pb
Nc
Consensus Value
(Uncertainty)d
sre
RSDrf
rg
SRh
RSDRi
Rj
C12:0
C14:0
C16:0
C16:1
C18:0
C18:1
C18:2
C18:3
C20:0
11
13
9
13
11
11
12
12
12
44
52
36
52
44
44
48
48
48
0.20 ( )
1.14 (0.05)
49.42 (0.25)
0.16 (0.08)
4.15 (0.10)
36.14 (0.77)
7.95 (0.29)
0.11 (0.07)
0.30 (0.08)
0
0.04
0.26
0.03
0.09
0.13
0.09
0
0.04
0
3.84
0.53
17.15
2.17
0.36
1.10
0
11.79
0
0.12
0.74
0.07
0.25
0.36
0.25
0
0.10
0
0.08
0.40
0.11
0.17
1.08
0.39
0.09
0.12
0
6.73
0.80
73.99
4.00
2.98
4.81
83.52
41.32
0
0.21
1.11
0.32
0.47
3.01
1.08
0.26
0.37
Fatty acid (FA) measurement using capillary column GC
Number of laboratories retained after eliminating outliers
c
Number of accepted test results (replicates)
d
Consensus value and uncertainty (%) generated as 95% CI
e
Repeatability standard deviation
f
Repeatability relative standard deviation
g
Repeatability limit
h
Reproducibility standard deviation
i
Reproducibility relative standard deviation
j
Reproducibility limit
b
278
J. Oleo Sci. 57, (5) 275-285 (2008)
Palm-Based Reference Materials for Fatty Acids Composition
signified that the FAC measurement was within the
acceptable variability. However, between-laboratory variation (RSDR) for C16:1, C18:3 and C20:0 were found to be
60.23 %, 60.38 % and 42.33 %, respectively. This is due to
the small percentage of these fatty acids, which generally
demonstrated large run-to-run variations between laboratories. Higher RSDR may be contributed by variations in
the instrument sensitivity, change of environmental conditions or uncontrolled change of instrument parameters17).
Such effects play a role, which may influence RSDR when
the samples are measured by different laboratories. Thus,
the measurement is still considered acceptable.
Table 2 highlights the statistical evaluation of FAC
quantification in palm olein. The RSDr was found to be in
the range from 0 % to 14.48 %, which is acceptable in
terms of variability of fatty acids measured. As expected,
the RSDR varied from 0.79 % to 60.80 %, where some of the
fatty acids (C12:0, C16:1 and C18:3) showed large run-torun variations due to their low contents of less than 0.5 %.
Results of the FAC quantification in palm stearin are tabulated in Table 3. The RSDr was found to be good, ranging
from 0 % to 17.15 %. The RSDR, however, varied from 0 %
to 83.52 %. This trend was similar to that in palm oil,
where C16:1, C18:3 and C20:0 contributed to the high RSDR
of 73.99 %, 83.52 % and 41.32 %, respectively.
In some cases, both RSDr and RSDR are calculated to be
0 %. This may be caused by two circumstances, where the
repeatability standard deviation (sr) of each individual laboratory retained after outliers elimination is observed to be
zero (0) or all the individual laboratory means are found to
be similar, thus resulting in the same overall laboratory
mean value. These conditions apply for C12:0 in both palm
oil (Table 1) and palm stearin (Table 3). In other cases, only
the RSDr is encountered to be 0 %. Although the sr of each
individual laboratory is zero, but the individual laboratory
means differ. This can be observed in C16:1 and C18:3 in
palm oil (Table 1), C16:1 and C20:0 in palm olein (Table 2)
and C18:3 in palm stearin (Table 3).
The FAC quantification of palm oil (Table 1), palm olein
(Table 2) and palm stearin (Table 3) may also be expressed
in the form of bar graph. Example of intra- and inter-laboratory variability of FAC measurement (C16:0 in palm oil) is
illustrated in Fig. 1. The bar graph consists of laboratory
codes with their individual means and standard deviations.
From that, the overall mean of all laboratory means with
its standard deviations were generated at 95 % CI after
outliers were eliminated through Cochran and Grubb tests.
These values were calculated according to ISO Guide 3518).
Pocklington and Wagstaffe5) reported that certification of
FAC in soya-maize oil blend (CRM No. 162) and beef-pig fat
blend (CRM No. 163) was only restricted to major fatty
acids since the determination of minor fatty acids (less
than 1 % of mass fraction) could not be undertaken to an
unacceptable degree of accuracy. They observed that the
Fig. 1 Example of Bar Graph for Quantifying C16:0 in Palm Oil.
The results plotted corresponded to four replications. Mean
of means indicate the average results of total individual
laboratory means.
279
J. Oleo Sci. 57, (5) 275-285 (2008)
A.H. Ahmad Tarmizi, W.L. Siew and A. Kuntom
measurement of minor fatty acids produced a wide range
of results and no attempt was made to attribute the uncertainties of those results. Therefore, the fatty acids measured were treated as indicative and expressed in terms of
arithmetic means.
3.2 Stability
Stability of the palm-based reference materials produced
was investigated with respect to their FAC and other general stability indicators such as FFA and TV. The test
results of four storage temperatures (-20, 0, 4 and
24) were compared with the reference materials that was
immediately ampoule-sealed and stored at -20 (t = 0).
Table 4, Table 5 and Table 6 summarise the one-year
storage stability of each individual fatty acid in palm oil,
palm olein and palm stearin. No detectable changes in the
relative proportions of fatty acids were perceived, even at
ambient temperature (24). Therefore, the stability (shelflife) of the reference materials produced for FAC measurement could be extended for more than a year.
Table 7 shows the average TV of the reference materials
over a 12-month storage period. Palm oil experienced
almost no increase of TV at -20, where TV was averaging
at 1.8 unit throughout the storage study. Similar pattern is
observed at 0, where TV was hovering at 1.7 unit to 2.2
unit. However, at 6 and 24, there were slight increases
of TV up to 2.5 unit and 3.1 unit, respectively.
Palm olein demonstrated a higher formation of TV at all
storage temperatures compared to palm oil (Table 7).
There was a slight increase of TV at -20 and 0 at 2.2
unit for both storage conditions. As expected, TV rose
from 1.7 unit to an average of 2.5 unit at 6 and 2.8 unit at
24. Similar trends were also observed in palm stearin. At
-20, 0 and 6, TV was doubled from its initial state,
whereby it increased up to 4-fold at 24 after one year
storage (Table 7).
The formation of TV during storage, especially at high
temperatures is expected. Peroxides are initially developed
and may break down into secondary products such as
hydrocarbons, aldehydes, ketones and small amount of
epoxides and alcohols1). In spite of the TV increase, the
maximum TV of all storage conditions were considered low
and acceptable within stability requirements of reference
materials. However, storage temperatures of -20 to 0
were preferable so as to ensure that the oxidation rate of
the reference materials could be minimised. Addition of
tert-butylhydroquinone, nitrogen flushing prior to sealing
and usage of dark amber glass ampoules will also help to
control the oxidation rate of the reference materials produced.
Triglyceride hydrolysis during storage would be accompanied by FFA development1). No FFA increase was detected at all temperatures in palm oil and palm olein after a 12month storage period (Table 8). Palm stearin demonstrated
a very small FFA increase at all storage temperatures.
This signified that the hydrolytic behaviour of the reference materials produced could be minimised and controlled.
4 CONCLUSION
Development of palm-based reference materials for fatty
acids composition has been achieved through inter-laboratory proficiency tests. Establishment of the consensus values (percentage of individual fatty acids) and their uncertainties at an acceptable level of 95 % confidence interval
have been attained using the SoftCRM 1.2.0 software.
ACKNOWLEDGEMENTS
The authors thank the Director-General of MPOB for
permission to publish this work; Director of Product Development and Advisory Services Division and Head of the
Analytical and Quality Development Unit for their suggestions and recommendations; collaborators of the inter-laboratory proficiency tests for their support and laboratory
technicians of the Analytical and Quality Development
Unit for their assistance.
References
1. Institute of Shortening and Edible Oils. Food fats and
oils. 9th edn., Washington D.C. (2006).
2. Ping, T.G. Analytical characteristics of crude and
refined palm oil and fractions. Eur. J. Lipid Sci. Technol. 109, 373-379 (2007).
3. Boyaci, I.K.; Karabulut, I.; Turan, S. Slip melting point
estimation of fat blends before and after interesterification based on their fatty acid compositions. J. Food
Lipids 10, 193-202 (2003).
4. Tan, Y.A. Quality monitoring (chemical and instrumental techniques). Proceeding of the 27 th Palm Oil
Familiarisation Programme (2007).
5. Pocklington, W.D.; Wagstaffe, P.J. The certification of
the fatty acid profile of two edible oil and fat materials.
BCR Information Reference Materials, Report EUR
11002 EN, Belgium (1987).
6. Boyaci, I.K.; Tekin, A.; Melih, .; Javidipour, I. Viscosity estimation of vegetable oils based on their fatty acid
composition. J. Food Lipids 9, 175-183 (2002).
7. Rabelo, J.; Batisha, E.; Cavaleri, F.V.W.; Meirelles,
A.J.A. Viscosity prediction for fatty systems. J. Am.
Oil Chem. Soc. 77, 1255-1262 (2000).
8. Morad, N.A.; Mustafa Kamal, A.A.; Panau, F.; Yew, T.W.
Liquid specific heat capacity estimation for fatty acids,
280
J. Oleo Sci. 57, (5) 275-285 (2008)
Palm-Based Reference Materials for Fatty Acids Composition
Changes of Individual Fatty Acids in Palm Oil.
Table 4
FAa
Tb ()
Storage Period (month)c
0
10
12
Mean
SDd
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.30
0.25
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.22
0.21
0
0
0.04
0.02
1.10
1.10
1.10
1.10
1.10
1.10
1.20
1.10
1.10
1.10
1.10
1.10
1.10
1.10
1.10
1.10
1.10
1.05
1.10
1.10
1.10
1.09
1.12
1.10
0
0.02
0.04
0
C12:0
-20
0
6
24
C14:0
-20
0
6
24
1.10
1.10
1.10
1.10
1.10
C16:0
-20
0
6
24
43.38
0.10
43.15
43.15
43.15
43.20
43.20
43.20
43.20
43.20
43.80
43.95
44.10
43.95
42.75
43.15
43.10
42.80
43.55
43.70
43.60
43.70
43.50
43.65
43.60
43.40
43.33
43.47
43.46
43.38
0.37
0.34
0.39
0.41
C16:1
-20
0
6
24
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.15
0.10
0.10
0.20
0.20
0.15
0.15
0.15
0.20
0.20
0.20
0.20
0.15
0.10
0.10
0.20
0.18
0.16
0.17
0.19
0.03
0.05
0.04
0.02
C18:0
-20
0
6
24
4.00
4.00
3.95
4.00
4.00
4.00
4.00
4.00
4.00
4.00
3.95
3.95
3.90
3.80
3.85
3.75
3.80
4.05
4.00
4.00
4.05
3.35
3.45
3.70
4.00
3.87
3.85
3.90
3.96
0.27
0.22
0.14
0.09
C18:1
-20
0
6
24
40.65
0.06
40.60
40.50
40.60
40.65
40.75
40.80
40.80
40.80
40.40
40.40
40.85
40.40
41.25
41.10
41.30
41.30
40.30
40.30
40.30
40.30
41.40
41.40
41.15
40.85
40.78
40.75
40.83
40.72
0.45
0.43
0.36
0.35
C18:2
-20
0
6
24
9.78
0.05
9.90
9.90
9.85
9.85
9.90
9.90
9.90
9.90
9.80
9.70
9.65
9.70
10.10
10.00
10.05
10.05
9.90
9.90
9.90
9.85
9.75
9.65
9.65
9.80
9.89
9.84
9.83
9.86
0.12
0.14
0.16
0.12
C18:3
-20
0
6
24
0.20
0.20
0.20
0.20
0.25
0.10
0.10
0.10
0.10
0.20
0.15
0.15
0.15
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.15
0.15
0.15
0.18
0.17
0.17
0.18
0.04
0.04
0.04
0.05
C20:0
-20
0
6
24
0.38
0.05
0.35
0.35
0.35
0.35
0.30
0.30
0.30
0.30
0.30
0.30
0.15
0.15
0.40
0.25
0.10
0.35
0.40
0.40
0.40
0.40
0.30
0.30
0.25
0.30
0.34
0.32
0.26
0.31
0.05
0.05
0.12
0.09
Fatty acid
Storage temperature
c
Mean of duplicate
d
Standard deviation
b
281
J. Oleo Sci. 57, (5) 275-285 (2008)
A.H. Ahmad Tarmizi, W.L. Siew and A. Kuntom
Table 5
FAa
Tb ()
Changes of Individual Fatty Acids in Palm Olein.
Storage Period (month)c
SDd
10
12
Mean
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0
0
0
0
1.10
1.10
1.10
1.05
1.00
1.10
1.00
1.05
1.00
1.00
1.00
1.00
1.10
1.10
1.10
1.10
1.10
1.05
1.10
1.10
1.07
1.08
1.07
1.08
0.05
0.04
0.05
0.03
C12:0
-20
0
6
24
C14:0
-20
0
6
24
1.00
1.00
1.10
1.10
1.10
C16:0
-20
0
6
24
39.88
0.10
40.30
40.00
39.90
39.80
40.05
40.00
39.90
39.95
40.25
40.35
40.25
40.30
39.90
39.45
39.40
39.35
40.00
40.20
40.20
40.10
41.05
40.70
40.45
40.55
40.26
40.12
40.02
40.01
0.42
0.42
0.37
0.42
C16:1
-20
0
6
24
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.25
0.20
0.15
0.20
0.20
0.20
0.19
0.20
0.21
0.20
0.02
0
0.02
0
C18:0
-20
0
6
24
3.90
3.70
3.80
3.80
3.75
3.70
3.70
3.70
3.80
3.80
3.80
3.80
3.80
3.55
3.55
3.70
3.55
3.90
3.90
3.80
3.85
3.60
3.50
3.70
3.70
3.71
3.71
3.75
3.74
0.13
0.16
0.05
0.11
C18:1
-20
0
6
24
43.38
0.05
42.90
43.20
42.70
42.55
43.20
43.30
43.40
43.20
43.05
42.95
43.05
43.00
43.20
43.80
43.60
44.05
43.45
43.30
43.25
43.30
43.10
43.45
43.35
43.30
43.15
43.33
43.23
43.23
0.18
0.28
0.31
0.49
C18:2
-20
0
6
24
10.68
0.05
11.00
11.00
11.05
10.90
10.90
10.90
10.90
10.80
10.70
10.80
10.70
10.75
11.15
11.05
11.10
11.00
10.55
10.50
10.60
10.55
10.35
10.40
10.55
10.45
10.78
10.78
10.82
10.74
0.30
0.27
0.23
0.21
C18:3
-20
0
6
24
0.20
0.30
0.30
0.35
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.25
0.25
0.30
0.25
0.20
0.20
0.20
0.20
0.15
0.20
0.20
0.20
0.22
0.23
0.24
0.21
0.05
0.04
0.07
0.02
C20:0
-20
0
6
24
0.40
0.30
0.30
0.40
0.30
0.30
0.30
0.30
0.30
0.40
0.30
0.35
0.35
0.35
0.35
0.50
0.30
0.40
0.40
0.40
0.40
0.30
0.30
0.30
0.30
0.34
0.33
0.38
0.33
0.05
0.04
0.08
0.04
Fatty acid
Storage temperature
c
Mean of duplicate
d
Standard deviation
b
282
J. Oleo Sci. 57, (5) 275-285 (2008)
Palm-Based Reference Materials for Fatty Acids Composition
Table 6
FAa
Tb ()
Changes of Individual Fatty Acids in Palm Stearin.
Storage Period (month)c
SDd
10
12
Mean
C12:0
-20
0
6
24
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0
0
0
0
C14:0
-20
0
6
24
1.15
0.06
1.20
1.20
1.20
1.20
1.20
1.20
1.20
1.20
1.25
1.20
1.10
1.20
1.20
1.20
1.20
1.20
1.20
1.20
1.20
1.20
1.15
1.15
1.15
1.15
1.20
1.19
1.18
1.19
0.03
0.02
0.04
0.02
C16:0
-20
0
6
24
49.50
0.20
49.40
49.50
49.35
49.45
49.40
49.40
49.40
49.40
50.60
50.55
50.55
50.25
49.15
49.00
49.00
49.00
49.80
49.60
49.65
49.60
50.05
50.20
50.05
51.10
49.73
49.71
49.67
49.63
0.53
0.57
0.56
0.47
-20
0
6
24
0.18
0.05
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.20
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.15
0.10
0.10
0.10
0.10
0.15
0.15
0.13
0.14
0.05
0.05
0.05
0.05
C18:0
-20
0
6
24
4.23
0.10
4.20
4.20
4.15
4.20
4.20
4.20
4.20
4.20
3.95
4.10
4.10
4.15
4.10
4.10
4.10
4.10
4.20
4.20
4.20
4.20
3.80
3.80
3.85
3.90
4.08
4.10
4.10
4.13
0.17
0.15
0.13
0.12
C18:1
-20
0
6
24
35.95
0.17
35.75
35.75
35.80
35.70
36.00
36.00
36.00
36.00
35.55
35.30
35.55
35.35
36.50
36.50
36.50
36.50
35.75
36.10
36.20
36.10
36.30
36.25
36.25
36.15
35.98
35.98
36.05
35.97
0.36
0.42
0.34
0.40
C18:2
-20
0
6
24
8.28
0.10
8.25
8.30
8.30
8.25
8.30
8.30
8.30
8.30
8.05
7.90
7.90
8.00
8.35
8.40
8.40
8.40
8.20
8.10
7.95
8.05
7.95
7.95
7.90
7.90
8.18
8.16
8.13
8.15
0.15
0.21
0.23
0.19
C18:3
-20
0
6
24
0.10
0.20
0.20
0.15
0.20
0.10
0.10
0.10
0.10
0.05
0.10
0.15
0.25
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.10
0.15
0.20
0.11
0.12
0.13
0.16
0.05
0.04
0.03
0.07
C20:0
-20
0
6
24
0.40
0.25
0.20
0.25
0.35
0.30
0.30
0.30
0.30
0.05
0.20
0.20
0.20
0.30
0.35
0.40
0.40
0.30
0.30
0.30
0.30
0.25
0.20
0.35
0.30
0.24
0.28
0.31
0.34
0.10
0.06
0.06
0.10
C16:1
Fatty acid
Storage temperature
c
Mean of duplicate
d
Standard deviation
b
283
J. Oleo Sci. 57, (5) 275-285 (2008)
A.H. Ahmad Tarmizi, W.L. Siew and A. Kuntom
Table 7 Changes of TV in Palm-Based Reference Materials.
Reference
Materials
a
b
Ta ()
Palm Oil
-20
0
6
24
Palm Olein
-20
0
6
24
Palm Stearin
-20
0
6
24
Storage Period (month)b
0
10
12
1.5
1.7
1.9
2.2
2.4
2.2
1.9
2.4
2.8
2.1
2.2
2.4
3.0
1.7
1.7
2.0
3.1
1.8
1.7
1.7
2.8
1.6
2.0
2.5
2.6
1.7
2.5
2.5
2.7
2.9
2.4
2.4
2.6
3.2
2.7
2.4
2.8
3.2
2.0
2.0
2.2
3.6
1.9
2.1
2.8
2.8
1.8
2.4
2.2
3.1
1.0
1.7
1.6
2.3
2.4
2.3
1.5
2.5
2.9
2.2
2.0
2.3
3.1
1.9
2.0
2.2
3.8
2.0
1.6
2.3
3.3
1.7
1.9
1.7
3.7
Storage temperature
Mean of triplicate
Table 8 Changes of FFA in Palm-Based Reference Materials.
Reference
Materials
a
b
Ta ()
Palm Oil
-20
0
6
24
Palm Olein
-20
0
6
24
Palm Stearin
-20
0
6
24
Storage Period (month)b
0
10
12
0.04
0.04
0.04
0.03
0.04
0.04
0.04
0.03
0.04
0.03
0.04
0.03
0.04
0.03
0.03
0.02
0.03
0.03
0.03
0.02
0.03
0.05
0.04
0.04
0.03
0.07
0.06
0.06
0.07
0.07
0.06
0.06
0.07
0.08
0.06
0.06
0.06
0.07
0.06
0.06
0.07
0.08
0.06
0.06
0.07
0.08
0.06
0.08
0.08
0.10
0.02
0.04
0.03
0.04
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.05
0.03
0.03
0.03
0.03
0.03
0.04
0.04
0.04
0.04
0.05
0.03
Storage temperature
Mean of triplicate
triacylglycerols and vegetable oils based on their fatty
acid composition. J. Am. Oil Chem. Soc. 77, 1001-1005
(2000).
9. Halvorsen, J.D.; Mammel Jr, W.C.; Clements, L.D. Density estimation for fatty acids and vegetable oils based
on their fatty acid composition. J. Am. Oil Chem. Soc.
70, 875-880 (1993).
10. Ainie, K.; Siew, W.L.; Tan, Y.A.; Nor Aini, I.; Mohtar, Y.;
Tang, T.S.; Nuzul Amri, I. MPOB test methodsA compendium of test on palm oil products, palm kernel
products, fatty acids, food related products and others. Malaysian Palm Oil Board (2004).
11. ISO 5508:1990. Animal and vegetable fats and oils
Analysis by gas chromatography (GC) of the methyl
284
J. Oleo Sci. 57, (5) 275-285 (2008)
Palm-Based Reference Materials for Fatty Acids Composition
12.
13.
14.
15.
esters of fatty acids. International Organization for
Standardization (1990).
Bonas G. SoftCRM version 1.2.0. Funded by Standards, Measurements and Testing Programmes (1997).
ISO 5725-2. Accuracy (trueness and precision) of measurement methods and results -Part 1 :Basic method
for the determination of repeatability and reproducibility of a standard measurement method. 1st
edn., International Organization for Standardization
(1994).
ISO 3534-1. Statistics, vocabulary and symbols-Part
1:Probability and general statistical terms. 1st edn.,
International Organization for Standardization (1993).
Firestone, D. Official methods and recommended
practices of the AOCS. 5th edn., Am. Oil Chem. Soc.,
Champaign (1998).
16. Dieffenbacher, A.; Pocillinton, W.D. Standard methods
for the analysis of oils, fats and derivatives. 7th edn.,
International Union and Pure Applied Chemistry,
Oxford (2002).
17. Azmil Haizam, A.T.; Elina, H.; Siew, W.L.; Ainie, K. and
Ooi, C.K. Commercialisation of standard reference
materials from palm oil products. MPOB 117th VIVA
Committee Meeting, VIVA Report No. 391/2007 (07),
Malaysian Palm Oil Board (2007).
18. ISO Guide 35. Certification of reference materials
General and statistical principle. 2nd edn., International Organization for Standardization, Switzerland
(1989).
285
J. Oleo Sci. 57, (5) 275-285 (2008)
S-ar putea să vă placă și
- Foundation Code 2004Document64 paginiFoundation Code 2004Patie Zheng100% (2)
- Foundation Code 2004Document64 paginiFoundation Code 2004Patie Zheng100% (2)
- Motor Load and Efficiency PDFDocument16 paginiMotor Load and Efficiency PDFwfjerrÎncă nu există evaluări
- Waste Oil Distillation MachineDocument12 paginiWaste Oil Distillation Machinenazif100% (1)
- Carboxylic AcidDocument21 paginiCarboxylic AcidShalsabila NHÎncă nu există evaluări
- Strobe Rocket Spindle For Other Rocket TypesDocument1 paginăStrobe Rocket Spindle For Other Rocket TypeseskawitzÎncă nu există evaluări
- Cbse Sample Papers For Class 11 Chemistry Download PDFDocument4 paginiCbse Sample Papers For Class 11 Chemistry Download PDFPIPARIYAÎncă nu există evaluări
- Ethanol Production From GrainDocument21 paginiEthanol Production From Grainrenger20150303Încă nu există evaluări
- Accounting Finance For EngineersDocument16 paginiAccounting Finance For EngineersSubashiиy PяabakaяaиÎncă nu există evaluări
- Accounting Finance For EngineersDocument16 paginiAccounting Finance For EngineersSubashiиy PяabakaяaиÎncă nu există evaluări
- 3 - Shortenings - Science and TechnologyDocument41 pagini3 - Shortenings - Science and TechnologyJulesÎncă nu există evaluări
- PRODUCTIONOF60000MTPAOFOLEOCHEMICALMETHYLESTERFROMRBDPALMKERNELOILDocument586 paginiPRODUCTIONOF60000MTPAOFOLEOCHEMICALMETHYLESTERFROMRBDPALMKERNELOILKevin Fernando PratamaÎncă nu există evaluări
- 5.pondasi FFB Conveyor & SterilzerDocument6 pagini5.pondasi FFB Conveyor & SterilzerSuhandra HendraÎncă nu există evaluări
- 4 - Technical Specifications For Piling Works - Pile Foundation For Tanks - ParadeepDocument48 pagini4 - Technical Specifications For Piling Works - Pile Foundation For Tanks - Paradeepvignesh_freeboterÎncă nu există evaluări
- Environmental Fact Sheet (# 33) Crude Palm Kernel Oil (CPKO)Document6 paginiEnvironmental Fact Sheet (# 33) Crude Palm Kernel Oil (CPKO)eefgtgÎncă nu există evaluări
- Blending of Thermal Coals: Section ContentsDocument17 paginiBlending of Thermal Coals: Section ContentsWulan Dwikusuma AsihÎncă nu există evaluări
- Bio Energy Presentation of Bio FuelDocument31 paginiBio Energy Presentation of Bio FuelDeep GreyÎncă nu există evaluări
- Experiment 1 Qualitative Analysis of CarbohydratesDocument14 paginiExperiment 1 Qualitative Analysis of CarbohydratesEko Nevrian90% (10)
- Palm Oil & Saturated Steam PropertiesDocument4 paginiPalm Oil & Saturated Steam PropertiesStefanusÎncă nu există evaluări
- Crude Palm OilDocument19 paginiCrude Palm OilmarpadanÎncă nu există evaluări
- LOTTE E&C - IntroduceDocument14 paginiLOTTE E&C - Introducemyusuf123Încă nu există evaluări
- Cornstarch ProjectDocument44 paginiCornstarch ProjectpadhaiÎncă nu există evaluări
- Chloride in Palm OilDocument4 paginiChloride in Palm OilFebrian IsharyadiÎncă nu există evaluări
- Dry FractionationDocument12 paginiDry FractionationRinovetz AlexandruÎncă nu există evaluări
- Reaction List v002Document5 paginiReaction List v002cecil3414Încă nu există evaluări
- Activation of Spent Bleaching Earth For Dehumidification ApplicationDocument8 paginiActivation of Spent Bleaching Earth For Dehumidification ApplicationWorld-Academic JournalÎncă nu există evaluări
- Lecture 3 PDFDocument53 paginiLecture 3 PDFCurtis Hotate100% (1)
- Bio Oil Empty Fruit BunchesDocument8 paginiBio Oil Empty Fruit Bunchesegananta100% (1)
- Thermal Conductivity of WaterDocument6 paginiThermal Conductivity of Waterhiddenkey1Încă nu există evaluări
- PolymerViscosity Lab ReportDocument14 paginiPolymerViscosity Lab ReportBrandeice Barrett100% (1)
- Contractual Methods of Analysis 2017Document11 paginiContractual Methods of Analysis 2017jaimeÎncă nu există evaluări
- IndustrialAndPreparativeResin CatalogueDocument26 paginiIndustrialAndPreparativeResin CatalogueI. Murali KrishnaÎncă nu există evaluări
- Substances Vs MixturesDocument2 paginiSubstances Vs MixturesEdelvina Lorenzo AlejoÎncă nu există evaluări
- Biodiesel Production Techniques PDFDocument4 paginiBiodiesel Production Techniques PDFatomixmanÎncă nu există evaluări
- Physico Chemical Properties of Palm StearinDocument77 paginiPhysico Chemical Properties of Palm StearinSeren VegaÎncă nu există evaluări
- FOSFA Technical Manual - Oils and FatsDocument15 paginiFOSFA Technical Manual - Oils and Fatssr51585559Încă nu există evaluări
- SNI Palm Kernel OilDocument8 paginiSNI Palm Kernel OilYouke Septiany SapanganÎncă nu există evaluări
- Biocatalysis and Agricultural Biotechnology: Garima Pande, Casimir C. Akoh, Robert L. ShewfeltDocument9 paginiBiocatalysis and Agricultural Biotechnology: Garima Pande, Casimir C. Akoh, Robert L. ShewfeltersierrasÎncă nu există evaluări
- Ruchi Soya IndustriesDocument8 paginiRuchi Soya IndustriesAkhilesh KumarÎncă nu există evaluări
- Product Spect - VPODocument2 paginiProduct Spect - VPOKomathi BalasupramaniamÎncă nu există evaluări
- Technical Manual 2021 AmendmentsDocument16 paginiTechnical Manual 2021 Amendmentslaboratorium operasionalÎncă nu există evaluări
- LP1 - Razmah Ghazali PDFDocument56 paginiLP1 - Razmah Ghazali PDFJessicalba LouÎncă nu există evaluări
- DR Seema ParohaDocument21 paginiDR Seema ParohaTEERATH RAJÎncă nu există evaluări
- Iodine Value Determination Porim Test MethodDocument2 paginiIodine Value Determination Porim Test MethodAdawiyah Ali100% (1)
- Table of Thermal Conductivity Values of Propylene Glycol and Water MixesDocument2 paginiTable of Thermal Conductivity Values of Propylene Glycol and Water MixesTung Trinh0% (1)
- Project Report On Citric Acid and Lemon Oil From LemonDocument7 paginiProject Report On Citric Acid and Lemon Oil From LemonEIRI Board of Consultants and PublishersÎncă nu există evaluări
- Exp 2-Dry FractionationDocument14 paginiExp 2-Dry FractionationFfmohamad NAdÎncă nu există evaluări
- Fuel Oil TreatmentDocument8 paginiFuel Oil TreatmentRague MiueiÎncă nu există evaluări
- AlfaLaval Corn Gluten Dewatering BrochureDocument2 paginiAlfaLaval Corn Gluten Dewatering BrochureI. Murali KrishnaÎncă nu există evaluări
- Ecoboard Spentwash PDFDocument23 paginiEcoboard Spentwash PDFMailhem Dr. Suhas GoreÎncă nu există evaluări
- Philippine Coconut Authority: Republic of The Philippines Department of AgricultureDocument12 paginiPhilippine Coconut Authority: Republic of The Philippines Department of Agricultureanthony santosoÎncă nu există evaluări
- Material BalanceDocument27 paginiMaterial BalanceJohn Bryan AldovinoÎncă nu există evaluări
- New Developments in Palm Oil FractionationDocument7 paginiNew Developments in Palm Oil FractionationALEJANDRO HERNANDEZÎncă nu există evaluări
- Technicalreport Alcoholtechnology PDFDocument0 paginiTechnicalreport Alcoholtechnology PDFAmol DeshmukhÎncă nu există evaluări
- Thermodynamics Palm Oil MillDocument9 paginiThermodynamics Palm Oil MillWilliam MansoÎncă nu există evaluări
- Afroz 2003Document7 paginiAfroz 2003Fatin AqilahÎncă nu există evaluări
- 31295019466605Document329 pagini31295019466605ctomeyÎncă nu există evaluări
- Lecture 1Document22 paginiLecture 1Curtis HotateÎncă nu există evaluări
- Palm Oil FractionationDocument2 paginiPalm Oil FractionationChiew Let Chong0% (1)
- A Direct Method For Fatty Acid Methyl Ester (FAME) SynthesisDocument40 paginiA Direct Method For Fatty Acid Methyl Ester (FAME) Synthesisanuradha.d.bhat9860Încă nu există evaluări
- Supply ChainDocument19 paginiSupply Chainmarkkishan100% (2)
- Deterioration of Bleachability of Palm Oil MillDocument5 paginiDeterioration of Bleachability of Palm Oil MillEffy SaifulÎncă nu există evaluări
- Poeb130 YosriDocument9 paginiPoeb130 YosriNa'imusyahmi Ghazali100% (1)
- Brief Refinery Write Up GRDocument11 paginiBrief Refinery Write Up GRShiv KumarÎncă nu există evaluări
- Proposal Defence - Farah Wahida Corrected Ss 2Document19 paginiProposal Defence - Farah Wahida Corrected Ss 2Farah Wahida JusohÎncă nu există evaluări
- Caustic Recovery With Hot WaterDocument1 paginăCaustic Recovery With Hot WaterNadeem NaseemÎncă nu există evaluări
- D1429-03-Test Methods For Specific Gravity of Water and BrineDocument7 paginiD1429-03-Test Methods For Specific Gravity of Water and BrinefabianÎncă nu există evaluări
- Liquid Urea-Formaldehyde Resin Manufacturing Industry-217599 - 4Document65 paginiLiquid Urea-Formaldehyde Resin Manufacturing Industry-217599 - 4Sanzar Rahman 1621555030Încă nu există evaluări
- Paradeep DFRDocument22 paginiParadeep DFRioeuserÎncă nu există evaluări
- FFB Crop ForecastDocument6 paginiFFB Crop ForecastAmeen ZennÎncă nu există evaluări
- Fatty Acid CompositionDocument5 paginiFatty Acid CompositionAnonymous MhTaJsÎncă nu există evaluări
- TAP Uleiuri False1Document12 paginiTAP Uleiuri False1Benn BennetÎncă nu există evaluări
- Standard For Essential Composition of VCO PDFDocument11 paginiStandard For Essential Composition of VCO PDFUbais AliÎncă nu există evaluări
- 117 Excerpts 2004Document7 pagini117 Excerpts 2004Anonymous DJrec2Încă nu există evaluări
- The Business Model CanvasDocument1 paginăThe Business Model CanvasAnonymous DJrec2Încă nu există evaluări
- Factor of Safety On The Geotechnical Information WebsiteDocument2 paginiFactor of Safety On The Geotechnical Information WebsiteAnonymous DJrec2Încă nu există evaluări
- External Friction Angle On The Geotechnical Information WebsiteDocument2 paginiExternal Friction Angle On The Geotechnical Information WebsiteAnonymous DJrec2100% (1)
- Bearing Capacity Factors On The Geotechnical Information WebsiteDocument2 paginiBearing Capacity Factors On The Geotechnical Information WebsiteAnonymous DJrec2Încă nu există evaluări
- Cohesion On The Geotechnical Information WebsiteDocument2 paginiCohesion On The Geotechnical Information WebsiteAnonymous DJrec2Încă nu există evaluări
- 0217 PresentationDocument73 pagini0217 PresentationHector RodriguezÎncă nu există evaluări
- CFD Simulation On CFBC BoilerDocument6 paginiCFD Simulation On CFBC BoilerAnonymous DJrec2Încă nu există evaluări
- Circulating Fluidized Bed Boiler Development Situation and ProspectsDocument4 paginiCirculating Fluidized Bed Boiler Development Situation and ProspectsAnonymous DJrec2Încă nu există evaluări
- Evolution of Management ThoughtDocument17 paginiEvolution of Management ThoughtAnonymous DJrec2Încă nu există evaluări
- BEM Code of Ethics - Ir. Yim Hon WaDocument53 paginiBEM Code of Ethics - Ir. Yim Hon WaAnonymous DJrec2Încă nu există evaluări
- BEM Code of EthicsDocument11 paginiBEM Code of EthicsAbdul Hakim AbdullahÎncă nu există evaluări
- Managing A Safe Work PlaceDocument3 paginiManaging A Safe Work PlaceAnonymous DJrec2Încă nu există evaluări
- Understanding MarketingDocument5 paginiUnderstanding MarketingAnonymous DJrec2Încă nu există evaluări
- Engineers in Construction Industry - Ir. Choo Kok BengDocument22 paginiEngineers in Construction Industry - Ir. Choo Kok BengAnonymous DJrec2Încă nu există evaluări
- Power Amps Efficiency PFDocument5 paginiPower Amps Efficiency PFAnonymous DJrec2Încă nu există evaluări
- Industrial LowbayDocument8 paginiIndustrial LowbayAnonymous DJrec2Încă nu există evaluări
- Dry-Type Transformers: Codes and Standards Enhancement (CASE) StudyDocument22 paginiDry-Type Transformers: Codes and Standards Enhancement (CASE) StudyAnonymous DJrec2Încă nu există evaluări
- HarmonicsDocument7 paginiHarmonicsAnonymous DJrec2Încă nu există evaluări
- Third Quarter 2016 Investment Outlook: Asset Class SectorDocument6 paginiThird Quarter 2016 Investment Outlook: Asset Class SectorAnonymous DJrec2Încă nu există evaluări
- Sportage SL Pen-010112 PDFDocument1 paginăSportage SL Pen-010112 PDFAnonymous DJrec2Încă nu există evaluări
- Basics of Power CableDocument7 paginiBasics of Power CableAnonymous DJrec2Încă nu există evaluări
- CiMB Research Report On REITDocument6 paginiCiMB Research Report On REITAnonymous DJrec2Încă nu există evaluări
- Adoc - Pub - Pengaruh Penambahan Bahan Kimia Pada Tanah LempungDocument12 paginiAdoc - Pub - Pengaruh Penambahan Bahan Kimia Pada Tanah LempungDewi rahmawatiÎncă nu există evaluări
- Column Manual: Metrosep A Supp 4 (6.1006.XX0 / 6.01021.XX0)Document50 paginiColumn Manual: Metrosep A Supp 4 (6.1006.XX0 / 6.01021.XX0)LollipopÎncă nu există evaluări
- Tutorial 7-Chemical Equilibrium and Ionic Equilibria Part IDocument2 paginiTutorial 7-Chemical Equilibrium and Ionic Equilibria Part IRazy NicholaiÎncă nu există evaluări
- Cambridge IGCSE™: Chemistry 0620/43Document11 paginiCambridge IGCSE™: Chemistry 0620/43krishaÎncă nu există evaluări
- Inorganica Chimica ActaDocument10 paginiInorganica Chimica ActaNikhil BhoumikÎncă nu există evaluări
- Zytel LC7602 BK010Document11 paginiZytel LC7602 BK010Spu XisterÎncă nu există evaluări
- Reversible Reactions: When You Heat Copper (II) Sulfate CrystalsDocument2 paginiReversible Reactions: When You Heat Copper (II) Sulfate CrystalsShahid Ur RehmanÎncă nu există evaluări
- Program Studi Kimia F.MIPA, Universitas UdayanaDocument6 paginiProgram Studi Kimia F.MIPA, Universitas UdayanaRyu- MikaÎncă nu există evaluări
- CHMBD 449 - Organic Spectral: AnalysisDocument43 paginiCHMBD 449 - Organic Spectral: AnalysisIleana ManciuleaÎncă nu există evaluări
- l4 Separation and Identification of Group 2a CationsDocument3 paginil4 Separation and Identification of Group 2a Cationsthegr8 GÎncă nu există evaluări
- Report Expt. 1 Chemical ReactionsDocument7 paginiReport Expt. 1 Chemical ReactionsNgô BắpÎncă nu există evaluări
- June 2018 Question Paper 31 PDFDocument16 paginiJune 2018 Question Paper 31 PDFKazi Ahnaf SaadÎncă nu există evaluări
- 20 PDFDocument82 pagini20 PDFWalid EbaiedÎncă nu există evaluări
- Lecture Planner - Chemistry - MANZIL For JEE 2024Document1 paginăLecture Planner - Chemistry - MANZIL For JEE 2024Rishi NairÎncă nu există evaluări
- Formulating For Extruding AbateDocument11 paginiFormulating For Extruding Abatehossny100% (1)
- Mrs. Melendez-Beltran Pre-AICE ChemDocument98 paginiMrs. Melendez-Beltran Pre-AICE ChemTravel UnlimitedÎncă nu există evaluări
- Experiment 7 (EDTA) - Lab ManualDocument3 paginiExperiment 7 (EDTA) - Lab ManualJoseph JoeÎncă nu există evaluări
- bufferDocument51 paginibufferdiah ayu romadhaniÎncă nu există evaluări
- FBISE Chapter 6Document12 paginiFBISE Chapter 6Ch NajamÎncă nu există evaluări
- Ultratech Cement: Particulars Test Results Requirements ofDocument1 paginăUltratech Cement: Particulars Test Results Requirements ofRajeshÎncă nu există evaluări
- Chemistry Practicals CompleteDocument22 paginiChemistry Practicals Completemoviemaza071Încă nu există evaluări
- Allopurinol PDFDocument3 paginiAllopurinol PDFGladdis Kamilah PratiwiÎncă nu există evaluări
- Safari PDFDocument18 paginiSafari PDFHussain KhanÎncă nu există evaluări