Documente Academic
Documente Profesional
Documente Cultură
Journal Article
Încărcat de
Oriol Kanu del BesòsTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Journal Article
Încărcat de
Oriol Kanu del BesòsDrepturi de autor:
Formate disponibile
Life History Patterns of Gambusia marshi (Poeciliidae) from Cuatro Cinegas, Mexico
Author(s): Gary K. Meffe
Source: Copeia, Vol. 1985, No. 4 (Dec. 10, 1985), pp. 898-905
Published by: American Society of Ichthyologists and Herpetologists (ASIH)
Stable URL: http://www.jstor.org/stable/1445239
Accessed: 01-10-2015 15:50 UTC
Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://www.jstor.org/page/
info/about/policies/terms.jsp
JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content
in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship.
For more information about JSTOR, please contact support@jstor.org.
American Society of Ichthyologists and Herpetologists (ASIH) is collaborating with JSTOR to digitize, preserve and extend
access to Copeia.
http://www.jstor.org
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
COPEIA, 1985, NO. 4
898
SELANDER,R. K., ANDT. S. WHITTAM.1983. Protein
polymorphism and the genetic structure of populations, p. 89-144. In: Evolution of genes and proteins. M. Nei and R. K. Koehn (eds.). Sinauer Assoc.
Inc., Sunderland, Massachusetts.
SHAKLEE,J.B., AND C. S. TAMARU. 1981. Biochemical and morphological evolution of Hawaiian bonefishes (Albula). Syst. Zool. 30:125-146.
SOKAL, R. R., AND F.J. ROHLF. 1969. Biometry. Freeman and Co., San Francisco, Calif.
TODD, T. N., G. R. SMITHAND L. E. CABLE. 1981.
Environmental and genetic contributions to morphological differentiation in ciscoes (Coregoninae)
of the Great Lakes. Can. J. Fish. Aquat. Sci. 38:5967.
UTTER, F. M. 1981. Biological criteria for definition
of species and distinct intraspecific populations of
anadromous salmonids under the U.S. Endangered
Species Act of 1973. Can. J. Fish. Aquat. Sci. 38:
1626-1635.
VAN VALEN,L. 1978. The statistics of variation. Evol.
Theory 4:33-43.
VRIJENHOEK,R. C., AND S. LERMAN. 1982. Heterozygosity and developmental stability under sexual
and asexual breeding systems. Evolution 36:768776.
1982. Milkfish fry seasonality on
Tarawa, Kiribati, its relationship to fry seasons elsewhere, and to a sea surface temperature (SST).
Aquaculture 26:265-271.
WAKE, D. B. 1981. The application of allozyme evidence to problems in the study of morphology, p.
257-270. In: Evolution today; Proc. 2nd Int. Congr.
Syst. Evol. Biol. G. G. E. Scudder and J. L. Reveal
(eds.). Carnegie Mellon University, Pittsburgh,
Pennsylvania.
WINANS, G. A. 1980. Geographic variation in the
milkfish Chanos chanos. I. Biochemical evidence.
Evolution 34:558-574.
WRIGHT,S. 1978. Evolution and the Genetics of Populations. Vol. 4. Variability Within and Among Natural Populations. University of Chicago Press, Chicago, Illinois.
WAINWRIGHT, T.
NORTHWEST
NATIONAL
TIONAL
AND ALASKA FISHERIES CENTER,
MARINE FISHERIES SERVICE, NA-
EAST,
AND ATMOSPHERIC
OCEANIC
NISTRATION,
2725
SEATTLE,
MONTLAKE
WASHINGTON
ADMI-
BOULEVARD
Ac98112.
cepted 26 Feb. 1985.
Copeia,1985(4),pp. 898-905
Life History Patterns of Gambusiamarshi
(Poeciliidae) from Cuatro Cienegas, Mexico
GARY K. MEFFE
The life history and ecology of Gambusia marshi, a poeciliid fish endemic to
the Cuatro Cienegas basin and Rio Salado drainage of Coahuila, Mexico, is
virtually unknown. Fishes collected in different seasons from natural lakes,
rivers and an artificial canal were analyzed for reproductive patterns, food choice
and parasitism. G. marshi reproduced seasonally, with fecundity increasing as a
function of female size. Reproduction is not strongly habitat dependent, although
reduced reproductive activity was noted in the canal. Superfetation was infrequent. Major food items of G. marshi were detritus and insects, with other invertebrates, plant material and juvenile fish also represented. Somatic condition
was little affected by habitat or season and most individuals were robust. Parasites
included two nematodes, a trematode and an acanthocephalan. G. marshi is a
habitat and food generalist, which perhaps accounts for its local abundance and
success within its limited range.
THE
Cuatro Cienegas region, an intermontane basin west of Monclova, Coahuila,
Mexico, is well known for its high degree of
endemism
biological
(Taylor and Minckley,
1966; Marsh, 1984). Aquatic endemics include
species of crustaceans, molluscs, fishes and tur-
tles (Minckley, 1969). Despite intense interest
in this biota, particularly in the fishes (reviewed
by Minckley, 1984), few comprehensive studies
have been conducted on basic ecology and life
histories of its component species. With growing awareness of the scientific value of this area,
? 1985 by the American Society of Ichthyologists and Herpetologists
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
MEFFE-GAMBUSIA MARSHI LIFE HISTORY
potentials for human-induced changes and resultant conservation concerns (Contreras-Balderas, 1984), it is appropriate to assemble life
history information regarding Cuatro Cienegas
fishes.
Gambusia marshi Minckley and Craddock
(Poeciliidae) is native to the Cuatro Cienegas
basin and the Rio Salado drainage of Coahuila.
Occurring in two color morphs, a light, spotted
phase and a striped, melanistic phase (Minckley,
1962), it is ". .. perhaps the most abundant fish
in the Cuatro Cienegas basin ..." (Minckley,
1969). Despite its abundance and general representation in collections, little has been published on G. marshi since its description more
than 20 years ago (Minckley, 1962). Included
are reports on laboratory hybridization with G.
affinis(Minckley, 1964), laboratory study of mate
choice by males (Arnold, 1966), a discussion of
distribution and occurrence within the Cuatro
Cienegas basin (Minckley, 1969, 1984), chromosome morphology (Campos and Hubbs,
1971) and details of male and female genital
morphologies (Peden, 1972a, b).
I investigated reproductive parameters, food
utilization and parasite occurrence in spotted
phase G. marshi over four seasons from three
major habitats of Cuatro Cienegas: shallow lakes
(lagunas), rivers and an artificial canal. Seasonal
and spatial (habitat) variation in life history of
G. marshi was thus obtained for a gross analysis
of its ecology.
MATERIALS AND METHODS
Specimens used.--All analyses were conducted
on preserved specimens housed in the Arizona
State University (ASU) Museum, collected by
various personnel from 1964-1970, but mostly
1964-1965. Because collections were not made
specifically for comprehensive life history studies, I selected habitats best represented and for
which the largest number of specimens was
available. Consequently, two habitat categories
included collections from several different localities, while the seasonal analysis included collections from several years. Seasonal designations are: winter = Dec.-Jan.; spring = April;
summer = June; autumn = Aug.-Sept.
The following collections were utilized (listed
are localities as in Minckley [1969], ASU museum number and collection date): LagunasSan Pablo Laguna, 625, 24 Jan. 1964; Laguna
Grande, 1746, 15 Apr. 1965; Ferrinios Laguna,
955, 9June 1964; Laguna Grande, 5967, 2 Sept.
899
1970. Rivers-Rio Salado, 2852, 21 Dec. 1966;
1725, 13 April 1965; Puente Colorado, 967, 10
June 1964; 5989,4 Sept. 1970. Canals-La Angostura, 620, 23Jan. 1964; 1731, 13 April 1965;
934, 7 June 1964; 2260, 13 Aug. 1965.
Length-frequencyanalysis.-All specimens from
each collection were sexed and placed in 1 mm
(standard length, SL) size categories. One to
three mature females were randomly selected
from each category for further analysis, so that
a total of 20-30 females representing the size
range of maturity were selected (three small
collections allowed analysis of only 18, 18 and
11 individuals). These fish were used in all subsequent procedures. Most collections were made
by 3.2 mm mesh seines, but some were by 6.3
mm mesh and mesh sizes may have influenced
size distributions in collections.
Reproductiveand somaticanalysis.-Females were
measured to the nearest 0.1 mm SL with a dial
caliper. Ovaries were removed and contents
counted under a dissecting microscope (7x).
Eggs and embryos were placed into one of six
categories, primarily for detection of superfetation: 1) immature ova-ova in the process of
yolking, but sub-mature in size; 2) mature ova/
fertilized egg-full-sized ova, unfertilized or recently fertilized; 3) primitive streak-primitive
streak present, but eyes not yet distinguishable;
4) early eyed-eyes present, but not full sized;
little dorsal pigmentation; 5) middle eyed-eyes
full sized; moderate to extensive dorsal pigmentation; and 6) late eyed-little
yolk remaining; near parturition.
Categories 2-6 were summed to obtain total
number of eggs and embryos carried, or "total
reproduction" (TR). Category 1 was considered
preparatory to actual reproduction since it included immature members. Subsequent to
counting, ovaries and their contents were placed
into individual tared aluminum pans, dried to
constant weight at 85-90 C for 24-28 hr and
weighed to the nearest 0.1 mg on a Mettler
analytical balance. The remainder of the fish,
minus the digestive tract (below), was similarly
treated and weighed. I thus obtained reproductive and somatic dry weights (RDW and
SDW), respectively. Since all fish were preserved in 10% formalin and stored in 35% isopropanol, potential effects of preservation on
length or weight should be equivalent among
groups.
A somatic condition factor was calculated for
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
COPEIA, 1985, NO. 4
900
WINTER
SPRI NC
AUTUMN
SUMMER
5040-
Y=1.147X - 26.6
r=8 896
:
-
20i *
10'Ih
D
a
I I II
V 1
,I
, tI
5040
RIVERS
Y =1.18X - 24.3
r = .837
Y =.045X2- 2.22X + 27.5
r = .917
Y=1.24X -25.0
r= 923
30-
Y .031X- .34
-r =.086 N.S.
Y =.240X - 5.3
r=.653
Y =.476X - 9.8
r = .698
Y=1,66X -38.2
r =930
2010<t
'tIT T,
r,
.I
. .
I I I I
I1I
I I I
32
36
,~
I . I.
50Y =.653X
r=.875
40CANA L
30-
- 11.3
Y= .073X2 - 3.93X
r=.862
56.3
.140X-3.6
r =.460
2010-
20 24 28 32 36 40 44
20
24
28
32
36
STANDARD
40'44
20 '2428'32'36
LENGTH
'4'4
44
24
28
40
44
(mm)
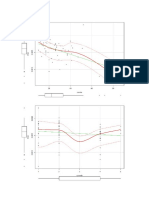
Fig. 1. Regressionsof total reproductionon standardlength for Gambusiamarshiby habitat and season.
The winter lagunaregressionincluded only reproductivelyactive fish. The spring lagunaregressiondoes not
include the extreme value at 41 mm SL, consideredan outlier. A regressionwasnot conducted for the winter
canal sample, since all observationswere zero and thus collinear. N.S. indicates statisticallynon-significant
product-momentcorrelation coefficients;the remainder are significantat P < .05 or better. Small arrows
indicate two identical observations.
each fish as SCF = (SDW/SL) x 100 and reproductive effort was calculated as RE = (RDW/
SDW + RDW) x 100, or the percentage of total
weight invested in reproduction. The relationship between dependent variables TR, SCF, or
RE and the independent variable SL was investigated using a regression analysis program
(BMDP5R, Dixon et al., 1981) on the ASU IBM
system. A linear model was used as a descriptor
of the relationship between the dependent variable and SL. In four cases a second order polynomial was used instead since it improved the
correlation coefficient by at least 10%. These
models are included simply as aids in visualizing
relationships between variables.
Food habits.-The entire length of the digestive
tract was removed (above) and opened lengthwise and contents were examined under a dissecting microscope (7-21x). Contents were
broadly classified as detritus, invertebrates, vascular plant material, turbellarian flatworms
(planarian-like), or fishes. Detritus consisted of
a variety of organic and inorganic materials including mud and debris, but was frequently invested with diatoms and unicellular or filamentous algae, as revealed under higher power (40100 x). Invertebrates consisted primarily of insects, but also included spiders, ostracods and
small unidentified crustaceans. Vascular plants
included small seeds and broken stems. Food
contents were quantified by visually estimating,
at 5% intervals, the percentage of gut filled by
each category.
Parasites.-Gut and coelom parasites were collected as encountered. No systematic search was
made for parasites.
RESULTS
Length-frequencyanalysis.-Male G. marshi generally matured between 18 and 20 mm SL, with
the smallest mature male 17.6 mm and the largest in the 31 mm size class. Females matured at
20-22 mm and ranged upward to 47 mm SL.
Fish from rivers were generally smaller than
those from canals or lagunas, but no consistent
seasonal size trends were noted.
Reproductiveand somaticanalyses.-Numbers of
ova/embryos carried by G. marshi were positively related to female size (Fig. 1). The smallest individual carrying an embryo was 22.0 mm
SL, but fish typically began to yolk ova in the
20-22 mm range. Reproduction was strongly
seasonal, with little activity in winter and autumn and peaks in spring and summer. An exception was females from lagunas, which were
reproductively active in all seasons although less
so in autumn and winter (Fig. 1). All embryos
in the winter (an.) laguna sample were in stage
2, indicating reproduction for that year was just
commencing.
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
MEFFE-GAMBUSIA MARSHI LIFE HISTORY
AUTUMN
SUMMER
SPRING
WINTER
901
504030-
LAGUNAS
Y=-.133X2+97X
Y -.698X + 47.5
N.S
r=-648
Y =.654X -13.5
r =.702
-147.1
Y=.390X
4.1
r=.353 N.S.
20??
t0-
r=.851
I I I
- .I
I'
>
20-
10-
J
,
t;- p I
f^T-.f
C)
.*
Y.618X-12.9
r=.576
Y=.336X-.85
r =.324 N.S.
Y=2.15X- 471
r .910
- Y=.128X-1.1
r= 117 N.S.
30-
,I
50-
30-
Y= 217X +5,9
r .189 NS.
Y -.147X2 9.2X-124.4
r=.858
Y -.014X +74
r. 239 N.S.
40-ANAL
1 .* ,
, 1 . *i
, . ,i .
50-
LI
..
'IVERS
,l
Y=.336X-8.1
r= .419
201020
24
28
32
36 40
44
20
24
28 32 36 40
STANDARD
44
20 24
LENGTH
28
32 36
(mm)
40
44
20
24
32
28
36
40
44
Fig. 2. Regressionof reproductiveeffort on standardlength for Gambusiamarshiby habitat and season.
Protocol as in Fig. 1.
Superfetation, for purposes here, is defined
as carrying ova/embryos in two or more nonconsecutive stages of development. Consecutive
stages (i.e., #2 and #3) were not considered instances of superfetation since they may represent slow development by some embryos, or an
error in classification by the observer. With this
definition only 6 of 251 (2.4%) mature females
superfetated; it was observed once in the canal
(spring, stages 1 and 6), four times in lagunas
(summer, stages 1, 2 and 6; autumn, stages 2
and 4, 2 and 5, and 1 and 5) and once in rivers
(spring, stages 1, 2 and 6). Superfetation is thus
rare in G. marshi.
Reproductive effort as a function of SL is
plotted in Fig. 2. The relation between RE and
SL, although inconsistent, is usually positive. A
low reproductive investment occurred in autumn and winter in all habitats except lagunas,
where the reproductive season was extended.
Generally low coefficients of determination (r2)
indicate that SL is a poor predictor of RE, or
conversely that RE is somewhat independent of
length.
Somatic condition factor as a function of SL
is plotted in Fig. 3. SCF's were consistent among
seasons and habitats, indicating that somatic
condition was little influenced by spatial or temporal change.
Food habits.--G. marshi is primarily a detritivore/insectivore (Fig. 4). The dominant item
AUTUMN
SUMMER
SPRING
WINTER
1.08
LAGUNAS
e
<(
LL
0
.6.42
Y=.034X-.63
r = .962
.38
Y =026r = 987
Y = .025X -.41
r=.990
.8-
Y .025X -.39
r=.963
Y =,028X -.50
r- = .968
I
1,4
Y =.021X -28
r-.921
_Y=.021X -.32
r = .942
--
.4.2
CANAL
1.0o-
26z
Y=.022X-.41
r-,952
< 1.02
0
.8- Y .024X- .45
-U
r =966
.6.4-
I,
Y =.030X -.50
r .993
, .
, .
Y =.023X -.35
r =.939
Y=.036X-.67
r =.957
,220
Fig. 3.
Fig. 1.
24
2 8 32
36 4 444
20
24 '28 32 36 40
STANDARD
44
20 24 28 32 36
L E N G T H (mm)
40
44
20
24
28
32
36 40
44
Regression of somatic condition factor on standard length for Gambusiamarshi.Protocol as in
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
COPEIA, 1985, NO. 4
902
WINTER
SPRING
SUMMER
FALL
LAGUNAS
70.0
47.3
70.8
RIVERS
17.8
CANAL
88.6
61.8
69.4
[I
EMPTY
PLANTS
FISH
FLATWORMS
83.8
[TO INVERTEBRATES
DETRITUS
[
Fig. 4. Pie diagramsshowing averageproportionof alimentarytractsfilled with each food item. Numbers
under diagramsare average percentage fullness of tracts.
in 9 of 12 populations was detritus, with invertebrates occasionally a major food item in summer or autumn. Other categories were sparsely
and intermittently represented.
The amount of each food item as a percentage of total food in guts is a better indicator of
actual food choice by G. marshi (Table 1) since
it ignores empty portions of the digestive tract.
Detritus constituted 37 to 90% of the diet, while
invertebrates made up 9 to nearly 60%. No seasonal trends were obvious other than an increase in utilization of invertebrates through
the year in lagunas. Overall food choice was
remarkably similar in lagunas and rivers, two
natural habitats, while detritus was more important in the artificial canal (Table 1). Invertebrate prey were identified whenever possible
and at least 12 taxa were utilized: Platyhelminthes (Planariidae), Arthropoda (Arachnida,
Ostracoda, unidentified crustaceans), Insecta
(Ephemeroptera, Odonata, Megaloptera, Hemiptera [Corixidae, Naucoridae], Coleoptera,
Diptera, Hymenoptera [red ants]).
Fishes as prey items were detected only from
lagunas and rivers and in quantifiable amounts
only in summer and autumn (Fig. 4). Based on
scale structure and pigmentation patterns, fish
eaten were almost certainly juvenile G. marshi.
Although the species is thus cannibalistic the
behavior appears to be rare.
Parasites.-Four parasite species were collected
from G. marshi: an intestinal trematode, a small
(5-10 mm) intestinal nematode, a large (20-100
mm) encysted coelomic nematode (possibly Contracaecumsp.), all unidentified and an acanthocephalan of the genus Paulisentis (Hoffman,
1967). Incidences of parasitism by season and
habitat (Table 2) indicate more heavily infected
fish in lagunas and lowest incidence of parasitism in the canal. The small nematode, when
present, often numbered several to 20 individuals per fish; other parasites generally occurred
singly. The only other report of G. marshi parasites is by Guajardo-Martinez (1984) who found
"... encysted and intestinal nematodes ...."
DISCUSSION
G. marshi is an abundant fish in the Cuatro
Cienegas basin and adjacent drainages (Minckley, 1962, 1969, 1984). It tolerates a wide range
of environmental conditions and adapts to a variety of situations, with relatively little effect on
life history parameters. The fish is by all measures a generalist, feeding on a variety of items
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
903
MEFFE-GAMBUSIA MARSHI LIFE HISTORY
TABLE 1.
PERCENTAGEOF Gambusia marshi DIET CONSISTING OF EACH FOOD CATEGORY, LISTED BY HABITAT
AND SEASON.
Detritus
Invertebrates
Winter
Spring
Summer
Autumn
Mean
90.3
52.4
40.1
37.7
55.1
8.9
46.9
50.3
59.1
41.3
River
Winter
Spring
Summer
Autumn
Mean
57.9
52.8
42.8
76.3
57.4
Canal
Winter
Spring
Summer
Autumn
Mean
Grand mean
76.6
90.5
77.5
83.1
81.9
64.8
Habitat
Laguna
Season
and existing in all major habitats of Cuatro Cienegas, from natural streams, marshes and lakes
to artificial canals.
Fish from three habitats exhibited similar patterns with respect to reproductive activities, but
with slightly different lengths of reproductive
season. Reproduction in lagunas began in Jan.,
when about one-third of the females contained
yolked ova or early embryos (Fig. 1). Reproduction was at a peak through autumn (Sept.)
and presumably declined thereafter. The reproductive season in lagunas apparently spans
9+ months.
Fish in rivers were reproductively inactive in
Dec. except for one individual beginning to yolk
ova and another with early embryos. Most fish
Flatworms
Plants
Fish
0
0.70
0
0
0.2
0.8
0
1.6
0.6
tr
0
9.6
1.6
2.8
42.1
41.5
54.2
21.0
39.7
0
0
0
0
0
0
5.7
1.5
2.7
2.5
0
0
1.5
0
0.4
22.3
9.3
18.7
10.6
15.3
32.1
1.1
0.2
0.3
1.3
0.7
0.3
0
0
3.5
5.0
2.1
1.7
0
0
0
0
0
1.1
were reproducing in spring and summer (Fig.
1), while only half remained active in Sept. River fish also appear to be reproductively active
from Jan.-Sept. but with smaller broods at the
beginning and end of the season.
Fish in the canal were all inactive near the
end of Jan., while full reproductive activity occurred through spring and summer (Fig. 1). By
mid-Aug. reproduction was on the decline, with
about two-thirds of the fish no longer carrying
offspring. Reproductive season in the canal was
shorter than elsewhere, probably lasting from
Feb. or March until Aug.
Overall, fish were reproductively active longest and carried the largest broods in lagunas,
while both parameters were depressed in the
TABLE2. PARASITES
COLLECTEDFROMGambusia marshi AT CUATROCIENEGAS.
Total number of fishes examined is in parentheses, below which is number of individuals infected by trematode, small nematode, large
nematode and acanthocephalanparasites,respectively.
Season
Winter
Lagunas
Rivers
Canal
(25)
8,0,0,0
(18)
0,0, 1
(26)
0,,0,00
Spring
0,0,
(32)
1,0,0,0
(11)
0,0,0
(25)
0,0,0,0
Summer
Autumn
(20)
4, 13,0,0
(24)
0,9,0,0
(25)
0,0,0,0
(18)
0,0,0, 1
(23)
1,00,0,0
(27)
1,0,3,0
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
COPEIA, 1985, NO. 4
904
canal. This is curious since fish in canals had
the highest average slope for SCF (.0283 vs .0274
and .0233 for lagunas and rivers, respectively)
and thus were not in poor somatic condition.
Reproduction in G. marshi is probably not primarily limited by energetic contraints, but is
rather a function of local environmental conditions.
SCF's were remarkably consistent among seasons and habitats (Fig. 3). Other than minor
mean differences just mentioned (not statistically significant) there was no clear evidence of
seasonal or habitat effects on robustness and
most fish examined appeared to be in good condition. There was, however, an unmeasured
seasonal effect on amount of fat present. Visceral fat was generally absent or at low levels in
reproductively active females (spring and summer) and was prevalent along the alimentary
tracts in autumn and winter. Storage apparently
begins when reproduction declines making highenergy fats available for the next reproductive
season.
Patterns of RE were erratic, but generally
paralleled total reproduction. In most cases, RE
increased with SL, although this was not always
significant (Fig. 2). RE was generally less dependent upon SL than was total reproduction,
indicating that energetic commitments to reproduction in G. marshi may be flexible.
Life history patterns of G. marshi are typical
of gambusiines in general (Krumholz, 1948,
1963; Hubbs and Springer, 1957; Hubbs, 1971;
Peden, 1973). Most examined to date reproduce seasonally, are generalists in food choice
and exhibit increased fecundity with increased
female size. Gambusiamarshi is more of a habitat
generalist than species such as G. amistadensis
(Peden, 1973), G. gaigei (Hubbs and Brodrick,
1963), G. geiseri (Hubbs and Springer, 1957), G.
georgei (Hubbs and Peden, 1969), G. heterochir
(Hubbs, 1957), G. krumholzi(Minckley, 1963) or
G. longispinis (Minckley, 1962), which are restricted to limited habitats or are otherwise rare
in Coahuila or adjacent southern Texas, but
probably is not as generalized as the cosmopolitan G. affinis. The apparently flexible nature of
G. marshi is likely responsible for its abundance
within its limited geographic range.
ACKNOWLEDGMENTS
W. L. Minckley's pioneering studies of the
Cuatro Cienegas fauna made this analysis possible. I thank him for introducing me to this
amazing region, for allowing me to exploit hardearned specimens collected by himself, his colleagues and students and for critically reviewing
the manuscript. The paper was improved by the
comments of Clark Hubbs and an anonymous
reviewer. Financial support during analysis of
fishes was provided by the Department of Zoology, ASU; manuscript preparation was aided
by a postdoctoral appointment at the University
of Florida. I thank Grace Kiltie for typing the
manuscript and B. DeMarias for assistance in
data analysis.
LITERATURECITED
ARNOLD,
E. T. 1966. Comparativestudiesof mating
behavior in gambusiin fishes (Cyprinodontiformes:
Poeciliidae). Unpubl. MS Thesis, Arizona State
University,Tempe, Arizona.
CAMPOS,H. H., AND C. HUBBS. 1971. Cytomor-
phology of six species of gambusiinefishes. Copeia
1971:566-569.
1984. Environmentalimpactsin CuatroCi6negas,Coahuila,Mexico:a commentary.J. Ariz.-Nev. Acad. Sci. 19:85-88.
CONTRERAS-BALDERAS,S.
DIXON, W. J., M. B. BROWN, L. ENGELMAN, J. W.
FRANE,
M. A.
HILL,
R. I.
JENNRICH
AND
J. D.
TOPOREK. 1981. BMDP statistical software. Uni-
versity of CaliforniaPress, Berkeley, California.
G. 1984. Preliminary survey
GUAJARDO-MARTINEZ,
of parasitesof CuatroCi6negas,Coahuila,Mexico.
J. Ariz.-Nev. Acad. Sci. 19:81-83.
HOFFMAN, G. L. 1967. Parasites of North American
freshwater fishes. University of California Press,
Berkeley, California.
HUBBS,C. 1957. Gambusia heterochir,a new poeciliid
fish from Texas, with an account of its hybridization
with G. affinis.Tulane Stud. Zool. 5:1-16.
1971. Competition and isolation mechanisms
in the Gambusia
affinisx G. heterochir
hybridswarm.
Bull. Tex. Mem. Mus. 19:1-47.
, AND H. J. BRODRICK. 1963. Current abundance of Gambusiagaigei, an endangered fish species.
Southwest. Natur. 8:46-48.
, ANDA. E. PEDEN. 1969. Gambusiageorgei sp.
nov. from San Marcos, Texas. Copeia 1969:357364.
,AND V. G. SPRINGER.1957. A revision of the
Gambusianobilis species group, with descriptions of
three new species, and notes on their variation,
ecology, and evolution. Tex. J. Sci. 9:279-327.
KRUMHOLZ, L. A. 1948. Reproductionin the western
mosquito fish, Gambusiaaffinis affinis (Baird and Girard), and its use in mosquito control. Ecol. Monogr. 18:1-43.
. 1963. Relationships between fertility, sex ratio, and exposure to predation in populations of the
mosquitofish Gambusiamanni Hubbs at Bimini, Bahamas. Int. Revue ges. Hydrobiol. 48:201-256.
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
MEFFE-GAMBUSIA MARSHI LIFE HISTORY
MARSH,P. C. 1984. Proceedings of the symposium
on the Cuatro Cienegas of Coahuila, Mexico. J.
Ariz.-Nev. Acad. Sci. 19:1-2.
W. L. 1962. Two new species of fishes of
MINCKLEY,
905
PEDEN,A. E. 1972a. Differencesin the external gen-
italiaof female gambusiinfishes.Southwest.Natur.
17:265-272.
1972b. The functionof gonopodialpartsand
behavioralpattern during copulation by Gambusia
(Poeciliidae).Canad.J. Zool. 50:955-968.
. 1973. Virtual extinction of Gambusiaamistadensisn. sp., a poeciliid fish from Texas. Copeia
1973:210-221.
the genus Gambusia
(Poeciliidae)from northeastern
Mexico. Copeia 1962:391-396.
. 1963. A new poeciliid fish (genus Gambusia)
from the Rio Grandedrainageof Coahuila,Mexico.
Southwest. Natur. 8:154-161.
. 1964. Hybridizationof two species of mos- TAYLOR,D. W., AND W. L. MINCKLEY.1966. New
world for biologists. Pacific Discov. 19:18-22.
quitofishes (Gambusia,Poeciliidae) in the laboratory. J. Ariz. Acad. Sci. 3:87-89.
1969. Environmentsof the Bolson of Cuatro DEPARTMENT OF ZOOLOGY, ARIZONA STATE
Cienegas,Coahuila,Mexico.Withspecialreference
UNIVERSITY, TEMPE, ARIZONA 85287. PRESto the aquaticbiota. Texas WesternPress, UniverENT ADDRESS: SAVANNAH RIVER ECOLOGY
sity of Texas Press, El Paso, Texas.
LABORATORY, DRAWER E, AIKEN, SOUTH
. 1984. Cuatro Cienegas fishes: research reCAROLINA
29801. Accepted 26 Feb. 1985.
view and a local test of diversityversushabitatsize.
Ariz.-Nev.
Sci.
Acad.
19:13-21.
J.
Copeia,1985(4),pp. 905-910
An Experimental Demonstration of the
Species-Recognition Role of Anolis Dewlap Color
JONATHAN B. Losos
Inter- and intraspecificencounters were staged in the laboratorybetween adult
males of the sympatricsibling species Anolis marcanoiand A. cyboteswhich differ
morphologically only in dewlap color. Levels of intraspecific aggression among
A. marcanoiwere high, but little interspecific aggressive behavior was observed.
The importanceof dewlap color for species-recognition was assessed by changing
the color of the dewlap of A. marcanoito appear like that of A. cybotesand viceversa. A. marcanoi in interspecific encounters between altered individuals exhibited an intermediate level of aggressive behavior. Intraspecific encounters
between pairs of A. marcanoi with altered dewlaps revealed no higher level of
aggression than the normal interspecific encounters. A system of species-recognition signals is proposed in which lizards examine more obvious signals, such
as dewlap color, at greater distances and more subtle signals, such as head-
bobbing patterns, at closer distances.
THE
sibling species Anolis marcanoi and A.
cybotesare trunk-ground ecomorphs (Williams, 1972) found sympatrically throughout
much of A. marcanoi's limited range in southwestern Dominican Republic. They are consistently separable morphologically only by the
color of the dewlap of males and the throat of
females: red in A. marcanoi, yellow or white in
A. cybotes(Williams, 1975). Electrophoretic studies, however, show them to be quite distinct and
reveal no hybrids (Webster, 1975). How the
species coexist is a puzzle. In some areas, they
are found on adjacent fenceposts (Hertz, 1980),
whereas in other localities only one species is
present. Although Hertz (1980) suggests that
A. marcanoi may be adapted to hotter, more
open microhabitats, field observations have
failed to discover any obvious difference (Williams, 1975; Hertz, 1980; pers. obs.). The existence of two such closely related species in
sympatry raises two questions amenable to laboratory investigation: does interspecific territorial behavior occur and, if not, how do males
distinguish conspecifics from non-conspecifics?
? 1985 by the American Society of Ichthyologists and
Herpetologists
This content downloaded from 139.184.14.159 on Thu, 01 Oct 2015 15:50:15 UTC
All use subject to JSTOR Terms and Conditions
S-ar putea să vă placă și
- The Biology and Management of Lobsters: Ecology and ManagementDe la EverandThe Biology and Management of Lobsters: Ecology and ManagementJ. Stanley CobbÎncă nu există evaluări
- Reproduction and Sexuality in Marine Fishes: Patterns and ProcessesDe la EverandReproduction and Sexuality in Marine Fishes: Patterns and ProcessesKathleen S. ColeÎncă nu există evaluări
- Beatles - (Guitar Songbook) Partitions 1962 - 1970Document283 paginiBeatles - (Guitar Songbook) Partitions 1962 - 1970Oriol Kanu del Besòs100% (6)
- A New Species of Pygmy Paroctopus Cephalopoda OctoDocument18 paginiA New Species of Pygmy Paroctopus Cephalopoda OctoYayan MardiansyahÎncă nu există evaluări
- Article: ZootaxaDocument14 paginiArticle: ZootaxaSabrina Nur Fitri 2004125073Încă nu există evaluări
- Artikel Filum MolluscaDocument10 paginiArtikel Filum MolluscaListiyani Putri AzzahroÎncă nu există evaluări
- Final Thesis ProposalDocument27 paginiFinal Thesis ProposalWnz Naive100% (2)
- Kuris1978 - Life Cycle, Distribution and Abundance of Carcinonemertes Epialti, A Nemertean Egg Predator of TheDocument18 paginiKuris1978 - Life Cycle, Distribution and Abundance of Carcinonemertes Epialti, A Nemertean Egg Predator of TheLuis Gamarra BustamanteÎncă nu există evaluări
- International Journal Primatology Definitive 10.1007Document39 paginiInternational Journal Primatology Definitive 10.1007Manuel RuizÎncă nu există evaluări
- Mesquita Et Al 2015 EcologyDocument1 paginăMesquita Et Al 2015 EcologyCarolina GarciaÎncă nu există evaluări
- Artículo Redalyc 44943437032Document23 paginiArtículo Redalyc 44943437032Fernanda Charqueño CelisÎncă nu există evaluări
- Sales Et Al 2011 - Feeding Ecology of Ameiva AmeivaDocument10 paginiSales Et Al 2011 - Feeding Ecology of Ameiva AmeivaRaul SalesÎncă nu există evaluări
- 27 Segura EpocaDocument12 pagini27 Segura EpocaVanessa Usbeck LópezÎncă nu există evaluări
- Artículo de RenacuajosDocument9 paginiArtículo de RenacuajosAndres Felipe BermudezÎncă nu există evaluări
- 1973, Davis - Geographic Variation in The Fishing Bat, Noctilio LeporinusDocument14 pagini1973, Davis - Geographic Variation in The Fishing Bat, Noctilio LeporinusLucas MourãoÎncă nu există evaluări
- Litter - Size - and - Embryo - Implantation Silva Et Al 2015 (OA)Document12 paginiLitter - Size - and - Embryo - Implantation Silva Et Al 2015 (OA)Tayssa MarquesÎncă nu există evaluări
- Vogt, 1988Document12 paginiVogt, 1988ianÎncă nu există evaluări
- Life History Traits of The Sand Stargazer Dactyloscopus Tridigitatus (Teleostei: Blennioidei) From South-Eastern Brazilian CoastDocument7 paginiLife History Traits of The Sand Stargazer Dactyloscopus Tridigitatus (Teleostei: Blennioidei) From South-Eastern Brazilian CoastLuiz Fernando Salvador Jr.Încă nu există evaluări
- Escholarship UC Item 5qm701p2Document21 paginiEscholarship UC Item 5qm701p2b.cuevas15Încă nu există evaluări
- Altig Johnston1989 PDFDocument30 paginiAltig Johnston1989 PDFITALO ALVARENGA GONCALVESÎncă nu există evaluări
- Trophic Ecology of Bottom Fishes Assemblage Along Coastal Areas of Thailand PDFDocument12 paginiTrophic Ecology of Bottom Fishes Assemblage Along Coastal Areas of Thailand PDFdaniralÎncă nu există evaluări
- 01 - 1620 - F1 MatheusDocument11 pagini01 - 1620 - F1 MatheusIlver AlabatÎncă nu există evaluări
- Lamonicaetal 2007Document7 paginiLamonicaetal 2007Oscar Daniel Medina BarriosÎncă nu există evaluări
- 1808-9798 (2007) 2 (31:araoga) 2 0 Co 2Document9 pagini1808-9798 (2007) 2 (31:araoga) 2 0 Co 2Isabella ValenciaÎncă nu există evaluări
- Foraging Behaviour and Feeding Ecology of The OtteDocument17 paginiForaging Behaviour and Feeding Ecology of The OtteDaniela Cristina IbanescuÎncă nu există evaluări
- Vitt Et Al. 2003 - (History and The Global Ecology of Squamate Reptiles)Document17 paginiVitt Et Al. 2003 - (History and The Global Ecology of Squamate Reptiles)Raissa SiqueiraÎncă nu există evaluări
- 2 - ROCHAetal2014-Specimen Collection An Essential ToolDocument2 pagini2 - ROCHAetal2014-Specimen Collection An Essential ToolThaliaSantosÎncă nu există evaluări
- Reproductive Biology of The Commercial Sea Cucumber Athyonidium Chilensis (Holothuroidea: Dendrochirotida) In..Document14 paginiReproductive Biology of The Commercial Sea Cucumber Athyonidium Chilensis (Holothuroidea: Dendrochirotida) In..Lopa LopaÎncă nu există evaluări
- Reproductive Biology (Histological & Ultrastructure) and Biochemical Studies in Ovaries of Mugil Cephalus From Mediterranean WaterDocument26 paginiReproductive Biology (Histological & Ultrastructure) and Biochemical Studies in Ovaries of Mugil Cephalus From Mediterranean WaterImran TaufikÎncă nu există evaluări
- jcb0306 PDFDocument22 paginijcb0306 PDFRizky IkwanÎncă nu există evaluări
- Baeza Et Al 2012 Mithrax AllometryDocument9 paginiBaeza Et Al 2012 Mithrax AllometryErick TristãoÎncă nu există evaluări
- Early Ontogeny of Aquarium-Raised Moenkhausia Sanctaefilomenae (Characiformes: Characidae)Document9 paginiEarly Ontogeny of Aquarium-Raised Moenkhausia Sanctaefilomenae (Characiformes: Characidae)Dustin MitchellÎncă nu există evaluări
- Species Diversity and Spatial Structure of Intertidal Mollusks in Padada, Davao Del Sur, PhilippinesDocument10 paginiSpecies Diversity and Spatial Structure of Intertidal Mollusks in Padada, Davao Del Sur, PhilippinesYayan MardiansyahÎncă nu există evaluări
- Fish Species Involved in Mass Washed Up Eggs in Chinchorro Beach, Arica - Chile During The Period ofDocument4 paginiFish Species Involved in Mass Washed Up Eggs in Chinchorro Beach, Arica - Chile During The Period ofNeyser QuispeÎncă nu există evaluări
- Liolaemus CuyanusDocument6 paginiLiolaemus CuyanusrociomilenaundecÎncă nu există evaluări
- A Survey of Tropical Ea - Rthworms: Taxonomy, Biogeography and Environmental PlasticityDocument28 paginiA Survey of Tropical Ea - Rthworms: Taxonomy, Biogeography and Environmental Plasticityvipulsingh161Încă nu există evaluări
- Haverkost and Gardner 2008 ADocument8 paginiHaverkost and Gardner 2008 At_haverkostÎncă nu există evaluări
- Eugerres MexicanusDocument12 paginiEugerres MexicanusFlavioÎncă nu există evaluări
- Duarte Et Al 2006Document17 paginiDuarte Et Al 2006Lachaquetadevardoc 1Încă nu există evaluări
- Upwellings SystemsDocument112 paginiUpwellings SystemsGiancarlo Moron CorreaÎncă nu există evaluări
- Springer: Springer Is Collaborating With JSTOR To Digitize, Preserve and Extend Access To Human EcologyDocument26 paginiSpringer: Springer Is Collaborating With JSTOR To Digitize, Preserve and Extend Access To Human EcologyBondÎncă nu există evaluări
- Sher Hameed Research ProposalDocument7 paginiSher Hameed Research ProposalYousaf JamalÎncă nu există evaluări
- Biologia Reprodutiva PDFDocument9 paginiBiologia Reprodutiva PDFclaudiousdecastroÎncă nu există evaluări
- Book Biolog and Culture of Percid FishesDocument897 paginiBook Biolog and Culture of Percid FishesHafez A MabroukÎncă nu există evaluări
- The Biology and Culture of TilapiasDocument429 paginiThe Biology and Culture of TilapiasDavid Salirrosas100% (3)
- Perbiche Nevesetal2015Document111 paginiPerbiche Nevesetal2015PriscilaÎncă nu există evaluări
- Ecology of BatsDocument15 paginiEcology of BatspomajoluÎncă nu există evaluări
- Effects of Environmental Variability On Trophic Interactions and Food Web Structure in A Pelagic Upwelling EcosystemDocument19 paginiEffects of Environmental Variability On Trophic Interactions and Food Web Structure in A Pelagic Upwelling EcosystemĐức HuyÎncă nu există evaluări
- Tecnica M. Esculenta R. OwerDocument4 paginiTecnica M. Esculenta R. OwerHernán LópezÎncă nu există evaluări
- (PAPER) Feeding Habitat of The Whale Shark Rhincodon Typus in The Northern Gulf of Mexico Determined Using Species Distribution ModellingDocument13 pagini(PAPER) Feeding Habitat of The Whale Shark Rhincodon Typus in The Northern Gulf of Mexico Determined Using Species Distribution ModellingTwpanambas g-driveÎncă nu există evaluări
- Vitt & Vangilder (1983) - Ecology of Snake Community in The Northeastern BrazilDocument24 paginiVitt & Vangilder (1983) - Ecology of Snake Community in The Northeastern BrazilEd MyersÎncă nu există evaluări
- Reptilian All The Way?: LetterDocument1 paginăReptilian All The Way?: LetterJamesComeyJustaBitchÎncă nu există evaluări
- Litter Size, Effects of Maternal Body Size and Date of Birth in South American Scorpions (Arachnida Scorpiones)Document12 paginiLitter Size, Effects of Maternal Body Size and Date of Birth in South American Scorpions (Arachnida Scorpiones)Iasmim SantosÎncă nu există evaluări
- Alexandre Specht - Agrotis Malefida - ROUND 1 Revisado Pelo Autor - JEFFDocument22 paginiAlexandre Specht - Agrotis Malefida - ROUND 1 Revisado Pelo Autor - JEFFsamusaran115Încă nu există evaluări
- Williner Et Al 2014Document9 paginiWilliner Et Al 2014veronica willinerÎncă nu există evaluări
- The Different Breeding Strategies of Penguins: A Review: Comptes Rendus Biologies March 2013Document13 paginiThe Different Breeding Strategies of Penguins: A Review: Comptes Rendus Biologies March 2013Gabriel FloreaÎncă nu există evaluări
- Seasonality, Developing Time and Protandry in Three Populations of The Neotropical Grasshopper Sphenarium Histrio in An Altitudinal GradientDocument21 paginiSeasonality, Developing Time and Protandry in Three Populations of The Neotropical Grasshopper Sphenarium Histrio in An Altitudinal GradientVíctor RamirezdÎncă nu există evaluări
- Urata - Etal - Larval Development of The Oriental Lancelet - BranchiostomaDocument11 paginiUrata - Etal - Larval Development of The Oriental Lancelet - BranchiostomaRochyRiquelmeÎncă nu există evaluări
- HyphomycetDocument10 paginiHyphomycetMohammad aminÎncă nu există evaluări
- Cano-Rocabayera Et Al 2022 Cadmium CuchasDocument11 paginiCano-Rocabayera Et Al 2022 Cadmium CuchasOriol Kanu del BesòsÎncă nu există evaluări
- 5 Ways To Make Outlook Work For You PDFDocument5 pagini5 Ways To Make Outlook Work For You PDFOriol Kanu del BesòsÎncă nu există evaluări
- Vegan TutorDocument43 paginiVegan TutorddaltonicoÎncă nu există evaluări
- Grapputo Et Al 2005 Potato BeetleDocument13 paginiGrapputo Et Al 2005 Potato BeetleOriol Kanu del BesòsÎncă nu există evaluări
- Chisholm SW - 1992Document26 paginiChisholm SW - 1992Oriol Kanu del BesòsÎncă nu există evaluări
- Spatial Heterogeneity in Freshwater Zooplankton VaDocument9 paginiSpatial Heterogeneity in Freshwater Zooplankton VaOriol Kanu del BesòsÎncă nu există evaluări
- ValidacióDocument1 paginăValidacióOriol Kanu del BesòsÎncă nu există evaluări
- Beatles - (Guitar Songbook) Partitions 1962 - 1970 PDFDocument283 paginiBeatles - (Guitar Songbook) Partitions 1962 - 1970 PDFOriol Kanu del Besòs100% (1)
- Life History, Population Dynamics and Production of Eastern Mosquitofish, Gambusia Holbrooki (Pisces, Poeciliidae), in Rice Fields of The Lower Mondego River Valley, Western PortugalDocument14 paginiLife History, Population Dynamics and Production of Eastern Mosquitofish, Gambusia Holbrooki (Pisces, Poeciliidae), in Rice Fields of The Lower Mondego River Valley, Western PortugalOriol Kanu del BesòsÎncă nu există evaluări
- FIsh Bioaccumulation Biomarker ETP2003 PDFDocument93 paginiFIsh Bioaccumulation Biomarker ETP2003 PDFOriol Kanu del BesòsÎncă nu există evaluări
- Title: Can Nitrate Pollution Alter The Cognitive Functions of A Widespread Invasive Species?Document2 paginiTitle: Can Nitrate Pollution Alter The Cognitive Functions of A Widespread Invasive Species?Oriol Kanu del BesòsÎncă nu există evaluări
- Swan2008 Ecoscience TreeOfHeavenDecompDocument9 paginiSwan2008 Ecoscience TreeOfHeavenDecompOriol Kanu del BesòsÎncă nu există evaluări
- Ancova RDocument21 paginiAncova ROriol Kanu del BesòsÎncă nu există evaluări
- Morphology of The Accessory Reproductive Glands of Sepia Pharaonis@jmor.20835Document12 paginiMorphology of The Accessory Reproductive Glands of Sepia Pharaonis@jmor.20835李孟芳Încă nu există evaluări
- Assisted Reproductive Technology by Rosemary C. AgboDocument19 paginiAssisted Reproductive Technology by Rosemary C. AgboRosemaryÎncă nu există evaluări
- Fecundity and Life-History Strategies in Marine InvertebratesDocument84 paginiFecundity and Life-History Strategies in Marine InvertebratesKyratsw KyriakouliÎncă nu există evaluări
- General Characters of Pteridophytes: Submitted By: Shafaque Waheed Submitted To: Dr. AshutoshDocument15 paginiGeneral Characters of Pteridophytes: Submitted By: Shafaque Waheed Submitted To: Dr. Ashutoshcristyruds234Încă nu există evaluări
- Exam Sci 101Document36 paginiExam Sci 101Xenia Mae FloresÎncă nu există evaluări
- Class: 10 BiologyDocument12 paginiClass: 10 BiologyRehan PervaizÎncă nu există evaluări
- REPRODUCTION-WPS OfficeDocument6 paginiREPRODUCTION-WPS OfficeGwzwwgÎncă nu există evaluări
- REPRODUCTION DEVELOPMENT - PRACTICE QUESTIONS-Student Copy3Document16 paginiREPRODUCTION DEVELOPMENT - PRACTICE QUESTIONS-Student Copy3salinaÎncă nu există evaluări
- Gametogenesis: Spermatogenesis & Oogenesis: Dr. Naveen Kumar MMMC ManipalDocument16 paginiGametogenesis: Spermatogenesis & Oogenesis: Dr. Naveen Kumar MMMC ManipalLim Yi QiÎncă nu există evaluări
- Science 4 Misosa - Animals Hatched From Eggs and Born AliveDocument6 paginiScience 4 Misosa - Animals Hatched From Eggs and Born AliveMA. KRISTINA MANLANGIT JUATON,Încă nu există evaluări
- Bio Exp 1 & 2Document5 paginiBio Exp 1 & 2Rittik Ranjan Prasad XII B67% (3)
- Hormonal Control of GametogenesisDocument29 paginiHormonal Control of GametogenesisWachiel Arhamz100% (2)
- sw8 chp04Document23 paginisw8 chp04api-115560904Încă nu există evaluări
- Gametogenesis and Fertilization: The Circle of SexDocument85 paginiGametogenesis and Fertilization: The Circle of SexAdityanair RA1711009010128Încă nu există evaluări
- Meiosis and The Sexual Life Cycle Reviewer NotesDocument23 paginiMeiosis and The Sexual Life Cycle Reviewer NotesJestoni BigaelÎncă nu există evaluări
- #10A 10B-Quiz#3Document15 pagini#10A 10B-Quiz#3Ovaun LatoucheÎncă nu există evaluări
- Third Periodical Test - Science 10 ANS KEYDocument4 paginiThird Periodical Test - Science 10 ANS KEYMerlita CahuloganÎncă nu există evaluări
- Bio11th Ncert Solutions MergedDocument175 paginiBio11th Ncert Solutions MergedRameshwer KambleÎncă nu există evaluări
- Unit 10 Study Guide KeyDocument7 paginiUnit 10 Study Guide KeyyawahabÎncă nu există evaluări
- Edexcel Biology AS Level Unit 2 Sample NotesDocument4 paginiEdexcel Biology AS Level Unit 2 Sample NotesMariam AhmedÎncă nu există evaluări
- Age of Gestation: First TrimesterDocument4 paginiAge of Gestation: First TrimesterBenjamin GabrielÎncă nu există evaluări
- Living Organisms Revision BookletDocument42 paginiLiving Organisms Revision BookletJo PatrickÎncă nu există evaluări
- Lelm 203Document4 paginiLelm 203umaidÎncă nu există evaluări
- INFERTILITYDocument31 paginiINFERTILITYShivam. KumarÎncă nu există evaluări
- Earth and Life Science Reproduction LessonDocument2 paginiEarth and Life Science Reproduction LessonZild Frigg CorbitaÎncă nu există evaluări
- LESSON PLAn FertilizationDocument5 paginiLESSON PLAn FertilizationJane Tolentino33% (3)
- Puberty & AdolescenceDocument15 paginiPuberty & Adolescencejean greyÎncă nu există evaluări
- 100 Weird Facts About The Human BodyDocument11 pagini100 Weird Facts About The Human Bodycryonics20039984Încă nu există evaluări
- Link Belt Wheel Loader l125 Electrical Schematic Hydraulic SchematicDocument22 paginiLink Belt Wheel Loader l125 Electrical Schematic Hydraulic Schematicchelseagomez020399mdi100% (125)
- AIPMT Examination Solutions 01MAY2016Code QCombinedDocument61 paginiAIPMT Examination Solutions 01MAY2016Code QCombinedkalloliÎncă nu există evaluări