Documente Academic
Documente Profesional
Documente Cultură
An Effect of Smoking On Wound Healing Following Extraction: A Critical Study
Încărcat de
IOSRjournalTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
An Effect of Smoking On Wound Healing Following Extraction: A Critical Study
Încărcat de
IOSRjournalDrepturi de autor:
Formate disponibile
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS)
e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 14, Issue 9 Ver. VII (Sep. 2015), PP 66-70
www.iosrjournals.org
An effect of smoking on wound healing following extraction: A
critical study
Dr. Kanwaldeep Singh Soodan, Dr. Pratiksha Priyadarshni, Dr. Kuljeet kaur
Abstract: Cigarette smoking has long been suspected to adversely affect wound healing but in Asian history
tobacco was attributed as a medicinal plant. It was often used to avert hunger during long hours of work. But in
reality, it causes various ill effects including pre-malignant lesions and cancers. Tobacco affects postoperative
wound healing following surgical and non-surgical tooth extractions, routine maxillofacial surgeries, implants
and periodontal therapies. Smoking tobacco is also associated with catecholamines release resulting in
vasoconstriction and decreased tissue perfusion. Smoking is believed to suppress the innate and host-immune
responses, affecting the function of neutrophils, the prime line of defence against infection. Thus, the association
between smoking and delayed healing of oral tissues following surgeries is evident.
Key words: Tobacco, healing effects, dry socket, dental extractions.
I.
Introduction
Dental extractions are routine dental procedures that are carried out in dental department. Removal of
tooth removal depends on the various factors including the post extraction instructions followed by patients. A
favourable architecture of alveolar ridge with sufficient alveolar bone volume is necessary to obtain functional
and esthetic prosthetic rehabilitation. Knowledge about healing process at extraction site is essential to avoid
insufficient bone volume.
Tobacco was introduced in India by Portuguese in 16th century. Today, use and production of tobacco
has increased to such an extent that it has become the second largest production in the world [1]. Nicotine is a
vasoconstrictor that decreases blood flow to the tissue, results in tissue ischemia and impairs healing of injured
tissue. Nicotine also decreases platelet adhesion, raising the risk of micro-vascular occlusion and tissue
ischemia. In addition, nicotine has well known harmful effects related to tissue healing such as inhibition of
angiogenesis, re-epithelialization and osteogenesis along with cellular healing such as inhibition of fibroblast
proliferation and adhesion and collagen synthesis [2-10].
The healing events in the tooth extraction socket terminate in the form of woven bone, which
ultimately remodels resulting in the restoration of defect [11]. In general, smoking exerts harmful effects on
bone and diminishes the blood filling of post-extraction socket and consequently has an adverse effect on
healing of extraction wound [12] .Cigarette smoking constitutes >4000 toxic contents in gas or particulate form.
Toxins of more interest are nicotine and carbon-monoxide that diminishes oxygen transport whereas hydrogen
cyanide inhibits the enzymes that are necessary for oxidative metabolism and oxygen transport at cellular level.
II.
Smoking and its effects on Healing
Wound healing is a dynamic process that involves number of phases i.e. inflammatory phase,
proliferation phase and maturation phase. Inflammatory phase is the bodys natural response to injury. After
injury, clot is formed. Once the hemostasis is achieved, blood vessels dilate to allow the essential cells,
antibodies, WBCs, growth factors, enzymes and nutrients to reach the injured area. Neutrophils and
macrophages come into their action. During proliferative phase, fibroblast lay down the collagen to form the
granulation tissue along with angiogenesis and later epithelialization occurs. Maturation phase involves
remodeling of collagen. Cellular activity reduces and the number of blood cells decreases in number.
When cigarette is inhaled, the toxins can directly poison the cilia or pass the cilia barrier, undergo
tissue absorption, enter into bloodstream and gain access to other parts of body. Cigarette smoke constitutes
number of toxic contents [Table 1].
Table 1: Cigarette smoke constitutes number of toxic contents in particulate and gaseous form.
Gas Phase
Particulate Phase
Carbon dioxide
Particulate matter
Carbon monoxide
Nicotine
Hydrogen cyanide
Catechol
Nitrogen oxides
Phenol
Acetone
N-nitrosonornicotine
Formaldehyde
Quinoline
Acrolein
Aniline
Ammonia
2-Toulidine
DOI: 10.9790/0853-14976670
www.iosrjournals.org
66 | Page
An effect of smoking on wound healing following extraction: A critical study
Pyridine
3-Vinylpyridne
N-Nitrosodimethylamine
N-Nitrosopyrolidine
Nickle
N-nitrosodiethandamine
Benzo[a]-Pyrene
2-Naphthylamine
With each cigarette smoked, approximately 20-30ml of carbon monoxide and 2-3mg of nicotine are
inhaled [13]. Carbon monoxide produced during combustion of tobacco also reduces capillary blood flow. A
clinical study has shown that a single cigarette can reduce the peripheral blood velocity by 40% in 1 hour [14].
Nicotine exerts several effects that influence wound healing. First, proliferation of RBCs, fibroblasts
and macrophages are diminished [13].Second, it increases platelet adhesiveness which causes microclots
formation raising the risk of micro-vascular occlusion and tissue ischemia [15]. Third, nicotine produces
cutaneous vasoconstriction. This vasoconstriction results from release of catecholamines which also raise heart
rate, blood pressure and oxygen demand [4].Various studies have shown that release of catecholamines results
in stimulation of chalone hormone formation that reduces wound healing by decreasing the rate of
epithelialisation [4].
The affinity of binding of carbon monoxide for hemoglobin is 200 times than that of oxygen.
Resulting, carbon monoxide inhibits binding of oxygen, reduces the oxygen carrying capacity of hemoglobin
which ultimately reduces the amount of oxygen to reach the periphery [13]. In addition, when
carboxyhemoglobin levels rise in blood, the oxygen dissociates curve shifts to the left that means oxygen is less
able to dissociate from RBCs and diffuse into the tissues, so the decrease in the levels of oxygen available for
tissue perfusion results into cellular hypoxia and diminished wound healing [16, 17]. Wound healing also
requires enzymes. Hydrogen cyanide inhibits enzyme system that is required for oxidative metabolism and
oxygen transport at cellular level [18]. All together, the ill effects of toxic substances of smoking have potential
to diminish the conditions that are required for proper wound repair and healthy scar formation.
III.
Clinical side effects of smoking
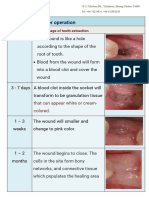
1) Dry socket
Cryer et al stated that smoking is associated with endogenous release of catecholamines resulting in
vasoconstriction and tissue perfusion [19]. Information on number of cigarettes smoked per day and the postoperative smoking habbit of each patient were recorded. According to them, heat released from burning tobacco
and tobacco along with its byproducts may act as contaminant in the surgical site together with the suction
applied to the cigarette that might dislodge the clot from the alveolus interfering healing of the socket. Larsen
evaluated the risk factors associated with development of localized osteitis after the extraction of 138 third
molars. He found use of tobacco is associated with increased incidence of dry socket compared to other factors
like age, sex, usuage of oral contraceptives and increased surgical time [20]. According to Meechan et al,
fibrinolytic activity caused by smoking decreases the blood supply to the surgical site after extraction and dry
socket was found common amongst these smoker patients [12]. Extractions were performed in 2417 patients and
incidence of painful socket was analyzed. It was observed that post-operatively poor filling of blood in socket,
were found more likely to develop painful socket (p>0.002). Post-operative socket filling with blood was greatly
reduced in smokers compared with non-smokers (p>0.001). Incidence of painful socket in heavy smokers (who
smokes 20 or more cigarettes per day) was higher compared with non-smokers (>0.005). Al-belasy et al
evaluated the risk of developing localized osteitis in water pipe (shisha) smokers [19]. They found that cigarette
and shisha smokers were 3-4 times more prone to develop dry socket than non-smokers. They found that
smoking on the day of surgery and increased frequencies of smoking significantly increase the incidence of dry
socket. According to Lopez Carriches et al, after extraction of third molar, smokers were more prone to develop
trismus than non-smokers. However, they didnt observe significant difference in relation of pain [21].
According to Sweet et al, the suction associated with cigarette might dislodge the blood clot from
alveolus socket and interrupt the healing. However, Alxender denies the fact that suction during smoking cause
physical dislodgement of the clot [22]. Al-belasy et al believed that dry socket is caused by destruction of clot
rather than physical dislodgement [19]. Though the exact mechanism by which smoking predisposes the socket
become painful remains unclear, smoking seems to be strictly associated with the occurrence of dry socket.
Effect of smoking upon healing following routine oral surgical procedures
Smoking is reported to be important factor responsible for post-operative infections leading to
hindrance in bone healing. A meta-analysis by Ward KD et al reported the magnitude of the association between
cigarette smoking and bone mass and showed that smokers presented reduce bone mass, compared with nonsmoker [23]. Saldanha et al in six months study reported that the smoking may affect remodeling process
following tooth extraction. In their study, alveolar bone height lost significantly more (1.5mm) in smokers as
compared to non-smokers (1mm). They also reported that radiographic bone density was more pronounced in
smokers than non-smokers. They believed that smoking increases the bone resorption at the fracture bone ends,
DOI: 10.9790/0853-14976670
www.iosrjournals.org
67 | Page
An effect of smoking on wound healing following extraction: A critical study
interfering with the osteoblastic function [24]. Cheynet et al in their study of infections complications of 60
mandibular osteotomies, smoking was observed as important risk factor responsible for post-operative infections
[25]. Levin et al observed greater complications following surgeries in smokers. They suggested that heat
released from cigarette smoke and toxic by-products of tobacco such as nicotine, hydrogen cyanide and carbon
monoxide could be risk factors affecting the success of dental procedures [26].
Interference of smoking on periodontal therapy
Cigarette smoking is significant risk factor for periodontal diseases and impairs healing after
periodontal surgeries [27]. Unlike usuage of smokeless tobacco that causes gingival recession at the site of
placement, the usuage of smoking tobacco causes widespread periodontal destruction. Tomar et al and Beck et
al conducted two population based epidemiological studies in which they found periodontitis is more common
in smokers than non-smokers [28, 29]. Number of clinical studies have been performed to compare the response
of smokers and non-smokers to different types of surgical and non-surgical periodontal therapies [29-33] and it
was found that smoking has a strong negative impact on regenerative therapy including osseous grafting [31],
guided tissue regeneration [34-37] or combination of these treatments [38] and 80% failure rate in the treatment
of furcation involvement defects. The majority studies found that gingival grafting for root coverage procedure
is less successful than non-smokers [39-41].
IV.
Discussion
Cigarette smoking affects the oral cavity in various ways ranging from staining of the teeth to more
serious diseases such as oral cancer. According to lots of data reviewed, smoking has various risk factors
including impaired wound healing. Treatment complications and failures in smokers inspired them for
investigation of relative risks associated with smoking and the mechanism for compromised wound healing.
Nadler reported that smoking reduced urinary levels of prostacyclin (PGI 2), a prostaglandin that causes
vasodilatation and decrease platelet aggregation [42]. Reus demonstrated that smoking causes vasoconstriction
and diminished blood flow in ears of nude mice exposed to tobacco smoke [43]. Mosely noticed that nicotine
impairs wound healing from 6th-10th day in the rabbit ear [4]. Harmful effects of nicotine were believed to
damage erythrocyte precursors as well as vasoconstriction and inhibition of epithelization. In 1976 Cryer,
reported that smoking is associated with epinephrine and nor-epinephrine release, resulting in vasoconstriction
and decreased tissue perfusion [23]. Rief Kohl noticed accelerated irreversible histological changes of
endarteritis obliterans in microvasculature of chronic smoker [44]. In animal studies, using controlled skin flaps,
exposure to cigarette smoke significantly raised the risk for skin slough and ultimately necrosis. Lawrence et al
investigated the survival of skin flap in rats exposed to cigarette smoke post-operatively [45]. They observed
impaired wound healing and decreased rate of flap survival as they attributed to vasoconstriction due to nicotine
as well as endogenous release of catecholamines , increased platelet aggregation leading to diminished flow,
increased hemoglobin and fibrinogen levels with resultant increased viscosity and increased levels of
carboxyhemoglobin resulting in reduced oxygen transport and changes in vascular endothelium of endarteritis.
Prevalence of ulcerative gingivitis, acute necrotizing ulcerative gingivitis and periodontitis is also
increased in smokers [46-48]. Clarke et al observed tissue ischemia as a etiology for these diseases based on
gingival vascular anatomy and the lack of collateral circulation to the papillary gingival [49, 50]. By using
thermal diffusion principle to measure gingival blood flow in rabbits, injection of epinephrine and nicotine as
well as their combinations resulted in significantly impaired gingival blood flow with some tendency for
tachyphylaxis to the effect of epinephrine but little to nicotine.
V.
Conclusion
The components of cigarette smoke clearly have an inhibitory effect on wound healing. Combined
effects of nicotine, carbon monoxide and hydrogen cyanide are tissue ischemia, cellular hypoxia, inhibition of
proliferation of epithelial cells, vasoconstriction, poisoning of enzymes and a decrease in oxygen carrying
capacity of blood cells required for wound healing. All the clinicians should take these complicating factors into
account and advise the patient to quit habit and inform them about prognosis of treatment.
References
[1].
[2].
[3].
[4].
[5].
Reddy KS, Gupta PC, editors. Report on Tobacco control in India. Ministry of Health and Family Welfare, New Delhi: Government
of India; 2004.
Daftari Tk, Whitesides jr Te, Heller Jg, Goodrich Ac, Mccarey Be, Hutton Wc. Nicotine on the revascularization of bone graft. An
experimental study in rabbits. Spine 1994; 19: 904-911.
Peacock EE, Van Winkle W. Wound repair. 2nd ed. Philadelphia: WB Saunders; 1976.
Mosely LH, Finseth F, Goody M. Nicotine and its effect in wound healing. Plast Reconstruction Surgery 1978; 61:570-575.
Sheveleva GA, Kiryuschenkov AP, Sheina NI, Silantyeva IV. Effects of nicotine on the mother embryo system under exposure
throughout the entire pregnancy. Farmacologiiani Toksikologiia 1984; 47:78-83.
DOI: 10.9790/0853-14976670
www.iosrjournals.org
68 | Page
An effect of smoking on wound healing following extraction: A critical study
[6].
[7].
[8].
[9].
[10].
[11].
[12].
[13].
[14].
[15].
[16].
[17].
[18].
[19].
[20].
[21].
[22].
[23].
[24].
[25].
[26].
[27].
[28].
[29].
[30].
[31].
[32].
[33].
[34].
[35].
[36].
[37].
[38].
[39].
[40].
[41].
[42].
[43].
[44].
Paulson RB, Shanfeld J, Mullet B, Cole J, Paulson JO. Prenatal smokeless tobacco effects on rat fetus. J Craniofac Genet Develop
boil 1994; 14:16-25.
Fang Ma, Frost Pj, Iida-Klein A, Hahn Tj. Effects of nicotine on cellular function in UMR 106-01 osteoblast like cells. Bone 1991;
12:283-286.
Peacock ME, Sutherland DE, Schuster GS, Brennan WAO, ONeal RB, Strong SL, Van dyke TE. The effect of nicotine on
reproduction and attachment of human gingival fibroblasts in vitro. J Periodontol 1993; 64:658-665.
Galvin Rj, Ramp Wk, Lenz Lg. Smokeless tobacco contains a non-nicotine inhibitor of bone metabolism. Toxicol Appl Pharmacol
1988; 95:292-300.
Ramp WK, Lenz LG, Galvin RJS. Nicotine inhibits collagen synthesis and alkaline phosphatase activity, but stimulates DNA
synthesis in osteoblast-like cells. Soc Exp Biol Med 1991; 197:36-43.
Evian CI, Rosenberg ES, Coselet JG, Corn H. the osteogenic activity of bone removed from healing extraction sockets in humans. J
Periodontol 1982:53:81-85.
Meechan JG, Macgregor ID, Rogers SN, Hobson RS, Bate JP, Dennison M. The effect of smoking on immediate post-extraction
socket filling with blood and on the incidence of painful socket. Br J Oral Maxillofac. Surg. 1988; 26:402-409.
The health consequences of smoking: cardiovascular disease. A report of the surgeon general. Rockville, Maryland: U.S.
Department of Health and Human Services, 1983.
Sherwin MA, Gastwirth CM. Detrimental effects of cigarette smoking on lower extremity wound healing. J Foot Surg. 1990:29:847.
Mayfield L, Soderholm G, Hallstorm H, Kullendorff B, Edwardsson S, Bratthall G, et al. Guided tissue regeneration for the
treatment of intraosseous defects using a bioabsorbable membrane: a controlled clinical study. J Clin. Periodontol 1998;25:585-95
Al-Belasy FA. The relationship of shisha (water pipe) smoking to postextraction dry socket. J Oral Maxillofac Surg 2004; 62:104.
Nolan J, Jenkins RA, Kurihara K, Schultz RC. The acute effects of cigarette smoke exposure on experimental skin flaps. Plast
Reconstr Surg 1985; 75:544-9.
Rees TD. The acute effects of cigarette smoke exposure on experimental skin flaps: a discussion. Plast Reconstr Surg 1985; 75:5501.
Mosely LH, Finseth F. Cigarette smoking: impairment of digital blood flow and wound healing in the hand.1977; 9:97-101.
Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic medication of smokingassociated hemodynamic and metabolic events. N Engl. J Med 1976; 295:573-7.
Sweet JB, Butler DP. The relationship of smoking to localized osteitis. J Oral Surg 1979; 37:732-5.
Larsen PE. Alveolar osteitis after surgical removal of impacted third molars: Identification of the patient at risk. Oral Surg Oral Med
Oral Pathol. 1992; 73:393-7.
Lo`pez-Carriches C, Go`mez-Font R, Martinez-Gonza`lez JM, Donado-Rodr`iguez M. Influence of smoking upon the postoperative
course of lower third molar surgery. Med Oral Patol Oral Cir Bucal 2006; 11:E56-60.
Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral desity. Calcify Tissue Int 2001; 68:259270.
Saldanha JB, Casati MZ, Neto FH, Sallum EA, Nociti FH Jr. Smoking may affect the alveolar process dimensions and radiographic
bone density in maxillary extraction sites: A prospective study in humans. J Oral Maxillofac Surg 2006; 64:1359-65.
Cheynet F, Chossegros C, Richard O, Ferrara JJ, Blanc JL. Infectious complications of mandibular osteotomy.Rev Stomatol Chir
Maxillofac 2001; 102:26-33.
Levin L, Schwartz-Arad D. The effects of cigarette smoking on dental implants and related surgery. Implant Dent 2005; 14:357-61.
Winn DM. Tobacco use and oral disease. J Dent Educ. 2001; 65:306-12.
Tomar SL, Asma S. Smoking- attributable periodontitis in the united states: Findings from NHANES III: National Health and
Nutrition Examination Survey. J Periodontol 2000; 71:743-51.
Beck JD, Cusmano L, Green-Helms W, Koch GG, Offenbacher S. a five year old study of attachment loss in community- dwelling
older adults: incidence density.J Periodontol Res 1997; 32:506-15.
Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. The effect of smoking on the response to periodontal therapy. J Clin
periodontal 1994; 21:91-7.
Preber H, Bergstrom J. Effect of non surgical treatment on gingival bleeding in smokers and non smokers. Acta Odontol Scand
1986; 44:85-9.
Grossi SG, Zambon J, Machtei EE, Schifferle R,Andreana S, Genco RJ,et al. Diabetics and smokers. J Periodontol 1996; 67:1094102.
Grossi SG, Zambon J, Machtei EE, Schifferle R, Andreana S, Genco RJ, et al. Effects of smoking and smoking cessation on healing
after mechanical periodontal thetrapy. J Am. Dent Assoc 1997; 128:599-607.
Rosen PS, Marks MH, Reynolds MA. Influence of
smoking on long-term clinical results of intrabony defects treated with
regenerative therapy. J Periodontol 1996; 67:1159-63.
Rosenberg ES, Cutler SA. The effect of cigarette smoking on the longterm success of guided tissue regeneration: A preliminary
study. Ann R Australas Coll Dent Surg 1994; 12:89-93.
Kaldahl WB, Johnson GK, Patil KD, Kalkwarf KL. Levels of cigarette consumption and response to periodontal therapy. J
Periodontol 1996;67:675-81
Trombelli L, Kim CK, Zimmerman GJ, Wikesjo UM. Retrospective analysis of factors related to clinical outcome of guided tissue
regeneration procedures in intrabony defects. J Clin Periodontol 1997; 24:366-71.
Luepke PG, Mellonig JT, Brunsvold MA. A clinical evaluation of a bioresorbable barrier with and without decalcified freeze-dried
bone allograft in the treatment of molar furcations. J Clin Periodontol 1997; 24:440-6.
Miller PD Jr. Root coverage with the free gingival graft: Factors associated with incomplete coverage. J Periodontol 1987; 58:67481.
Muller HP, Eger T, Schorb A. Gingival dimensions after root coverage with free connective tissue grafts. J Clin Periodontol 1998;
25: 424-30.
Zucchelli G, Clauser C, De Sanctis M, Calandriello M. Mucogingival versus guided tissue regeneration procedures in the treatment
of deep recession type defects. J Periodontol 1998; 69:138-45.
Nadler JL, Velasco JS, Horton R: Cigarette smoking inhibits prostacyclin formation. Lancet 1: 1248, 1983.
Reus WF, Robson MC, Zachary L, et al: Acute effects of tobacco smoking on blood flow in the cutaneous microcirculation. Br J
Plast Surg 37:213, 1984.
DOI: 10.9790/0853-14976670
www.iosrjournals.org
69 | Page
An effect of smoking on wound healing following extraction: A critical study
[45].
[46].
[47].
[48].
[49].
[50].
Riefkohl R, Wolfe JA, Cox EB, et al: Association between cutaneous occlusive vascular disease, cigarette smoking. and skin slough
after rhytidectomy. Plast Reconstr Surg 77:592, 1986.
Lawrence WT, Murphy RC, Robson MC, et al: The detrimental effect of cigarette smoking on flap survival; An experimental studv
in the rat. Br J Plast Sure. 37:2 16. 1984
Rees TD, Liverett DM, Guy CL: The effect of cigarette smoking on skin-flap survival in the face-lift patient. Plast Reconstr Surg
73:911, 1984
Craig S, Rees TD: The effects of smoking on experimental skin flaps in hamsters. Plast Reconstr Surg 75:842, 1985
Pindborg JJ: Tobacco and gingivitis I; Statistical examination of the significance of tobacco in the development of
ulceromembranous gingivitis and in the formation of calculus. J Dent Res 26:261. 1947
Pindborg JJ: Tobacco and gingivitis II; Correlation between consumption of tobacco, ulceromembranous gingivitis, and calculus. J
Dent Res 28:460, 1949
DOI: 10.9790/0853-14976670
www.iosrjournals.org
70 | Page
S-ar putea să vă placă și
- "I Am Not Gay Says A Gay Christian." A Qualitative Study On Beliefs and Prejudices of Christians Towards Homosexuality in ZimbabweDocument5 pagini"I Am Not Gay Says A Gay Christian." A Qualitative Study On Beliefs and Prejudices of Christians Towards Homosexuality in ZimbabweInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Socio-Ethical Impact of Turkish Dramas On Educated Females of Gujranwala-PakistanDocument7 paginiSocio-Ethical Impact of Turkish Dramas On Educated Females of Gujranwala-PakistanInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Attitude and Perceptions of University Students in Zimbabwe Towards HomosexualityDocument5 paginiAttitude and Perceptions of University Students in Zimbabwe Towards HomosexualityInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- A Proposed Framework On Working With Parents of Children With Special Needs in SingaporeDocument7 paginiA Proposed Framework On Working With Parents of Children With Special Needs in SingaporeInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- A Review of Rural Local Government System in Zimbabwe From 1980 To 2014Document15 paginiA Review of Rural Local Government System in Zimbabwe From 1980 To 2014International Organization of Scientific Research (IOSR)Încă nu există evaluări
- The Role of Extrovert and Introvert Personality in Second Language AcquisitionDocument6 paginiThe Role of Extrovert and Introvert Personality in Second Language AcquisitionInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- The Impact of Technologies On Society: A ReviewDocument5 paginiThe Impact of Technologies On Society: A ReviewInternational Organization of Scientific Research (IOSR)100% (1)
- An Evaluation of Lowell's Poem "The Quaker Graveyard in Nantucket" As A Pastoral ElegyDocument14 paginiAn Evaluation of Lowell's Poem "The Quaker Graveyard in Nantucket" As A Pastoral ElegyInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Assessment of The Implementation of Federal Character in Nigeria.Document5 paginiAssessment of The Implementation of Federal Character in Nigeria.International Organization of Scientific Research (IOSR)Încă nu există evaluări
- Comparative Visual Analysis of Symbolic and Illegible Indus Valley Script With Other LanguagesDocument7 paginiComparative Visual Analysis of Symbolic and Illegible Indus Valley Script With Other LanguagesInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Edward Albee and His Mother Characters: An Analysis of Selected PlaysDocument5 paginiEdward Albee and His Mother Characters: An Analysis of Selected PlaysInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Investigation of Unbelief and Faith in The Islam According To The Statement, Mr. Ahmed MoftizadehDocument4 paginiInvestigation of Unbelief and Faith in The Islam According To The Statement, Mr. Ahmed MoftizadehInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Relationship Between Social Support and Self-Esteem of Adolescent GirlsDocument5 paginiRelationship Between Social Support and Self-Esteem of Adolescent GirlsInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Importance of Mass Media in Communicating Health Messages: An AnalysisDocument6 paginiImportance of Mass Media in Communicating Health Messages: An AnalysisInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Role of Madarsa Education in Empowerment of Muslims in IndiaDocument6 paginiRole of Madarsa Education in Empowerment of Muslims in IndiaInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Topic: Using Wiki To Improve Students' Academic Writing in English Collaboratively: A Case Study On Undergraduate Students in BangladeshDocument7 paginiTopic: Using Wiki To Improve Students' Academic Writing in English Collaboratively: A Case Study On Undergraduate Students in BangladeshInternational Organization of Scientific Research (IOSR)Încă nu există evaluări
- Designing of Indo-Western Garments Influenced From Different Indian Classical Dance CostumesDocument5 paginiDesigning of Indo-Western Garments Influenced From Different Indian Classical Dance CostumesIOSRjournalÎncă nu există evaluări
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Alvogyl Patient Information Leaflet S 05 06 047 11 00Document1 paginăAlvogyl Patient Information Leaflet S 05 06 047 11 00walatangÎncă nu există evaluări
- Do Postoperative Antibiotics Decrease The Frequency of InflammatoryDocument9 paginiDo Postoperative Antibiotics Decrease The Frequency of InflammatoryCindy MendivelsoÎncă nu există evaluări
- Bone DiseasesDocument191 paginiBone DiseasesDrRobin SabharwalÎncă nu există evaluări
- NBDE Dental Boards Oral SurgeryDocument30 paginiNBDE Dental Boards Oral SurgeryJoyce Lim100% (1)
- Стоматология extractionDocument3 paginiСтоматология extractionmilenalyulinaÎncă nu există evaluări
- ExodontiaDocument74 paginiExodontiaYusra AqeelÎncă nu există evaluări
- Manifestasi Oral No 3 PDFDocument3 paginiManifestasi Oral No 3 PDFAgum AripratamaÎncă nu există evaluări
- Instruc ExtractionDocument13 paginiInstruc ExtractionMrunal DoiphodeÎncă nu există evaluări
- LITERATURADocument3 paginiLITERATURAMarija ČandrlićÎncă nu există evaluări
- Njopeka AN, Mwangosi IEAT: Original ArticleDocument6 paginiNjopeka AN, Mwangosi IEAT: Original ArticleTôn Thất Đam TriềuÎncă nu există evaluări
- Dry Socket PDFDocument7 paginiDry Socket PDFLuthfanita Afrisa FamiÎncă nu există evaluări
- LECTURE ExodontiaDocument113 paginiLECTURE ExodontiaNuzhat Noor Ayesha89% (9)
- Ceu RevalidaDocument421 paginiCeu RevalidaKENT DANIEL SEGUBIENSEÎncă nu există evaluări
- All ORE OSCEs PDFDocument123 paginiAll ORE OSCEs PDFAna100% (1)
- 7 Complications of Tooth Extraction PDFDocument10 pagini7 Complications of Tooth Extraction PDFlyli Star AngeloÎncă nu există evaluări
- Medoral 21 E696Document7 paginiMedoral 21 E696andreeaÎncă nu există evaluări
- ChlorhexidineDocument11 paginiChlorhexidineJuan José SantosÎncă nu există evaluări
- Oral Maxillofacial SurgeryDocument19 paginiOral Maxillofacial Surgeryluis castroÎncă nu există evaluări
- Mental Dental Oral SurgeryDocument21 paginiMental Dental Oral SurgeryAmanda AmaroÎncă nu există evaluări
- 2 OS To PrintDocument12 pagini2 OS To PrintDENTAL REVIEWER ONLY0% (1)
- SurgeryDocument109 paginiSurgeryEman ShahinÎncă nu există evaluări
- Healing of Extraction SocketDocument21 paginiHealing of Extraction SocketSushant Pandey100% (1)
- Dry Socket PresentationDocument10 paginiDry Socket PresentationThaer ZabenÎncă nu există evaluări
- Post Extraction AbstractDocument8 paginiPost Extraction AbstractThangam VeluÎncă nu există evaluări
- Extraction 1Document9 paginiExtraction 1CristinaÎncă nu există evaluări
- Questions 1 - 100Document15 paginiQuestions 1 - 100Vicky Cezar Villanueva50% (2)
- A General Overview of Post Extraction Complicationsprevention Management and Importance of Post Extraction AdvicesDocument13 paginiA General Overview of Post Extraction Complicationsprevention Management and Importance of Post Extraction AdvicesBilqist Savannah PutriÎncă nu există evaluări
- Dry SocketDocument6 paginiDry SocketSharah PutriÎncă nu există evaluări
- 1 PDFDocument6 pagini1 PDFadinda rachmaliaÎncă nu există evaluări
- Osteomyelitis: Dr. Amit Gupta Reader Department of Oral PathologyDocument77 paginiOsteomyelitis: Dr. Amit Gupta Reader Department of Oral PathologyAMIT GUPTAÎncă nu există evaluări