Documente Academic
Documente Profesional
Documente Cultură
11 Chapter Reaction Kinetics Text Book Exercise
Încărcat de
Sajid AzeemDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
11 Chapter Reaction Kinetics Text Book Exercise
Încărcat de
Sajid AzeemDrepturi de autor:
Formate disponibile
1st year chemistry n0tes new
CHAPTER11 REACTION KINETICS
TEXT BOOK EXERCISE
Multiple choice questions
(i)
In a zero order reaction, the rate is independent of
(a)
Temperature of reaction.
(b)
Concentration of reactants.
(c)
Concentration of products
(d)
None of these
(ii)
If the rate equation of a reaction 2A + B product is , rate
2
=k[A] [B]. and A is present in large excess, then order of reaction
is
(a)
1
(b)
2
(c)
3
(d)
none of these
(iii)
The rate of reaction
(a)
Increases as the reaction proceeds.
(b)
Decreases as the reaction proceeds.
(c)
Remains the same as the reaction proceeds.
(d)
May decrease or increase as the reaction proceeds.
(iv)
With increase of 10oC temperature, the rate of reaction doubles.
This increase in rate of reaction is due to:
(a)
Decrease in activation energy of reaction.
(b)
Decrease in the number of collisions between reactant
molecules.
(c)
Increase in activation energy of reactants.
(d)
Increase in number of effective collisions.
(v)
The unit of the rate constant is the same as that of the rate of
reaction in
(a)
First order reaction
(b)
Second order reaction
(c)
Zero order reaction
ww
w.a
llon
line
fre
e.c
om
Q1.
www.allonlinefree.com
1st year chemistry n0tes new
Q3.
(i)
(ii)
(iii)
(iv)
(v)
Ans.
Q4.
Ans.
(i)
(ii)
om
e.c
fre
An.
line
(v)
llon
(iv)
w.a
(ii)
(iii)
ww
Ans.
(v)
Q2.
(i)
(d)
Third order reaction
(i)
b
(ii)
a
(iii)
b
(iv)
d
c
Fill in the blanks with suitable words.
The rate of an endothermic reaction_______wiht the
increase in temperature.
All radioactive disintegration nuclear is of ________order.
For a fast reaction the rate constant is relatively _______and
half-life is ________.
The second order reaction becomes ________if one of the
reactants is in large excess.
Arrhenius equation can be used to find out_________of a
reaction.
(i)
increases
(ii)
first (iii)
large : small
(iv)
First order
(v)
energy of activation
Indicate TRUE or FALSE as the case may be.
The half-life order reaction increases with temperature.
The reactions having zero activation energies are
instantaneous.
A catalyst makes a reaction more exothermic.
There is difference between rate law and the law of mass
action.
The order of reaction is strictly determined by the
Stoichiometry of the balanced equation.
(i)
False (ii)
True (iii)
False (iv)
True
(v)
False
What is chemical kinetics? How do you compare chemical
kinetics with chemical equilibrium and thermodynamics?
Chemical thermodynamics can be used:
To predict whether or not a reaction will proceed to the
right, as written.
To predict the extent to which a reaction will proceed before
reaching a condition of equilibrium.
2
www.allonlinefree.com
1st year chemistry n0tes new
To enable the amount of energy theoretically required by or
released during reactions to be calculated,
Thermodynamics cab tells us where the position of equilibrium lies
between the reactants and the products. It enables us to calculate the
equilibrium constants. It has the following limitations.
(i)
It can only predict the us where possibility of a reaction but not
its success.
(ii)
It cannot tell us about the rate at which a reaction will occur. It is
because time is not a thermodynamic variable.
(iii)
It provides no information about the mechanism of the reaction.
This is because the change in any state function is path
independent.
Chemical kinetics can be used:
(i)
To determinc the rate of reaction, that is the rate at which
products are formed or the rate at which reactions are used up in
the reaction.
(ii)
To understand the factors that affects the rate of a reaction.
(iii)
To find the mechanism of a reaction.
Example:
When gaseous H2 and O2 are mixed, thermodynamics tell
us that H2O should be formed, since H2O is more energetically stable
than H2 and O2. It is a well known fact that at room temperature, a
mixture of H2 and O2 will not produce H2O. However, if the mixture is
sparked, water is produced with explosive violence, thereby proving the
prediction of thermodynamics. However, it does not give any indication
of how fast the reaction will proceed.
Q5.
The fast of a chemical reaction with respect to products is written
with positive sing, but with respect to reactants is written with a
negative sing. Explain it with reference to the following
hypothetical reaction.
aA + bB
cC + dD
Ans. The concentration of reactants decrease with time whereas the
concentration of products increases with time.
ww
w.a
llon
line
fre
e.c
om
(iii)
www.allonlinefree.com
1st year chemistry n0tes new
The rate of disappearance of reactant is always negative. A
negative sign in the rate expression indicates that the
concentration of the reactant decreases with time.
For the general reaction:
om
aA + bB
cC + dD
The rate of reaction is given by
Ans.
ww
w.a
llon
line
fre
e.c
Rate =- .
=- .
=+ .
=+ .
Remember that in order to have a unique value of the reaction rate
(independent of the concentration term chosen), it is necessary to divide
each concentration change by its coefficient in the balanced equation for
the reaction.
Q6.
What are instantaneous and average rates? Is it true that the
instantaneous rate of a reaction at the beginning of the reaction is
greater than average rate and becomes far less than the average rate
near the completion of reaction?
Q7.
Differentiate between
(i)
Rate and rate constant of a reaction
Rate of reaction
Rate constant of
reaction
1. The proportionality
constant in rate law
equation which relates
concentration to the rate
of reaction is called rate
constant of reaction.
2. It is independent of
the concentration of the
The change
in concentration of
a
reactant
or
product per unit
time is called the
rate of reaction.
2.
The reaction
takes place in one
1.
www.allonlinefree.com
1st year chemistry n0tes new
reactant.
3.
The 3.
Its units depend
mechanism
upon the order of reaction
involves
the and differs according to
formation of an order of reaction.
intermediate
substance.
(ii)
Homogeneous and heterogeneous catalyses
Ans.
Homogeneous catalyses
Heterogeneous catalysis
1. The process in which the 1. The process in which the
catalyst and
catalyst and the reaction are in
the reactants are in the same different phases is called
phase is called homogeneous heterogeneous catalysis.
catalysis.
2. The reaction takes place at
2. The reaction takes place in a phase boundary. The gaseous
one phase. The catalyst is molecules react at the surface of
uniformly distributed and is the solid catalyst.
itself in the same phase.
3.
The mechanism involves
3.
The mechanism involves adsorption. Molecules are bound
the formation of an intermediate to active sites on a solid
substance.
surface.
(iii)
Fast step and the rate determining step
Ans.
Fast step
Rate determining step
1. A relatively rapid step of 1.
The slowest step of the
the reaction mechanism is reaction mechanism is called
called fast step.
rate determining step.
2. The overall rate of reaction 2. The rate of this step
is independent if this step.
determines the overall rate of
3. The number of molecules of reaction.
each reactant taking part in this 3. The number of molecules of
step does not appear in the rate each reactant taking part in this
ww
w.a
llon
line
fre
e.c
om
phase.
www.allonlinefree.com
1st year chemistry n0tes new
ww
w.a
llon
line
fre
e.c
om
equation.
step appears in the rate equation.
Remember that except the slow step, all other steps of the reaction
mechanism are normally faster steps. The terms slow and fast are
relative. They don not necessarily imply that the reaction itself is slow or
fast.
(iv)
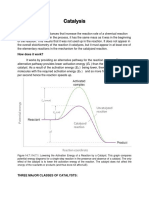
Enthalpy change of reaction and energy of activation of reaction
Ans.
Enthalpy change of reaction
Energy of activation of
reaction
1. The heat change when the 1. The minimum amount of
reaction is carried out at energy , in addition to the
constant pressure is called average kinetic energy. Which
enthalpy change of reaction.
the reactant molecules must
2.
It is the amount of heat have for effective collisions is
evolved or consumed in the called activation energy of
course of reaction.
reaction.
3. It is given the symbol, H 2. It is the minimum amount
of energy required to initiate a
reaction.
3. It is given the symbol, Ea
Q8.
Justify the following statements
(i)
Rate of chemical reaction is an ever changing parameter under
the given conditions.
Ans. The rate of chemical reaction change with time. It has a
maximum value at the beginning of the reaction, then gradually
decreases and finally becomes zero when the reactants are totally
converted into products.
(ii)
The reaction rate decreases every moment but rate constant k
of the reaction is constant quantity, under the given conditions.
Ans. The rate of chemical reaction is not uniform. It depends upon the
molar concentrations of reactants. The rate of reaction is directly
proportional to concentrations of reactants. Since the concentration
of reactants decreases every moment, so the rate of reaction, then
6
www.allonlinefree.com
1st year chemistry n0tes new
e.c
om
gradually decreases with time. It has a maximum value at the start
of the reaction, then gradually decreases and finally becomes zero.
The constant, k of the reaction is independent of concentration.
So, it remains constant under the given conditions. However, it
changes with temperature.
(iii)
50% of a hypothetical first order reaction completes in one
hour. The remaining 50% needs more than one hour to complete.
Ans. Calculation of k:
For a first order reaction
fre
T1/2 =
llon
line
1 hour =
k=0693 hours-1
Calculation of time for remaining 50%
Completion of reaction:
For first order reaction rate equation:
K=
log
ww
w.a
t=
log
Where [A]o is initial concentration and [A] is concentration at any time t.
[A]=
t=
t=
=
t =5.6457 hours
Hence remaining 50% needs more than one hour to complete.
(iv)
The radioactive decay is always a first order reaction.
Ans. Since the half-life of a first order reaction in constant and is
independent of initial concentration of the reaction, so the radioactive decay is always a first order reaction.
7
www.allonlinefree.com
1st year chemistry n0tes new
The unit of rate constant of a second order reaction is dm3 mol1 -1
s , but the unit of rate of reaction is mol dm-3s-1.
Ans. For second order reaction: A+B
Products
2
Rate =k[A]
Units of rate are: mol dm-3s-1.
Units of concentration of A: mol dm-3
Therefore,
mol dm-3s-1=k(mol dm-3)2
mol dm-3s-1=(mol2 dm-6)
om
(v)
e.c
k=
=mol-1dm3s-1
So, the units of rate constant of 2nd order reaction are: dm3 mol1 -1
fre
The sum of the coefficients of a balanced chemical equation is
not necessarily important to give the order of a reaction.
Ans. The order of a chemical reaction is the sum of the exponents of
the concentration terms in the rate equation. It cannot be written by
merely looking at the balanced chemical equation. Usually, the
powers of concentration terms in the rate equation are different
from coefficients of reactants in the balanced equation. The order
of a reaction can be determined only by experiment. For example,
for N2O5, the rate is proportional to [N2O5] and its power is one
although the coefficient of N2O5in the balanced equation is two.
N2O5
4NO2+O2
Rate = k [N2O5]
A reactant whose concentration does not affect the reaction rate
is not included in the rate equation. The total number of molecules
or atoms taking part in a reaction can never be zero or in fraction
while the order of reaction can be zero or in fraction.
(vii)
The order of a reaction is obtained from the rate expression of a
reaction and the rate expression is obtained from the experiment.
Ans.
The rate expression can be only being determined
experimentally. It expresses actual dependence of the rate on the
concentrations of the reactants. A reactant whose concentration
ww
w.a
llon
line
(vi)
www.allonlinefree.com
1st year chemistry n0tes new
ww
w.a
llon
line
fre
e.c
om
does not affect the reaction rate is not included in the rate equation.
The order of reaction is the number of reacting molecules whose
concentrations alter as a result of a chemical reaction. Hence the
order of a reaction is obtained from the rate equation and the rate
expression is obtained from the experiment.
Q9.
Explain that half-life method for measurement of the order of a
reaction can help us to measure the order of even those reactions
which have a fractional order.
Q10. A cure is obtained when a graph is plotted between time on xaxis and concentration on y-axis. The measurement of the slopes of
various points give us the instantaneous rates of reaction. Explain
with suitable examples.
Q11. The rate determining step of a reaction is found out from the
mechanism of that reaction. Explain it with few examples.
Q12. Discuss the factors which influence the rates of chemical
reactions.
Q13. Explain the following facts about the reaction.
2NO(g) + 2H2(g)
2H2O(g) +N2(g)
(i)
The changing concentrations of reactants change the
rates of this reaction.
(ii)
Individual orders with respect to NO and H2 can be
measured.
(iii)
The overall order can be evaluated by keeping the
concentration of one of the substances constant.
Q14. The collision frequency and the orientation of molecules are
necessary conditions for determining the proper rate of reaction.
Justify the statement.
Q15. How does Arrhenius equation help us to calculate the energy of
activation of a reaction?
Q16. Define the following terms and give examples.
(i)
Homogeneous catalysis
(ii)
Heterogeneous catalysis
(iii)
Activation of a catalyst
9
www.allonlinefree.com
1st year chemistry n0tes new
ww
w.a
llon
line
fre
e.c
om
(iv)
Auto-catalysis
(v)
Catalytic poisoning
(vi)
Enzyme catalysis.
Q17. Briefly describe the following with examples:
(i)
Change of physical state of a catalyst at the end of
reaction.
Ans. There may be change in physical state such as the particle size or
change in the colour of the catalyst at the end of reaction. For
example, granular MnO2 used as a catalyst in the thermal
decomposition of KC1O3 is left as fine powder at the end of
reaction.
(ii)
A very small amount of a catalyst may prove sufficient
to carry out a reaction.
Ans. Since a catalyst is not used up in the reaction, a very small
amount of catalyst is required. It can catalyses the reaction over
and over again. Sometimes a trace of a metal catalyst is required
to affect very large amount of reactants. For example, 1 mg of fine
platinum powder can convert 2.5 dm3 of H2 and 1.25 dm3 of O2 to
water. Dry HC1 and NH3 combine in the presence of trace of
moisture to give dense white fume of NH4C1.
(iii)
A finely divided catalyst may prove more effective.
Ans. A catalyst is more effective when it is present in a finely divided
form than it is used in bulk. With the increase of fine subdivision,
the free surface area is increased. As a result, the active sites on the
surface are increased. Consequently, the activity of the catalyst is
also enhanced. For example, a lump of platinum will have much
less catalytic activity than colloidal platinum. Finely divided nickel
is a better catalyst than lumps of solid nickel.
(iv)
Equilibrium constant of a reversible reaction is not
changed in the presence of a catalyst.
Ans. A catalyst for the forward reaction is also a catalyst for the
reverse reaction. A catalyst speeds up the rate of both the forward
10
www.allonlinefree.com
1st year chemistry n0tes new
and reverse reactions equally. So the equilibrium constant (k= )
for the reaction remains the same. A catalyst helps the equilibrium
to e established earlier. For example, the reaction of N 2 and H2 to
from NH3 is very slow. In the presence of the catalyst, the
equilibrium
ww
w.a
llon
line
fre
e.c
om
N2(g) + 3H2(g)
2NH2(g)
Is reached much sooner but the percentage yield remains
uncharged.
(iv)
A catalyst is specific in its action.
Ans. A catalyst cans catalyst only a specific reaction. When a
particular catalyst works for one reaction it may not necessarily
work for any other reaction. It may increase the rate of one
reaction but not increase the rate of another reaction. For example,
MnO2 can catalyse the decomposition of KC1O3 but not that of
NH3. The decomposition of NH3 is catalysed by a hot tungsten
wire.
2KC1O3(g)
2KC1(s) + 3O2(g)
2NH3(g)
N2(g) + 3H2(g)
Remember that transition metals catalyse reaction of different
types.
For example, the decomposition of aqueous solutions of
hydrogen peroxide (H2O2) is catalysed by MnO2 or colloidal
platinum.
2H2O2(l)
2H2O(l) + O2(g)
Q18. What are enzymes? Give examples in which they act as catalyst.
Mention the characteristics of enzyme catalysis.
Q19. In the reaction of NO and H2, it was observed that equimolecular
mixture of gases at 340.5 mm pressure was half changed in 102
seconds. In another experiment with an initial pressure of 288 mm
of Hg, the reaction was half completed in 140 seconds. Calculate
the order of reaction.
Ans. Initial pressure of reactants = Initial concentrations of reactants.
11
www.allonlinefree.com
1st year chemistry n0tes new
Given:
a1=340.5 mm
A2=288 mm
Order of reaction, n=?
om
t1=102 seconds
t2=140 seconds
fre
e.c
Formula used:
=1 + 1.89=2.89
n =3
Hence the reaction of third order.
Q20. A study of chemical kinetiecs of a reaction
A + B
products
Gave the following data at 25oC. Calculate the rate law
w.a
llon
n =1 +
line
n =1 +
ww
Exp.
[A]
[B]
Rate
1
1.00
0.15
-6
4.2 x 10
2
2.00
0.15
-6
8.4 x 10
3
1.00
0.2
-6
5.6 x 10
Solution:
Examination of the data shows that when the
concentration of B is kept constant 0.15. The concentration of A is
doubled form 1.00 to 2.00; the rate doubles from 4.2 x 10 -6 tp 8.4 x 10-6.
12
www.allonlinefree.com
1st year chemistry n0tes new
(i)
What is the value of the factor at 25oC.
Ea=50kJ mol-1 =50000 Jmol-1
R=8.3143 J K-1 mol-1
ww
w.a
Ans.
llon
line
fre
e.c
om
Mathematically, the rate of reaction with respect to A is directly
proportional to the first power of the concentration of A. this is,
Rate [A]
Examination of the experiment 1 and 3 shows that when the
concentration of A is kept constant 1.00, the concentration of B is
increased from 0.15 to 0.2, the rate of the reaction with respect to B is
also increased form 4.1 x10-6 to 5.6 x10-6. Mathematically, the rate of
reaction with respect to B is directly proportional to the first power of
the concentration of B. that is,
Rate [B]
Since the rate of a reaction is proportional to the product of
concentration of the reactants, so,
Rate [A][B]
Rate=k[A][B]
This equation is known as the rate law for the reaction.
Q21. Some reaction taking places around room temperature have
activation energies around 50kJ mol-1.
At 25oC, the factor =
=
=1.72 x10-9
(ii)
Calculate this factor at35oC, and 45oC note the increase
in this factor for every 10oC rise in temperature.
Ans.
At 25oC, the factor
=3.314 x10-9
At 25oC, the factor =
=
=6.12 x 10-9
(iii)
prove that for every 10oCrise in temperature, the factor
doubles and so rate constant also doubles.
Ans.
At 25oC, the factor
=1.72 x 10-9
13
www.allonlinefree.com
1st year chemistry n0tes new
At 35oC, the factor
=3.314 x 10-9
(i)
line
fre
e.c
om
At 45oC, the factor =6.12 x 10-9
Since for every 10oC rise in temperature, the factor becomes
doubles and so rate constant also doubles.
Q22. H2 and 12 react to produce HI. Following data for rate constant at
various temperatures (K) have been collected.
Temperature(K)
Rate constant(
3
-1 -1
cm mol s )(K)
500
6.814 x 10-4
550
2.64 x 10-2
600
0.56 x 10o
650
7.31 x 10o
700
66.67 x 10o
Pot a graph between
llon
axis.
(ii)
Measure the slope of this straight line and calculate the
energy for actives this reaction.
8326.32: 160.6 Jmol-1.
ww
w.a
Ans.
on x-axis and log k on the y-
14
www.allonlinefree.com
S-ar putea să vă placă și
- Chemsheets A2 1029 (Catalysis)Document17 paginiChemsheets A2 1029 (Catalysis)Jon HadleyÎncă nu există evaluări
- PhotocatalysisDocument57 paginiPhotocatalysisdrndryl92% (13)
- Chapter # 11 Reaction KineticsDocument24 paginiChapter # 11 Reaction KineticsAnoshKhanÎncă nu există evaluări
- 11 Chapter Reaction Kinetics Text Book Exercise PDFDocument14 pagini11 Chapter Reaction Kinetics Text Book Exercise PDFBilal KhanÎncă nu există evaluări
- Melc 130 138 Chemical KineticsDocument36 paginiMelc 130 138 Chemical KineticsAlayna AlejagaÎncă nu există evaluări
- General Chemistry 2: Quarter 3 - Module 4Document16 paginiGeneral Chemistry 2: Quarter 3 - Module 4Rose Ann Carlos100% (3)
- Chemical Kinetics 1Document114 paginiChemical Kinetics 1Ashish KrishnaÎncă nu există evaluări
- Adama Science and Technology UniversityDocument34 paginiAdama Science and Technology UniversityAme ShumetaÎncă nu există evaluări
- Order of ReactionDocument6 paginiOrder of ReactionSherenaiah GacoteÎncă nu există evaluări
- Module 7 Reaction Rates Factors Affecting Reaction Rates SendDocument54 paginiModule 7 Reaction Rates Factors Affecting Reaction Rates SendAndrea ReyesÎncă nu există evaluări
- General Chemistry 2: Quarter 3 - WEEK 4Document13 paginiGeneral Chemistry 2: Quarter 3 - WEEK 4RODEL AZARESÎncă nu există evaluări
- Chapter 8 - Chemical EquilibriaDocument10 paginiChapter 8 - Chemical EquilibriaHikmaÎncă nu există evaluări
- Kinetics LPDocument41 paginiKinetics LPHarkritSinghÎncă nu există evaluări
- 1 Rates of Reaction NotesDocument7 pagini1 Rates of Reaction Notesapi-369690183Încă nu există evaluări
- K1 KineticsDocument11 paginiK1 KineticsEmmanuel ManteyÎncă nu există evaluări
- Rate Laws and Reaction KineticsDocument6 paginiRate Laws and Reaction KineticsThala SkÎncă nu există evaluări
- Chapter Fifteen Chemical Kinetics: A + B C + DDocument17 paginiChapter Fifteen Chemical Kinetics: A + B C + DanandyelwalÎncă nu există evaluări
- Chemical KineticsDocument39 paginiChemical Kineticsneel-amberÎncă nu există evaluări
- Chemistry Pre-U Chemistry Sem 1 Chap 5 PDFDocument85 paginiChemistry Pre-U Chemistry Sem 1 Chap 5 PDFJIANHUI0160% (1)
- Dr. Pedro Julio VillegasDocument57 paginiDr. Pedro Julio VillegasSheikh Samir HassanÎncă nu există evaluări
- Chemical KineticsDocument53 paginiChemical KineticsEuann MagtibayÎncă nu există evaluări
- Unit-4 KineticsDocument34 paginiUnit-4 KineticsAbi RÎncă nu există evaluări
- Chapter-04 Chemical KineticsDocument11 paginiChapter-04 Chemical Kineticsshrey4602Încă nu există evaluări
- KINETICS IN 40Document11 paginiKINETICS IN 40Srijan GoyalÎncă nu există evaluări
- SS 2 Week 3Document71 paginiSS 2 Week 3Denzel MusaÎncă nu există evaluări
- Factors That Influence Reaction RatesDocument26 paginiFactors That Influence Reaction Ratesyokehong tanÎncă nu există evaluări
- Chemistry G-11, Short Noteswith Practice Quetions 2012Document73 paginiChemistry G-11, Short Noteswith Practice Quetions 2012Chrstina Alazar100% (1)
- Rates of Reaction NotesDocument13 paginiRates of Reaction NotesNia RamÎncă nu există evaluări
- COLISION THEORY - CopyDocument85 paginiCOLISION THEORY - Copyactive learning educationÎncă nu există evaluări
- CBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageDocument14 paginiCBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageBHAVYA BÎncă nu există evaluări
- Chemical Kinetics 1234 FinalDocument22 paginiChemical Kinetics 1234 FinalJayesh SavaliyaÎncă nu există evaluări
- Chemical KineticDocument40 paginiChemical KineticHamzaÎncă nu există evaluări
- Introduction To Reaction Kinetics, Hazle CoxDocument83 paginiIntroduction To Reaction Kinetics, Hazle CoxcachorroingenieroÎncă nu există evaluări
- Chapter 23 - Reaction KineticsDocument11 paginiChapter 23 - Reaction KineticsnuofanxiaÎncă nu există evaluări
- Kinetics: Factors Affecting Reaction RatesDocument118 paginiKinetics: Factors Affecting Reaction RatesHarsh PipariyaÎncă nu există evaluări
- 21 Chemical Kinetics Formula Sheets Getmarks AppDocument8 pagini21 Chemical Kinetics Formula Sheets Getmarks AppRockstarÎncă nu există evaluări
- XII - CHEMICAL KINETICS - Module 2Document5 paginiXII - CHEMICAL KINETICS - Module 2Rahul Joseph ThomasÎncă nu există evaluări
- Chemical KineticsDocument13 paginiChemical KineticsMichael John DianaÎncă nu există evaluări
- 1 Chemical Kinetics and EquilibriumDocument77 pagini1 Chemical Kinetics and EquilibriumApril Mergelle Lapuz100% (1)
- Unit 6Document53 paginiUnit 6Muktaar HassenÎncă nu există evaluări
- End Term ALLDocument31 paginiEnd Term ALLJulie Anne CristalesÎncă nu există evaluări
- Chapter 14 - Modified by Rabeay - 2022Document90 paginiChapter 14 - Modified by Rabeay - 2022s-islam.safwatÎncă nu există evaluări
- 1 Lab Handout PDFDocument5 pagini1 Lab Handout PDFKhud SarÎncă nu există evaluări
- 4 Rates of Reaction Review With AnswersDocument3 pagini4 Rates of Reaction Review With Answersapi-369690183Încă nu există evaluări
- Chemical Kinetics in Biology: GoalsDocument28 paginiChemical Kinetics in Biology: GoalsDrJayasimha GoudÎncă nu există evaluări
- Week 5 Chemical KineticsDocument60 paginiWeek 5 Chemical KineticsLuke BelmarÎncă nu există evaluări
- LAS General Chemistry 2 Reinforcement ActivitiesDocument16 paginiLAS General Chemistry 2 Reinforcement ActivitiesMarlon C. CambayÎncă nu există evaluări
- Experiment 5 Chemical Kinetics: Rate Reaction: I. Experiment's Date: April, 11 2017 II. ObjectiveDocument17 paginiExperiment 5 Chemical Kinetics: Rate Reaction: I. Experiment's Date: April, 11 2017 II. Objectiveyokehong tanÎncă nu există evaluări
- Reaction Kinetics ExplainedDocument31 paginiReaction Kinetics ExplainedchweetomahiÎncă nu există evaluări
- Kinetic Chemistry: Rates of Reaction and Theories Explaining ThemDocument83 paginiKinetic Chemistry: Rates of Reaction and Theories Explaining Themmusafir24Încă nu există evaluări
- Chemical Kinetics in Biology - Analyzing Reaction RatesDocument69 paginiChemical Kinetics in Biology - Analyzing Reaction RatesDaniel YakubovichÎncă nu există evaluări
- Reaction Rate: A+B ABDocument5 paginiReaction Rate: A+B ABFaisal Mohad Al SakhenÎncă nu există evaluări
- Chemical KineticsDocument31 paginiChemical Kineticsakbar azamÎncă nu există evaluări
- Chemistry Class 10 Chapter 9Document11 paginiChemistry Class 10 Chapter 9Rahim BakhshÎncă nu există evaluări
- Combine PDFDocument101 paginiCombine PDFZeni MalikÎncă nu există evaluări
- Bahria College Karachi Chapter # 8: Introduction To Chemical KineticsDocument15 paginiBahria College Karachi Chapter # 8: Introduction To Chemical KineticsNayan RoyÎncă nu există evaluări
- Chem Kinetics Lec 1 PDFDocument32 paginiChem Kinetics Lec 1 PDFKinza ShahzadÎncă nu există evaluări
- Chemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarDocument36 paginiChemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarAnindya BhattacharyaÎncă nu există evaluări
- English WB IgcseDocument23 paginiEnglish WB Igcsetoleen playzÎncă nu există evaluări
- Chapter No 6 - Chemical KineticsDocument45 paginiChapter No 6 - Chemical KineticsTanish SalviÎncă nu există evaluări
- Chapter No 04 9th Class NotesDocument5 paginiChapter No 04 9th Class NotesSajid AzeemÎncă nu există evaluări
- 1 ChapteR Short Question With AnswerDocument16 pagini1 ChapteR Short Question With AnswerSajid AzeemÎncă nu există evaluări
- NTS TestDocument4 paginiNTS TestSajid AzeemÎncă nu există evaluări
- Chapter No 03 9th Class NotesDocument6 paginiChapter No 03 9th Class NotesSajid AzeemÎncă nu există evaluări
- 6 Chapter Chemical Bonding Text Book ExerciseDocument20 pagini6 Chapter Chemical Bonding Text Book ExerciseSajid Azeem100% (2)
- Student Computer Class PaperDocument2 paginiStudent Computer Class PaperSajid AzeemÎncă nu există evaluări
- Student Computer Class PaperDocument2 paginiStudent Computer Class PaperSajid AzeemÎncă nu există evaluări
- 9th Class Chemistry Important Solved Short Notes For Exam 2013Document34 pagini9th Class Chemistry Important Solved Short Notes For Exam 2013HAFIAZ MUHAMMAD IMTIAZ100% (4)
- 9th Class Math Unit 5 LessonDocument30 pagini9th Class Math Unit 5 LessonSajid AzeemÎncă nu există evaluări
- 8 Chapter Chemical Equilibrium Text Book ExerciseDocument18 pagini8 Chapter Chemical Equilibrium Text Book ExerciseSajid Azeem0% (1)
- English L T Iqbalkalmati Blogspot Com PDFDocument41 paginiEnglish L T Iqbalkalmati Blogspot Com PDFfatima100% (1)
- Simple Harmonic Motion ExplainedDocument13 paginiSimple Harmonic Motion ExplainedsajidazeemÎncă nu există evaluări
- 4 Chapterliquids and Solids Short Question With AnswersDocument18 pagini4 Chapterliquids and Solids Short Question With AnswersSajid AzeemÎncă nu există evaluări
- 10 Chapter Electrochemistry Text Book ExerciseDocument31 pagini10 Chapter Electrochemistry Text Book ExerciseSajid AzeemÎncă nu există evaluări
- 1 ChapteR Short Question With AnswerDocument16 pagini1 ChapteR Short Question With AnswerSajid AzeemÎncă nu există evaluări
- 1ST Chapter Text Book QuestionsDocument20 pagini1ST Chapter Text Book Questionsfaysal8080Încă nu există evaluări
- 10 Phy Unit 10 NewDocument13 pagini10 Phy Unit 10 NewsajidazeemÎncă nu există evaluări
- 10h Class SummaryDocument1 pagină10h Class Summarysajidazeem100% (1)
- 10 Phy Unit 10 NewDocument13 pagini10 Phy Unit 10 NewsajidazeemÎncă nu există evaluări
- WebsiteDocument1 paginăWebsiteSajid AzeemÎncă nu există evaluări
- Simple Harmonic Motion ExplainedDocument13 paginiSimple Harmonic Motion ExplainedsajidazeemÎncă nu există evaluări
- ORIGINALDocument109 paginiORIGINALAli AhsanÎncă nu există evaluări
- Efficient Heterogeneous Fenton-Like Catalysis of Fe-Doped SAPO-44Document9 paginiEfficient Heterogeneous Fenton-Like Catalysis of Fe-Doped SAPO-44NamÎncă nu există evaluări
- Chemical Kinetics: The Rates and Mechanisms of Chemical ReactionsDocument21 paginiChemical Kinetics: The Rates and Mechanisms of Chemical ReactionsOyinkansola OsiboduÎncă nu există evaluări
- Activity of Solid Acid Catalysts For Biodiesel Production: A Critical ReviewDocument23 paginiActivity of Solid Acid Catalysts For Biodiesel Production: A Critical ReviewMạnh BùiÎncă nu există evaluări
- Ronnie VangDocument213 paginiRonnie VangsuncesjajnoÎncă nu există evaluări
- 4 Chemical KineticsDocument96 pagini4 Chemical Kineticsforojaypee2002Încă nu există evaluări
- MCQS Special TopicsDocument18 paginiMCQS Special TopicsPhoton Online Science AcademyÎncă nu există evaluări
- Ozone - 1 s2.0 S2666821120300314 MainDocument18 paginiOzone - 1 s2.0 S2666821120300314 MainStefania IordacheÎncă nu există evaluări
- Hydrothermal MethodDocument16 paginiHydrothermal Methodavni vatsÎncă nu există evaluări
- Understanding Catalysis and Reactor DesignDocument43 paginiUnderstanding Catalysis and Reactor DesignLê MinhÎncă nu există evaluări
- Applied Catalysis B: Environmental: Matej Hu S, Venkata D.B.C. Dasireddy, Neja Strah Stefan Ci C, Bla Z LikozarDocument12 paginiApplied Catalysis B: Environmental: Matej Hu S, Venkata D.B.C. Dasireddy, Neja Strah Stefan Ci C, Bla Z LikozarAminÎncă nu există evaluări
- Mass Transfer Slides - CHE304 - Chapter 24Document53 paginiMass Transfer Slides - CHE304 - Chapter 24RehabÎncă nu există evaluări
- Copper ECSADocument7 paginiCopper ECSANusrat RashidÎncă nu există evaluări
- CATALYSISDocument7 paginiCATALYSISJudy Ann Leyco BartolataÎncă nu există evaluări
- Study On Decolorization of Methylene Blue by H2O2 Catalyzed With IronDocument6 paginiStudy On Decolorization of Methylene Blue by H2O2 Catalyzed With IrondumitriuÎncă nu există evaluări
- Physical Chemistry of Surfaces: Arthur W. AdamsonDocument12 paginiPhysical Chemistry of Surfaces: Arthur W. AdamsonAbeera Hassan ChohanÎncă nu există evaluări
- CHEN 305 Lecture Note 1Document23 paginiCHEN 305 Lecture Note 1Ibrahim garba yaroÎncă nu există evaluări
- Deutschmann NatGasCS01Document8 paginiDeutschmann NatGasCS01vazzoleralex6884Încă nu există evaluări
- 35 Book Rev CatrinescuDocument3 pagini35 Book Rev CatrinescuHammad ShamshadÎncă nu există evaluări
- Visual Encyclopedia of Chemical Engineering Catalysts: General InformationDocument7 paginiVisual Encyclopedia of Chemical Engineering Catalysts: General InformationVanÎncă nu există evaluări
- Clusters, Surfaces, and Catalysis: Gabor A. Somorjai, Anthony M. Contreras, Max Montano, and Robert M. RiouxDocument7 paginiClusters, Surfaces, and Catalysis: Gabor A. Somorjai, Anthony M. Contreras, Max Montano, and Robert M. RiouxDorisÎncă nu există evaluări
- Catalysis: A Brief Review On Nano-Catalyst: August 2014Document11 paginiCatalysis: A Brief Review On Nano-Catalyst: August 2014Kapil KhandelwalÎncă nu există evaluări
- Kinetic Study of Esterification Reaction Using Ion Exchange ResinDocument89 paginiKinetic Study of Esterification Reaction Using Ion Exchange Resinsumit potdarÎncă nu există evaluări
- Introduction To Heterogeneous Catalysis: Letcture-1Document14 paginiIntroduction To Heterogeneous Catalysis: Letcture-1hamoodahÎncă nu există evaluări
- SyllabusDocument22 paginiSyllabusSohael AftabÎncă nu există evaluări
- CHE 2001 - Chemical Reaction Engineering: Dr. K. SivagamiDocument137 paginiCHE 2001 - Chemical Reaction Engineering: Dr. K. SivagamiAmol RastogiÎncă nu există evaluări
- Grade 11 Answer Key Chemistry Revision SheetsDocument8 paginiGrade 11 Answer Key Chemistry Revision Sheetskirki pÎncă nu există evaluări