Documente Academic
Documente Profesional
Documente Cultură
8 Chapter Chemical Equilibrium Text Book Exercise
Încărcat de
Sajid AzeemDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
8 Chapter Chemical Equilibrium Text Book Exercise
Încărcat de
Sajid AzeemDrepturi de autor:
Formate disponibile
1st year chemistry n0tes new
CHAPTER 8
CHEMICAL EQUILIBRIUM
ww
w.a
llon
line
fre
e.c
om
TEXT BOOK EXERCISE
Q1.
Multiple Choice questions
(i)
For which system does the equilibrium constant, Kc has units of
(concentration)-1
(a)
N2 + 3H2
2NH3
(b)
H2 + I2
2HI
(c)
2NO2
N2 O4
(d)
2HF
H2 + F2
(ii)
Which statement about the following equilibrium is correct
2SO2 + O2
2SO3
(a)
The value of Kp falls with a rise in temperature
(b)
The value of KP falls with increasing Pressure
(c)
Adding V2 O5 catalyst increases the equilibrium yield of
SO3
(d)
The value of Kp is equal to Kc
(iii) The pH of 10-3 mol/dm3 of an equous solution of H2SO4 is
(a)
3
(b)
2.7
(c)
2
(d)
1.5
10
2
-6
(iv)
The solubility product of AgCI is 2 x 10 mol dm . The
maximum concentration of Ag+ ions in the solution is
(a)
2 x 10-10 mol dm-3
(b)
1.41 x 10-10 mol dm-3
(c)
1 x 10-10 mol dm-3
(d)
4 x 10-20 mol dm-3
(v)
An ecxcess of aqueous silver nitrate is added to aqueous barium
chloride and precipitate is removed by filtration. What are the
main ions in the filtrate?
(a)
Ag+ and NO3-1 only
(b)
Ag+ and Ba2+ and NO3-1
(c)
Ba2+ and NO3-1 only
1
www.allonlinefree.com
1st year chemistry n0tes new
(i)
Ans.
Q3.
(i)
(ii)
om
e.c
fre
(iv)
line
(iii)
llon
(ii)
ww
w.a
Ans.
Q2.
(i)
(d)
Ba2+ and NO3-1 and CI-1
i)c
ii)a
iii)b
iv)b
v)b
Fill in the blanks
Law of mass action states that the rate at which a reaction
proceeds is directly proportional to the product of the active
masses of the ______.
In an exothermic reversible reaction, _________in temperature
will shift the equilibrium towards the forward direction.
The equilibrium constant for the reaction 2O3
3O2 is 1055 at
25oC, it tells that ozone is _______at room temperature.
In a gas phase reaction, if the number of moles of reactants are
equal to the number of moles of the products, KC of the reaction is
______to the Kp.
Buffer solution is prepared by by mixing together a weak
base and its salt with _______ or a weak acid and its salt with
________.
i)reactants
ii)decrease
iii)unstable
iv)equal
v)strong acid, strong base
Label the sentences True or False
When a reversible reaction attains equilibrium both
reactants and products are present in a reaction mixture.
The Kc of the reaction
A+B
C+D
is given by
Kc =
Therefore it is assumed that [A] = [B]=[C]=[D]
(iii)
A catalyst is a compound, which increases the speed of the
reaction and consequently increases the yield of the product.
(iv)
Ionic product Kw of pure water at 25oC is 10-14 dm-6 and is
represented by an expression
Kw =[H+][OH-]=10-14 mol2 dm-6
(v)
AgCI is a sparingly soluble ionic solid in water. Its solution
produces excess of Ag+ and CI- ions.
2
www.allonlinefree.com
1st year chemistry n0tes new
Ans. i)True
iii)False
iv)True
v)False
Explain the terms reversible reaction and state of
equilibrium
See Section 8.1 and 8.1.2
(b)
Define and explain the law of mass action and drive the
expression for the equilibrium constant KC.
See Section 8.1.3
(c)
Write KC for the following reactions
(i)
Sn2+(aq) + 2Fe3+(aq)
Sn4+(aq) + 2Fe2+(aq)
(ii)
Ag+(aq) +Fe2+(aq)
Ag(s) + Fe3+(aq)
(iii)
N2(g)+ O2(g)
2NO(g)
(iv)
4NH3(g) 5O2(g)
4NO(g) +6H2O(g)
(v)
PCI5(g)
PCI3(g) +CI2(g)
(i)
Kc= (ii)
Kc= (iii) K =
(a)
Q5.
Kc= (v) Kc=
ww
w.a
(iv)
llon
line
fre
e.c
om
Q4.
ii)False
(a) Reversible reactions attain the position of equilibrium,
which is dynamic in nature and not static. Explain it.
See Section 8.1.1
(b)
Why do the rates of forward reactions slow down when
a reversible in nature and not static. Explain it.
See Section 8.1.1
Q6.
When a graph is plotted between time on X-axis and the
concentration of reactants and products on Y-axis for a
reversible reaction, the curve becomes parallel to time axis at a
certain stage.
(a)
At what stage the curves become parallel?
3
www.allonlinefree.com
1st year chemistry n0tes new
Kc =
ww
w.a
Kp is given by
llon
line
fre
e.c
om
(b)
Before the curves become parallel, the steepness of
curves falls? Give reasons.
(c)
The rate of decrease of concentrations of the reactants
and rate of increase of concentrations of any of products
may or may not be equal for various types of reactions
before the equilibrium time. Explain it.
See section 8.1.1
Q7.
(a)
Write down the relationship of different types of
equilibrium constants. i.e. Kc and Kp for the following general
reactions.
aA + bB
cC +dD
See section 8.1.2
(b)
Decide the comparative magnitudes of Kc, Kp for the
following reactions.
Synthesis of NH3
N2(g) +3H2(g)
2NH3(g)
KC is given by
Kp =
For this reaction, change in number of moles is given by
n =number of mole of product-number of moles of reactants
=2 (1+3)=-2
Hence
Kp=Kc x (RT)-2
Or
Kp = Kc x
Thus
if T is such that RT > 1, then Kp < Kc
If T is such that RT< 1, then Kp > Kc
Dissociation of PCI5
PCI5(g)
PCI3(g)+ CI2(g)
4
www.allonlinefree.com
1st year chemistry n0tes new
Kc is given by according to law of Mass of Action
Kc =
Kp is given by
om
Kp =
ww
w.a
llon
line
fre
e.c
For this reaction, change in number of moles is given by
n =number of mole of product-number of moles of reactants
=2 1 =1
Kp =Kc x (RT)
Thus If T is such that RT > 1, then Kp > Kc
If T is such that RT< 1, then Kp <Kc
Q8.
(a)
Write down KC for the following reversible reactions.
Suppose that the volume of reaction mixture in all the cases is
V dm3 at equilibrium stage.
(i)
CH3 COOH+ CH3 CH2 OH
CH3COOC2H5+ H2O
(ii)
H2 +12
2HI
(iii)
2HI
H2 +I2
(iv)
PCI5
PCI3 + CI2
(v)
N2 + 3H2
2NH3
See section 8.1.3
(b)
How do you explain that some of the reactions
mentioned above are affected by change of volume at
equilibrium stage?
Q9.
Explain the following two applications fo equilibrium
constant. Give examples.
(i)
Direction of reaction
(ii)
Extent of reaction
See section 8.1.5
Q10. Explain the following with reasons.
(a)
The change of volume disturbs the equilibrium position
for some of the gaseous phase reactions, but not the
equilibrium constant.
5
www.allonlinefree.com
1st year chemistry n0tes new
The change of temperature disturbs both the
equilibrium position and the equilibrium constant of a
reaction.
(c)
The solubility of glucose in water is increased by
increasing the temperature.
Q11. (a)
What is ionic product of water? How does this value
vary with the change in T? Is it true that this value is 75 times
when the T of water increased form 0oC to 100oC.
Ionic Product of water is given by the equation
Kw [H+][OH-]
Value of Kw increases with increase in temperature. It is
because increase in temperature increase the ionization of H2O.
Thus, more H+ or OH- ions are produced. Hence value of Kw
increase.
e.g.
At 25oC
Kw =1 x 10-14
and
At 100oC
Kw =7.5 x 10-14
Further
At 0oC
(Kw)o =0.1 x 10-14_____(1)
At 100oC
(Kw)100 =7.5 x 10-14_____(2)
Divide eq (2) by eq (1)
ww
w.a
llon
line
fre
e.c
om
(b)
(b)
(c)
=75
or
(KW) 100 =75 x (Kw)0
Hence, KW at 100oC is 75 times more than at 0oC
What is the Justification for the increase of ionic
product with temperature?
Value of Kw increase with increase in temperature. It is
because increase in temperature increase the ionization of
H2O. Thus, more H+ or OH- ions are produced. Hence value
Kw increases.
How do you prove that at 25OC in 1 dm3 of water, there
are 10-7 moles of H3O+?
6
www.allonlinefree.com
1st year chemistry n0tes new
ww
w.a
llon
line
fre
e.c
om
At 25OC
Kw =[H3O+][OH-14 ____(1)
Since ionization of water gives equal no. of H3O+ and OHions
therefore
[H3O+]=[OH-]
Hence, eq (1) can be written as
Kw =[H3O+][H3O+]=10-14
Or
[H3O+]2=10-14
Taking square root on both sides
[H3O+]2=10-7 mol/dm3
Hence, at 25oC, water has 10-7 mole/dm3 of H3O+ ions.
Q12. (a)
Define pH and pOH. How are they related with pKw.
(b)
What happens to the acidic and basic properties of
aqueous solutions when pH varies form 0 to 14.
(c)
Is it true that the sum of pHa and pKb is always equal to
14 to all temperatures for any acid? If not why?
Q13. (a)
What is Lowry bronsted idea of acids and bases?
Explain conjugate acids and bases.
(b)
Acetic acid dissolves in water and gives proton to water.
But when dissolves in H2SO4, it accepts proton. Discuss
the role of acetic acid in both cases.
CH3 COOH + H2O
CH3 COO- + H3O+
However, H2SO4 is a stronger acid than acetic acid, therefore,
H2SO4 donates proton and acts as an acid while acetic acid accepts
proton and acts as a base.
H2SO4+ CH3 COOH
HSO4- + CH3 COOH2+
Q14. In the equilibrium PCI5(g)
PCI3(g) + CI2(g)
H=90 kJ/
mol
(a)
The position of equilibrium
(b)
Equilibrium constant?
If
(i)
Temperature is increased
(ii)
Volume of the container is decrease.
7
www.allonlinefree.com
1st year chemistry n0tes new
Catalyst is added
CI2 is added
See section 8.2
Explain your answer.
Q15. Synthesis of NH3 by Habers process is an exothermic
reaction.
N2 + 3H2
2NH3
H=92.46 kJ/ mol
(a)
What should be the possible effect of change of
temperature at equilibrium stage?
(b)
How does the change of pressure or volume shifts the
equilibrium position of this reaction?
(c)
What is the role of the catalyst in this reaction?
(d)
What happens to equilibrium position of this reaction
if NH3 is removed form the reaction vessel form time to time.
See Section 8.2.1
Q16. Sulphuric acid is the king of chemicals. It is produced by the
burning of SO2 to SO3 through an exothermic reversible
process.
(a)
Write the balanced reversible reaction
(b)
What is the effect of pressure change on this reaction?
(c)
Reaction is exothermic but still the temperature of
o
400-500 C is required to increase the yield of SO3. Give
reasons.
See Section 8.2.2
Q17. (a)
What are buffer solutions? Why do we need them in
daily life?
See Section 8.2
(b)
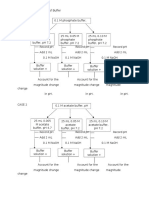
How does the mixture of sodium acetate and acetic acid
give us the acidic buffer?
See Section 8.7
(c)
Explain that a mixture of NH4OH and NH4CI gives us
the basic buffer?
See Section 8.7
ww
w.a
llon
line
fre
e.c
om
(iii)
(iv)
www.allonlinefree.com
1st year chemistry n0tes new
How do you justify that the greater quantity of
CH3COONa in acetic acid decrease the dissociating
power of acetic acid and so the pH increases.
CH3COOH is a weak acid and ionizes very small, while
CH3COONa is a strong electrolyte and it ionizes in water to
greater extent and provides acetate ions.
CH3COOH +H2O
CH3COO- + H3O+
CH3COONa
CH3COO- + Na+
Thus CH3COONa decreases the ionization of
CH3COOH due to common CH3COO- ion and pH of solution
increases.
(d)
Explain the term buffer capacity.
See Section 8.7.1
Q18. (a)
What is the solubility product? Derive the
solubility product expression for sparingly soluble compounds,
AgCI, Ag2CrO4 and PbCI2.
See Section 8.8
(b)
How do you determine the solubility product of a
substance when its solubility is provided in grams/100 g
of water?
See Section 8.8
(c)
How do you calculate the solubility of a substance from
the value of solubility product.
See Section 8.8
Q19. Kc value for volume for the following reaction is 0.076 at
520oC
2HI
H2
+
I2
Equilibrium mixture constants [HI] =0.08 M, [H2] =0.01 M.
To this mixture more HI is added so that its new concentration is
0.096 M. what will be the concentration of [HI], [H2] and [I2] when
equilibrium is re-established.
Solution
2HI
H2
+
I2
ww
w.a
llon
line
fre
e.c
om
(d)
www.allonlinefree.com
1st year chemistry n0tes new
Equilibrium conc.
0.08
0.01
0.01
0.096
0.01
0.01
0.096 2x
0.01
0.01
(mol/dm3)
Initial conc.
After adding more HI
Equilibrium conc.
When equilibrium is re-established
(mol/dm3)
e.c
According to law of mass action
Kc =
om
(mol/dm3)
=0.016
fre
Kc =
=0.016
line
llon
Taking square root on both sides
=0.126
ww
w.a
0.01 + x=0.126 (0.096 2x)
0.01 + x =0.0121 0.252 x
x + 0.252 x=0.0121 0.01
1.252 x=0.0021
x ==
x=0.00168 mol/dm-3
Thus
Concentrations when equilibrium is re-established are
[H2] =0.01 + x =0.01 +0.00168
=0.01168 mol dm-3
[I2] =0.01 + x =0.01 +0.00168
=0.01168 mol dm-3
[HI] =0.096 - 2 x =0.096 -2x0.00168 =0.0926 mol dm-3
Q20. The equilibrium constant for the reaction between acetic and
ethyl alcohol is 4. A mixture of 3 moles of acetic acid and one
10
www.allonlinefree.com
1st year chemistry n0tes new
om
mole of C2H5OH is allowed to come to equilibrium. Calculate
the amount of ethyl acetate at equilibrium stage in number of
moles and grams. Also, calculate the masses of reactants left
behind.
Solution
CH3COOH + C2H5OH
CH3 COOC2H5 +H2O
Initial conc.
3
1
0
0
(mol/dm3)
1x
3- x
e.c
Equilibrium conc.
(mol/dm3)
fre
According to law of mass action
Kc=
line
Kc=
ww
w.a
llon
X2 =4(3 x) (1 x)
X2 =4(3 3x) (1 x)
X2 =4(3 4x + x2)
X2 =12 16x + 4 x2)
Or
12 16x + 4x2 x2 =0
3x2 16x + 12=0
It is quadratic equation and can be solved by using quadric
formula
Here
a=3 ,
b = 12
,
c = 12
Thus
x=
x=
x=
x=
11
www.allonlinefree.com
1st year chemistry n0tes new
x=
Either x=
or
x=
(b)
Solution
ww
w.a
llon
line
fre
e.c
om
x= 4.43 mol dm-3
or
x= 0.9 mol dm-3
x= 4.43 is not possible as it is greater than the initial
concentrations of reactants, therefore , x= 0.9 mol dm-3
Therefore
Moles of ethyl acetate =x=0.9 moles
Mass of ethyl acetate = 0.9x88=79.46g.
Moles of water =x=0.9 moles
Mass of water =0.9 x 18=16.2 g.
Moles of acetic acid = 3 x =3 0.9 =2.1 moles
(left behind)
Moles of ethyl alcohol = 1 x =1 0.9= 0.1 moles
(Left behind)
Mass of ethyl alcohol =0.1 x 46=4.6g.
(left behind)
Q21. study the equilibrium
H2O + CO
H2 + CO2
(a)
Write an expression of Kp
Kp=
When 1 mole of steam and 1 mole of CO are allowed to
reach equilibrium, 33.3% of the equilibrium mixture is
hydrogen. Calculate the value of Kp. state the units of Kp.
Initial conc.
H2O +
1
CO
1
H2 + CO2
0
0
1x
1x
(mol/dm3)
Equilibrium conc.
(mol/dm3)
Total no. of moles at equilibrium =1 x + 1 x + x + x=2
Hence
12
www.allonlinefree.com
1st year chemistry n0tes new
% of H2 =
33.3=
or
no of moles of H2
om
Hence
=0.67 moles
line
fre
e.c
At equilibrium
Moles of H2 =x=0.67 moles
Moles of CO2 =x=0.67 moles
Moles of H2O =1 x = 1 - 0.67= 0.33 moles
Moles of CO =1 x = 1 - 0.67= 0.33 moles
Hence, Kc is given as
Kc =
Kc =
llon
Since Kp =Kc (RT) and n =n products -n rectants =0, therefore
Kp=Kc=4
ww
w.a
Q22. Calculate the pH of
(a)
10-4 moles/ dm3 of HCI
HCI ionizes as
HCI
H+ + CISince HCI is a strong acid, and it is 100% dissociated. Hence 104
mol/dm3 of HCI produces 10-4 mol/dm3 of H+ ions.
Thus
[H+] = 10-4 mol/dm3
So
pH = - log [H+]
pH = - log [10-4]
pH = 4
(b)
10-4 moles/dm3 of Ba(OH)2
Ba(OH)2 ionizes as
Ba(OH)2
Ba+2 + 2 OH13
www.allonlinefree.com
1st year chemistry n0tes new
ww
w.a
llon
line
fre
e.c
om
Since Ba(OH)2 is a strong base it is 100% dissociated.
Hence 10-4 mol/dm3 of Ba(OH)2 produces 2 x 10-4 mol/dm3 of OH- ions.
Thus
[OH-]= 2 x 10-4 mol/dm3
So
pOH= - log[OH-]
pOH= - log[2 x 10-4]
pOH= 3.699
Since
pH + pOH=14
Therefore
pH= 14 pOH
=14 3.699 =10.301
(c)
1 mol/dm3 of H2X, which is 50% dissociated
H2X ionizes as
H2X
2H+ + X1mole of H2X produces 2 moles of H+ ions if 100% dissociated
However, since H2X is 50% dissociated therefore 1 mole of H2X
produces 1 mole of H+ ion
Thus
[H+]=1 mol/dm3
So
pH= -log [H+]
pH= -log [1]
pH= 0
(d)
1 mol/dm3 of NH4 OH which is 1% dissociated
NH4 OH ionizes as
NH4OH
NH4+ + OHIt shows that 1 mole of NH4OH produces 1 mole of OHions.
NH4OH is only 1% dissociated
Hence
14
www.allonlinefree.com
1st year chemistry n0tes new
% dissociation =
x 100
mol of OH-
x1=0.01 mol/dm3
om
1=
x 100
Thus
[OH-] =0.01 mol/dm3
Since
fre
pOH = - log [OH-]
pOH = - log [0.01]
pOH = 2
e.c
So
ww
w.a
llon
line
pH + pOH =14
Therefore
pH=14 pOH
=14 2=12
Q23. (a)
Benzoic aicd C6H5COOH is weak mono-basic acid
(Ka=6.4 x10-5mol/dm3). What is the pH of a solution
containing 7.2 g of sodium benzoate in one dm3 of o.02
mol/dm3 benzoic acid.
Mass of sodium benzoate
=7.2 g /dm3
Formula
of
sodium
benzoate
is
C6H5COONa
Mol. Mass of sodium benzoate
=144 g/mol
Moles of sodium benzoate
= =0.05 mol/dm3
Moles of benzoic acid
=0.02 mol/dm3
Ka of benzoic acid
6.4 x 10-5 mol/dm3
Thus
pKa = - log Ka= - log (6.4 x10-5 ) =4.2
Hence, according to Hendersons eq.
15
www.allonlinefree.com
1st year chemistry n0tes new
pH =pKa + log
pH =4.2 + log
or
llon
line
fre
e.c
om
pH = 4.2 + 0.39
pH =4.59
(b)
A buffer solution has been prepared by mixing 0.2 M
CH3COONa and 0.5 M CH3 COOH in 1 dm3 of solution.
calculate the pH of solution. pKa of acid is 4.74 at 25oC.
How the value of pH will change by adding 0.1 mole 0.1
mole of NaOH and 0.1 mole mol HCI respectively.
Solution
[CH3 COOH]
=0.5 M
[CH3 COONa]
=0.2 M
pKa of CH3 COOH
=4.74
pH
=?
Since pH =pKa + log
pH =pKa + log
ww
w.a
pH =4.74 + log
pH =4.74 - 0.4
pH =4.34
When 0.1 mole of NaOH is added
NaOH is strong base. It dissociates completely. Therefore, it
produces 0.1 moles of OH- ions. Thus, 0.1 moles of OH- ions
reacts with 0.1 moles of CH3 COOH. Hence, out of 0.5 moles of
CH3 COOH, 0.4 moles of CH3 COOH are behind.
On the other hand, due to slat formed by the neutralization
reaction, conc. of salt (CH3 COONa) is increased from 0.2 moles
to 0.3 moles.
Hence, new conc. will be
16
www.allonlinefree.com
1st year chemistry n0tes new
COONa]
Thus
[CH3 COOH]
=0.3M
pH =pKa + log
=0.4 M
[CH3
om
pH =4.74 + log
llon
line
fre
e.c
pH =4.74 - 0.12
pH =4.62
Addition of 0.1 mole of HCI
HCI is a strong acid. It dissociates completely. Therefore, it
produces 0.1 moles of H+ ions. Thus, 0.1 moles of H+ ions react
with 0.1 moles of CH3COO- ions. Hence, out of 0.2 moles of salt
(CH3COO-Na+), 0.1 moles of salt are left behind.
On the other hand, conc. of acid (CH3COOH) is increased
form 0.5 moles to 0.6 moles.
Hence, new conc. will be
[CH3 COOH]
=0.6 M
[CH3
COONa]
=0.1M
Thus pH =pKa + log
ww
w.a
pH =4.74 + log
pH =4.74 - 0.78
pH =3.96
(See Section 8.7.1 for complete understanding of this numerical)
Q24. Solubility of CaF2 in water at 25OC is found to be 2.05 x 10-4
mol dm-3. What is the value of Ksp at this T.
Solubility of CaF2 = 2.05 x 10-4 mol dm-3
According to balanced chemical eq.
CaF2(aq)
Ca2+(aq) + 2F-(aq)
At initial stage 2.05 x 10-4
0
0
3
(mol/dm )
After solubility 0
2.05 x 10-4 2x2.05 x 10-4
Hence Ksp=[Ca+2][F-]
17
www.allonlinefree.com
1st year chemistry n0tes new
e.c
om
Ksp=[0.05 x 10-4][2 x 2.05 x 10-4]2
Ksp=3.446 x 10-11 mol3 dm-9
Q25. The solubility product of Ag2CrO4 is 2.6 x 120-2 at 25oC.
Calculate the solubility of the compound.
Ksp of Ag2 CrO4 =2.6 x 10-2
We know
Ag2CrO4(aq)
2Ag+
CrO42-(aq)
Initial stage (mol/dm3)
Ag2CrO4
0
0
At equilibrium (mol/dm3)
Ag2CrO4
2S
S
line
Ksp =[Ag+]2[CrO42-]
Ksp=[2S]2[S] =2.6 x 10-2
4[S]3 =2.6 x 10-2
fre
Hence
[S]=
ww
w.a
llon
or
[S]=0.1866 mol/dm3
Hence at equilibrium
[Ag+] =2 x 0.1866 mol/dm3 =0.3732 mol/dm3
and
[CrO42- ]
=0.1866 mol/dm3
Since
1 mole of Ag2CrO4 gives 1 mole of CrO42-ions, hence
Solubility of Ag2CrO4=[CrO42-]=0.1866 mol/dm3
18
www.allonlinefree.com
S-ar putea să vă placă și
- Dissolution of Benzoic AcidDocument2 paginiDissolution of Benzoic AcidAngela Calatayud100% (1)
- HWK 5Document1 paginăHWK 5Kelly SmithÎncă nu există evaluări
- Limiting Reactants and Percent Yield WorksheetDocument2 paginiLimiting Reactants and Percent Yield Worksheetanj pianoÎncă nu există evaluări
- Chem 102 FinalDocument12 paginiChem 102 FinalAlex GampelÎncă nu există evaluări
- Enthalpy PogilDocument4 paginiEnthalpy Pogilapi-213793181Încă nu există evaluări
- Chemistry 6821: General Certificate of Education June 2003 Advanced Extension AwardDocument10 paginiChemistry 6821: General Certificate of Education June 2003 Advanced Extension AwardDaniel ConwayÎncă nu există evaluări
- Equilibrium With Fried Eggs of PbI2-1Document2 paginiEquilibrium With Fried Eggs of PbI2-1Joel Tr100% (1)
- Chem162 SpectSeries Report 111314Document4 paginiChem162 SpectSeries Report 111314charwill12340% (4)
- Chemistry 20L Practice Final.Document13 paginiChemistry 20L Practice Final.Bahjat Irani100% (1)
- BT HPTDocument31 paginiBT HPTLinh NguyenÎncă nu există evaluări
- HW 4Document2 paginiHW 4kimyakimya89Încă nu există evaluări
- UNIFAC - Smith, VanNess, Abbott PDFDocument7 paginiUNIFAC - Smith, VanNess, Abbott PDFAlejandra InsuastyÎncă nu există evaluări
- 8 e 1 3 Stations Water QualityDocument5 pagini8 e 1 3 Stations Water Qualityapi-2930845090% (1)
- Determination of Phosphoric Acid Content in Cola BeveragesDocument13 paginiDetermination of Phosphoric Acid Content in Cola BeveragesNicole Pavlichich100% (1)
- Module 6 Chemistry NotesDocument19 paginiModule 6 Chemistry NotesspamÎncă nu există evaluări
- Sample QuestionsDocument3 paginiSample QuestionsBrai'Lly COncordiaÎncă nu există evaluări
- Chemical Equlibria Test-1Document4 paginiChemical Equlibria Test-1newlifelabsÎncă nu există evaluări
- CHE 1000 Tutorial Sheet 11-Acid Base Equilibrium, Buffers and SolubilityDocument4 paginiCHE 1000 Tutorial Sheet 11-Acid Base Equilibrium, Buffers and SolubilityReagan HamweembaÎncă nu există evaluări
- Chapter 8 Practice Test Answers 4u1Document1 paginăChapter 8 Practice Test Answers 4u1helloblargÎncă nu există evaluări
- Taller de GasesDocument20 paginiTaller de GasesAle Cruz DÎncă nu există evaluări
- 4.4, 4.5 Exam Questions MsDocument9 pagini4.4, 4.5 Exam Questions MsswanderfeildÎncă nu există evaluări
- Calculate The Vapor Pressure at 25C of An Aqueous Solution That Is 5Document7 paginiCalculate The Vapor Pressure at 25C of An Aqueous Solution That Is 5Charsea ReighÎncă nu există evaluări
- Chemistry 6821: General Certificate of Education June 2004 Advanced Extension AwardDocument16 paginiChemistry 6821: General Certificate of Education June 2004 Advanced Extension AwardQuach Pham Thuy TrangÎncă nu există evaluări
- TruechemDocument10 paginiTruechemaÎncă nu există evaluări
- Chapter 7 Practice TestDocument2 paginiChapter 7 Practice TesthelloblargÎncă nu există evaluări
- Fill in The Table Below:: Empirical Formula WorksheetDocument2 paginiFill in The Table Below:: Empirical Formula WorksheetSherida GibbsÎncă nu există evaluări
- Physical Chemistry 1 Prob SetDocument8 paginiPhysical Chemistry 1 Prob SetArrianne Jaye MataÎncă nu există evaluări
- Chapter 20.0-Polymer Soalan (74-76)Document5 paginiChapter 20.0-Polymer Soalan (74-76)ChemMatrikÎncă nu există evaluări
- 102 MSJC 13Document11 pagini102 MSJC 13noelÎncă nu există evaluări
- Chemistry Level M Couse Question Document PDFDocument46 paginiChemistry Level M Couse Question Document PDFJoe ToubiaÎncă nu există evaluări
- Unit Practice Test: Gas Laws: Multiple ChoiceDocument8 paginiUnit Practice Test: Gas Laws: Multiple Choiceanj pianoÎncă nu există evaluări
- Moles 2Document15 paginiMoles 2yvg95Încă nu există evaluări
- Homework 2: U KQ Where:u Btu H FT Q FT H K ConstantDocument6 paginiHomework 2: U KQ Where:u Btu H FT Q FT H K ConstantkatelynÎncă nu există evaluări
- Chapter 3 Cre MCQDocument10 paginiChapter 3 Cre MCQRohit Ramesh KaleÎncă nu există evaluări
- Chem Olympiad 2019 Exam Paper AnswersDocument9 paginiChem Olympiad 2019 Exam Paper AnswersPaulette LaurenteÎncă nu există evaluări
- Chemical Kinetics Methodology, RDRDocument7 paginiChemical Kinetics Methodology, RDRKhayzel MelanoÎncă nu există evaluări
- Chemistry EUEE 2013 (14) - 151269132054Document12 paginiChemistry EUEE 2013 (14) - 151269132054mintesnot udessa100% (1)
- Aea Chem SpmsDocument23 paginiAea Chem Spmssdd104Încă nu există evaluări
- Test Bank GasesDocument38 paginiTest Bank GasesMohammed AhmedÎncă nu există evaluări
- 2011 Enthalpy Tutorial (With Ans)Document11 pagini2011 Enthalpy Tutorial (With Ans)kahwahcheong100% (1)
- Class XI Assignment States of MatterDocument2 paginiClass XI Assignment States of MatterCheryl ChaudhariÎncă nu există evaluări
- PLTL Ch. 16 AssignmentDocument6 paginiPLTL Ch. 16 AssignmentJules BrunoÎncă nu există evaluări
- Inorganic Chemistry: Period 3 ElementsDocument5 paginiInorganic Chemistry: Period 3 ElementsUng Hie HuongÎncă nu există evaluări
- Chemistry Urt ExamDocument9 paginiChemistry Urt ExamAmira AbdallahÎncă nu există evaluări
- Tutorial + Solutions 27 August 2010Document2 paginiTutorial + Solutions 27 August 2010Jailene Gómez CollazoÎncă nu există evaluări
- Physics em Class 10 PSR Digital BooksDocument86 paginiPhysics em Class 10 PSR Digital BooksrajeshÎncă nu există evaluări
- Tutorial 3 & 4 - Equilibria & Application of Rates and EquilibriumDocument5 paginiTutorial 3 & 4 - Equilibria & Application of Rates and EquilibriumAhmad Taufiq Mohd ZaidÎncă nu există evaluări
- Microsoft Word - CH 12 Worksheet 2-2 - DocDocument7 paginiMicrosoft Word - CH 12 Worksheet 2-2 - DocAhmad RezaÎncă nu există evaluări
- Chapter 8 Practice Test 4u1Document1 paginăChapter 8 Practice Test 4u1helloblargÎncă nu există evaluări
- Ch123 Exam II Practice Exam Spring2011Document7 paginiCh123 Exam II Practice Exam Spring2011christopher92530% (1)
- Assignment 151Document5 paginiAssignment 151Hai Xuan DoÎncă nu există evaluări
- Rate LawsDocument20 paginiRate LawsReginal MoralesÎncă nu există evaluări
- CBSE Class 12 Chemistry Coordination Compounds PDFDocument2 paginiCBSE Class 12 Chemistry Coordination Compounds PDFDeepa PaulÎncă nu există evaluări
- Problems ElectrochemistryDocument11 paginiProblems ElectrochemistryorlandompsilvaÎncă nu există evaluări
- Practice Question For Second Term 111 1Document18 paginiPractice Question For Second Term 111 1Ramina TamangÎncă nu există evaluări
- CBSE Class 12 Chemistry Sample Paper Solution Set 1Document9 paginiCBSE Class 12 Chemistry Sample Paper Solution Set 1Sidharth SabharwalÎncă nu există evaluări
- DrVince G10 Physics AFontDocument223 paginiDrVince G10 Physics AFontsayakolinn maungÎncă nu există evaluări
- Tutorial 1 SolutionsDocument20 paginiTutorial 1 Solutionsanushka shagunÎncă nu există evaluări
- Chemistry - Mccord - Exam 1Document9 paginiChemistry - Mccord - Exam 1Miguel MartinezÎncă nu există evaluări
- CH 6 PracticeDocument11 paginiCH 6 PracticeMichel zakhariaÎncă nu există evaluări
- Energetics - SL - 01: (Total 1 Mark)Document20 paginiEnergetics - SL - 01: (Total 1 Mark)Abhinaya PolakaÎncă nu există evaluări
- NCERT Exemplar Class 11 Chemistry Chapter 7 EquilibriumDocument19 paginiNCERT Exemplar Class 11 Chemistry Chapter 7 EquilibriumAnidhya TiwariÎncă nu există evaluări
- Chem Sample PaperDocument4 paginiChem Sample PaperridahÎncă nu există evaluări
- 9th Class Chemistry Important Solved Short Notes For Exam 2013Document34 pagini9th Class Chemistry Important Solved Short Notes For Exam 2013HAFIAZ MUHAMMAD IMTIAZ100% (4)
- NTS TestDocument4 paginiNTS TestSajid AzeemÎncă nu există evaluări
- Chapter No 03 9th Class NotesDocument6 paginiChapter No 03 9th Class NotesSajid AzeemÎncă nu există evaluări
- Chapter No 04 9th Class NotesDocument5 paginiChapter No 04 9th Class NotesSajid AzeemÎncă nu există evaluări
- English L T Iqbalkalmati Blogspot Com PDFDocument41 paginiEnglish L T Iqbalkalmati Blogspot Com PDFfatima100% (1)
- Tick The Correct OptionDocument2 paginiTick The Correct OptionSajid AzeemÎncă nu există evaluări
- 6 Chapter Chemical Bonding Text Book ExerciseDocument20 pagini6 Chapter Chemical Bonding Text Book ExerciseSajid Azeem100% (2)
- Tick The Correct OptionDocument2 paginiTick The Correct OptionSajid AzeemÎncă nu există evaluări
- 9 Math Unit 5Document30 pagini9 Math Unit 5Sajid AzeemÎncă nu există evaluări
- 1 ChapteR Short Question With AnswerDocument16 pagini1 ChapteR Short Question With AnswerSajid AzeemÎncă nu există evaluări
- 4 Chapterliquids and Solids Short Question With AnswersDocument18 pagini4 Chapterliquids and Solids Short Question With AnswersSajid AzeemÎncă nu există evaluări
- WebsiteDocument1 paginăWebsiteSajid AzeemÎncă nu există evaluări
- 11 Chapter Reaction Kinetics Text Book ExerciseDocument14 pagini11 Chapter Reaction Kinetics Text Book ExerciseSajid AzeemÎncă nu există evaluări
- 10 Chapter Electrochemistry Text Book ExerciseDocument31 pagini10 Chapter Electrochemistry Text Book ExerciseSajid AzeemÎncă nu există evaluări
- Short Questions: Physics For 10 Class (Unit # 10)Document13 paginiShort Questions: Physics For 10 Class (Unit # 10)sajidazeemÎncă nu există evaluări
- 1 ChapteR Short Question With AnswerDocument16 pagini1 ChapteR Short Question With AnswerSajid AzeemÎncă nu există evaluări
- 1ST Chapter Text Book QuestionsDocument20 pagini1ST Chapter Text Book Questionsfaysal8080Încă nu există evaluări
- Short Questions: Physics For 10 Class (Unit # 10)Document13 paginiShort Questions: Physics For 10 Class (Unit # 10)sajidazeemÎncă nu există evaluări
- 10h Class SummaryDocument1 pagină10h Class Summarysajidazeem100% (1)
- 10 Phy Unit 10 NewDocument13 pagini10 Phy Unit 10 NewsajidazeemÎncă nu există evaluări
- 10 Phy Unit 10 NewDocument13 pagini10 Phy Unit 10 NewsajidazeemÎncă nu există evaluări
- Hanna Instruments Catalog v30Document772 paginiHanna Instruments Catalog v30Aqua Technology GroupÎncă nu există evaluări
- Q2 SHS - PT - Gen CHEM 2 - 2022 - 23Document8 paginiQ2 SHS - PT - Gen CHEM 2 - 2022 - 23Diane GuilaranÎncă nu există evaluări
- Metalworking Brochure HuntsmanDocument58 paginiMetalworking Brochure Huntsman12bisiÎncă nu există evaluări
- Chlorine, Free: Usepa DPD Method Method 10245 0.05 To 4.00 MG/L CL (MR) Powder PillowsDocument6 paginiChlorine, Free: Usepa DPD Method Method 10245 0.05 To 4.00 MG/L CL (MR) Powder PillowsClydeA.Sardoncillo100% (1)
- Step7 Corrosion GuideDocument18 paginiStep7 Corrosion GuideGuha ArnabÎncă nu există evaluări
- Pka ConceptsDocument16 paginiPka ConceptsGasper Fernandes100% (1)
- Lesson in Acid and BasesDocument5 paginiLesson in Acid and BasesmarcÎncă nu există evaluări
- TBL Spec 56206 P 1426009Document2 paginiTBL Spec 56206 P 1426009Banty BantyÎncă nu există evaluări
- Du-Zinc 019Document7 paginiDu-Zinc 019KaRenthLuNaÎncă nu există evaluări
- Inspection of Quality Control Laboratories PDFDocument18 paginiInspection of Quality Control Laboratories PDFHamid HamidÎncă nu există evaluări
- Electrochem Reduction of 1-Nitroalkenes To Amine Success! - )Document9 paginiElectrochem Reduction of 1-Nitroalkenes To Amine Success! - )electrosioux100% (1)
- The Water-Quality Map: Nick StaresinicDocument15 paginiThe Water-Quality Map: Nick StaresinicJaan LiÎncă nu există evaluări
- ComprDocument41 paginiComprAllBetaÎncă nu există evaluări
- Critical Reviews in Food Science and NutritionDocument11 paginiCritical Reviews in Food Science and NutritionJuan Guillermo Morales SaldarriagaÎncă nu există evaluări
- Degradation of A Cationic Dye (Rhodamine 6G) Using Hydrodynamic CavitationDocument45 paginiDegradation of A Cationic Dye (Rhodamine 6G) Using Hydrodynamic CavitationThành Định LêÎncă nu există evaluări
- Patent Number Document Id Date Published: Process For Preparing Organic Fluorine CompoundsDocument6 paginiPatent Number Document Id Date Published: Process For Preparing Organic Fluorine CompoundsJyot VakhariaÎncă nu există evaluări
- SchematicDocument4 paginiSchematicCai PascualÎncă nu există evaluări
- PHS 3E Manual 20190418Document16 paginiPHS 3E Manual 20190418Teguh_Yudha_Adi_WijaÎncă nu există evaluări
- Acid Bases SaltDocument4 paginiAcid Bases SaltPhạm Quốc HảiÎncă nu există evaluări
- 8 Chapter Chemical Equilibrium Text Book ExerciseDocument18 pagini8 Chapter Chemical Equilibrium Text Book ExerciseSajid Azeem0% (1)
- Preparation of Aluminium OxideDocument21 paginiPreparation of Aluminium OxideSarath Jose K100% (1)
- Nutrient Management in Recirculating Hydroponic Culture: On What We Want The Plant To Take UpDocument14 paginiNutrient Management in Recirculating Hydroponic Culture: On What We Want The Plant To Take UpAnthony ContrerasÎncă nu există evaluări
- Acid and Alkali: 6.1 Properties of Acids and AlkalisDocument2 paginiAcid and Alkali: 6.1 Properties of Acids and AlkalisKalesware MuniandyÎncă nu există evaluări
- Equilibriumchemistry Cheat SheetDocument2 paginiEquilibriumchemistry Cheat SheetShradha SharmaÎncă nu există evaluări
- H Chemistry All 2012Document40 paginiH Chemistry All 2012Ross TaylorÎncă nu există evaluări
- K-Means Algorithm Grouping For Visualizing Potential of Hydrogen (PH) Data in Kali Lamong RiverDocument7 paginiK-Means Algorithm Grouping For Visualizing Potential of Hydrogen (PH) Data in Kali Lamong RiverachmadniamÎncă nu există evaluări
- Acids and BasesDocument18 paginiAcids and BasesSunnyMoon21Încă nu există evaluări