Documente Academic
Documente Profesional
Documente Cultură
Swelling Pressure Calculated From Mineralogical Properties
Încărcat de
Nabucodonosor1980Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Swelling Pressure Calculated From Mineralogical Properties
Încărcat de
Nabucodonosor1980Drepturi de autor:
Formate disponibile
Clays and Clay Minerals, Vol. 33, No. 6, 501-509, 1985.
SWELLING PRESSURE CALCULATED FROM
MINERALOGICAL PROPERTIES OF A
JURASSIC OPALINUM SHALE, SWITZERLAND
FRITZ T. MADSEN AND MAX MLrLLER-VONMOOS
Institute of Foundation Engineering and Soil Mechanics
Laboratory for Clay Mineralogy, Federal Institute of Technology
CH-8093, Ziirich, Switzerland
Abstract--Nineteen drill core samples of lower Dogger opalinum shale from wells drilled in connection
with a tunnel project near Brugg, northern Switzerland, were investigated. The shale is a well known
swelling rock that has caused problems in underground construction work. Swelling pressures determined
under constant volume conditions to obtain maximum values were 0.7 to 2.2 N/ram 2. The samples
contained 37-59% clay-size material and about 35% quartz, 7-18% carbonate minerals, and about 5%
feldspar, pyrite, and organic matter. In addition to kaolinite, illite, and chlorite, the clay-size fraction also
contained mixed-layer illite/smectite with about 30% swelling layers.The specific surface area of the clay
fraction was 135 m2/g. The specific surface charge of the clay (6.7 x 104 esu/cm2), the ion concentration
in the pore fluid of the specimen after the swelling test (10-2 mmole/cm3), the valence of the ions in the
double layer of the clay particles (+ 1), and the half distance between the clay plates in the specimen (815/~) allowed the calculation of the swelling pressure for each sample according to the Gouy double layer
theory.
The mean value of the calculated swelling pressures was found to be of the same order of magnitude
as the measured values, indicating that the technique can be used where cylindrical or rectangular specimens are not available for direct measurement.
Key Words--Gouy double layer theory, Mineralogical composition, Shale, Soil mechanics, Surface area,
Surface charge, Swelling pressure.
INTRODUCTION
In recent years m a n y Swiss railway tunnels have had
to be repaired due to damage caused by swelling rocks.
I n new highway tunnels swelling rocks have also caused
great difficulties (Grob, 1976; Einstein, 1979). The rock
m o v e m e n t in the tunnels is mostly in the form of heave
of the floor, followed by a convergence of the abutments. From laboratory tests and in-situ observations
it is known that the prevention of swelling strains induces considerable compressive stresses, the so-called
swelling pressure. Swiss rocks particularly susceptible
to swelling are Tertiary marlstones (molasse) which
contain ~30% montmorillonite in the clay fraction,
the Triassic Keuper shale which contains corrensite
and anhydrite, and a lower Dogger opalinum shale
(named after the ammonite Leioceras opalinum) containing mixed-layer minerals illite/montmorillonite.
In connection with a new tunnel project near Brugg
in northern Switzerland, the swelling pressure of 19
samples o f o p a l i n u m shale was investigated. The measurements were made on drill cores from between 15and 155-m depth. The variation of the results could
not be explained on the basis of sample depths, and it
was assumed that differences in the mineralogical composition could be the reason for the different swelling
pressures. Therefore, the mineralogical composition of
the specimens was investigated to allow the calculation
Copyright 9 1985 The Clay MineralsSociety
of the swelling pressure from mineralogical parameters
according to the Gouy double layer theory.
SWELLING PRESSURE T H E O R Y
In clay minerals, the electric double layer consists of
a negative surface charge and a compensating counterion charge, which is accumulated in the liquid near the
surface of the clay particle. The positive counter-ions
are electrostatically attracted by the negative charged
surface. The concentration of the positive ions near the
surface is high, and it decreases with increasing distance
from the surface. Simultaneously, a deficiency of negative ions exists near the surface, inasmuch as these
ions are electrostatically repelled by the clay particle.
The distribution of the ions as a function of the distance
from the clay surface can be calculated according to
the electrostatic and diffusion theory using thb PoissonBoltzmann equation (Gouy, 1910, 1917; Chapman,
1913).
According to the double layer theory (Verwey and
Overbeek, 1948; Bolt, 1955, 1956; van Olphen, 1977),
the interacting force between two double layers can be
derived from the ion concentration midway between
the clay surfaces, which is determined by the value of
the electric potential. The repulsive pressure (Langmuir, 1938) is given by the osmotic pressure midway
between the surfaces with respect to that of the equi-
501
502
Clays and Clay Minerals
Madsen and Miiller-Vonmoos
l i b r i u m solution. Because these o s m o t i c pressures are
d e t e r m i n e d by the ion concentration, the pressure can
be calculated directly f r o m the excess c o n c e n t r a t i o n
m i d w a y between the clay surfaces. T h i s excess concentration is equal to
n(exp(u) - 1) + n ( e x p ( - u ) - 1) = 2n(cosh u -
1)
d2~ _ 81rneu sinh(ve~/kT),
dx 2
e
which describes the v a r i a t i o n o f the field strength as a
hyperbofic function o f the potential. Substituting y =
8ornery z .
ue~b/kT, z = ue~b0/kT, ~ = Xx, and x 2 - - gaves
ekT
and the pressure is
d2y
-
p = 2nkT(cosh u -
1),
n+ = n+* exp(-p+eff/kT) and
(2)
n_ = n_* exp(v_e~b/kT),
in which n+ and n = local concentrations o f p o s i t i v e
and negative ions, n+* and n_* = concentrations o f positive and negative ions far away f r o m the surface (potential = 0), u+ and p_ = valences o f the ions, e = elem e n t a r y charge, and ~k = electric potential. T h e local
density o f charge p in a distance x is
p = v+en+ - u_en_.
Because the v a l e n c e o f the ions o f the s a m e sign as
the surface charge is n o t v e r y i m p o r t a n t , u+ is a s s u m e d
to equal ~_; thus, n+* = n_* = n. Then,
0
ue(n+
n_).
(3)
C o m b i n i n g Eqs. (2) and (3) gives
0 = ( - 2 n v e ) s i n h (ve~/kT),
(4)
which describes the local density o f charge as a hyperbolic function o f the potential. T h e local density o f
charge and the local electric potential are also related
by the Poisson e q u a t i o n
d2~b
dx 2
41r0
--,
r
d( ~
(1)
where p = repulsive pressure, n = ion concentration far
f r o m the clay surface, u = electric potential m i d w a y
between the clay surfaces, k = B o l t z m a n n constant,
and T = absolute temperature.
T h e ion c o n c e n t r a t i o n n, which here is equal to the
ion c o n c e n t r a t i o n in the p o r e fluid o f the swelling speci m e n , and the t e m p e r a t u r e T can be easily determined.
T h e electric potential u c a n n o t be m e a s u r e d directly,
but m u s t be calculated according to the double layer
theory as follows: F o r e q u i l i b r i u m c o n d i t i o n s in the
double layer, the average local c o n c e n t r a t i o n o f positive and negative ions at a distance x f r o m the clay
surface can be expressed as a f u n c t i o n o f the average
electric potential at that distance according to Boltzm a n n ' s theorem:
(5)
which describes the v a r i a t i o n o f the field strength as a
function o f the local density o f charge, e is the dielectric
constant o f the m e d i u m (water). C o m b i n i n g Eqs. (4)
a n d (5), the f u n d a m e n t a l e q u a t i o n for the d o u b l e layer
is obtained,
(6)
sinh y,
(ra)
where ~b0 = surface potential. In the m i d w a y plane between two clay surfaces, at a distance d f r o m either
surface, the field strength is zero. T h e potential m i d w a y
between the surfaces is ~ba, and u = ue~ba/kT.
Integrating Eq. (6a) once with the b o u n d a r y condition that for x = d, y = u a n d dy = 0 gives
d y = - ( 2 cosh y - 2 cosh u) ~
d~
(7)
T h e second integration between the limits z and u for
y or 0 and d for x gives
yz
(2 cosh y - 2 cosh u) -~ dy = -
yo
d~ = - x d .
Evaluating this elliptic integral using tables (Jahnke
and E m d e , 1933) gives
(;)
x d = 2 exp -
+o u, ar=ex0(
where the functions F~ and F : are o b t a i n e d using the
tables.
T h e charge o f the clay particle is a result o f cation
substitutions in the crystal. Therefore, the surface charge
o f the particle is a constant. Thus, the potential m i d w a y
between the clay surfaces u is a function o f the surface
potential z and the h a l f distance d and z is a f u n c t i o n
o f the surface charge D a n d d.
T h e surface charge D is given by
D = -
yo
p dx
and according to Eq. (5)
dff
As y = ve~k/kT and ~ = Xx, Eq. (9) is t r a n s f o r m e d to
\ 27r ]
\d~]o"
Vol. 33, No. 6, 1 9 8 5
Calculation of swelling pressure from mineralogical properties
Combining Eqs. (9a) and (7) gives as y = z for ~ = 0
D=\
('nkT~ ~
21r ] ( 2 c o s h z -
2cosh
u)O.5
.
Therefore, for a constant charge,
(2 cosh z - 2 cosh u)~
~ = constant
independent o f the distance between the clay surfaces.
To calculate the change o f the midway potential u with
varying distance 2d at a given electrolyte concentration
n, X sets o f values for z and u are required for which
(2 c o s h z -
2 cosh 0) 0.5= D ( 27r ~o.5= constant.
\cnkT/
Knowing the values o f surface charge D, ion concentration n, valence v, temperature T, and dielectric constant ~, it is possible to calculate the midway potential
u as a function o f the particle distance, and also to
calculate the repulsive pressure p as a function o f the
particle distance.
The steps o f the calculation are:
(8a-ne~ ~
~kT ]
(1)
(2)
(dye,
\-~]o
according to
d(~)
47revD which is obo = ekTx
tained from Eq. (9a) with n = x2*kT"
8~re2v2'
(3) z f~ ch~
values~
acc~176
(d~) =0
- ( 2 cosh z - 2 cosh u)~
(4) d with values o f x, z, and u, according to Eq. (8);
(5) p(d) for the values ofu(d), according to Eq. (1).
The half distance d was calculated as follows: If, as
assumed, all water in the saturated sample was associated with the clay particles, the half distance d was
the quotient o f the specific water volume o f the sample
after the measurement o f the swelling pressure and the
specific surface area o f the sample, i.e.,
d=
specific water volume
specific surface area
EXPERIMENTAL
Apparatus
The swelling pressure apparatus as shown in Figure
1 consisted essentially o f a loading device capable of
continuous adjustment to maintain the specimen at
constant height as the swelling process develops. The
adjustment was m a d e by tightening the upper nuts,
whereas the force required to resist the axial displacement was measured by the pressure cell. The displacement, i.e., the swelling strain, was measured by the two
503
micrometer dial gauges. The specimen was e m b e d d e d
tightly in a stainless steel ring for rigid radial restraint.
Porous plates at the top and b o t t o m o f the specimen
allowed the water access. A stainless steel plate, 1.5cm thick, at the top o f the upper porous plate, ensured
the rigid application o f the force. The specimen assembly was contained in a 15-cm diameter cell, capable o f
being filled with water to a level above the top o f the
specimen.
Mineralogical investigations
F r o m the theoretical considerations, the following
parameters o f the specimen must be known in order
to calculate its swelling pressure: valence o f the counter-ions in the double layer (v), ion concentration far
away from the clay surface, i.e., pore water o f the specimen (n), surface charge o f the clay (D), and the half
distance between the clay surfaces (d). After measuring
the swelling pressure, which took about 14 days for
each sample, the valence g and the ion concentration
n were determined by analyzing the water from the cell
of the swelling pressure apparatus (Figure 1). The
amount of N a and K was measured with a flame
photometer; Ca 2 and Mg 2+ were obtained by the complexometric method. One half of the swelling specimen
was used to determine the water content by drying it
for 24 hr at 105"C. The other half o f the swelling specimen was dried at room temperature and used for
the remainder of the determinations according to Figure 2.
The external specific surface area was measured by
the one-point BET method with N 2. The specimen was
degassed for 18 hr at 150~ and 10 -6 tort before the
adsorption was measured. To ensure a representative
composition of the specimens being investigated, a mechanical sample splitter was used to divide the bulk
specimen. The external specific surface area was used
in the calculation o f the total specific surface area (external plus internal area). The carbonate content was
determined by means o f an evacuated system using the
m e t h o d o f Hutchinson and MacLennan as reported in
Piper (1944). The carbonate, mainly CaCO3, was treated with HC1; CO2 was passed into a N a O H solution,
and after precipitation with BaC12, it was determined
by titration. The carbonate content was used to recalculate the original sample composition after determining the size o f clay fraction from the decarbonated
sample.
Before the amount o f the clay fraction (<0.002 mm)
was obtained by the pipet-method, it was necessary to
eliminate the carbonates. F o r this purpose ~ 5 g o f
sample was treated for 10 min in an ultrasonic bath
with a frequency o f 28 kHz and an amplitude o f 0.028
m m (MiiUer-Vonmoos, 1971), and then boiled in a p H
5 sodium acetate-acetic buffer solution. After removing the organic matter with H202, the noncrystalline
504
Clays and Clay Minerals
Madsen and Miiller-Vonmoos
Material and
preparation
Determinations
Data for calculotion of the
swelling pressure
water
{ amount of Na"
I amount of K
{ amount ~- C& 2 ]~
valency v and ion
[ concentrotion n
t amount of Mg.2
swelling specimen
{
crushing < 2mm
sample' dividing
removal of
I
texture
r external surface
BET
IF{
ultrasonics
decarbonating
k
- fraction ~ 0.002 mm
I
removal of organic
matter
water content
general x-ray
investigation
ir
half distance d
I [ carbonate content
t amount - 0,002 mm
,j
I
,
x-ray
tota~ surface
(glycerol)
surface charge D
J cation exch
Figure 2. Procedure for the determination of the mineralogical data.
Figure 1. Apparatus for measuring the swelling pressure.
Measurement of the swelling pressure
coatings on the clay particles were eliminated by extraction with dithionite-citrate (Mehra and Jackson,
1960). The clay fraction was then saturated with Ca z+
for the further investigations. The clay minerals were
characterized by X-ray powder diffraction (XRD)
(CuKa radiation) using orientated specimens. The
specimens were treated with glycerol and with hydrazine.
The internal specific surface area of the clay fraction
was determined by glycerol sorption on a Mettler-thermobalance (Madsen, 1977) and recalculated using the
above obtained a m o u n t of the clay fraction to the original sample composition. About 100 nag of the clay
fraction was treated with glycerol and heated to 300~
at l'C/minute. Between 100~ and 170~ the loss of
weight was about 0.5 mg/min. At 170~ the loss of
weight slowed, and between about 200 ~ and 2300C it
was ~0,08 rag/rain. Above 230~ a loss of weight of
~0.5 rag/rain was again observed. It was assumed that
at about 200"C all glycerol except a monolayer was
lost. The specific surface area of the clay was calculated
assuming a value of 1967 mVg for a monolayer thickness of 4.5/k for glycerol. The cation-exchange capacity
of the clay fraction was determined by the method of
Mackenzie (1951) using NH4 +.
The swelling pressure was measured according to the
recommendations of the International Society for Rock
Mechanics (1979). The test measures the pressure necessary to constrain the rock specimen at constant volume when it is immersed in water. The measurements
were made on 8-cm diameter drilling cores from between 15 m and 155 m depth. The cores were sawed
into discs with a thickness of 3 cm. The disc was fitted
into the stainless steel ring, and the apparatus was assembled. A small axial stress, about 0.025 N / r a m 2, was
applied to the specimen, and the cell was flooded with
water to cover the top of the porous plate. Because the
chemistry of the water in the rock mass from which
the samples were obtained was unknown, distilled water
was used in all tests. The swelling heave was measured
by the dial gauges, and after a heave of about 0.01 to
0.02 mm, the original height of the sample was reestablished by increasing the axial stress. The value of
the stress, o*, at which no further swelling strain was
observed, was designated the swelling pressure.
RESULTS
Clay data
The determination of the a m o u n t of Na +, K +, Ca2+,
and Mg 2+ from the water in the swelling pressure cell
Vol. 33, No. 6, 1 9 8 5
Calculation of swelling pressure from mineralogical properties
lllit~
001
K~o1,.it~
ooi
Chlor
<
HydrazinktoJ?nite
~?
Chlorite 00e
<
W
505
-40
~112~
M~~
l~176 mLo
11010lite
[Ihte Chlo,~te
O0
-35A
W
o,~te
Cblor~t~
00z
O 9
30
zo
Figure 4. X-ray powder ditfractograms of the clay fraction
using OLKa radiation. Left: air-dried specimen; middle: glycerol-treated specimen; fight: hydrazine-treated specimen.
(3.
Z
rr
iii
FX
?o
20
35
AMOUNT<0.002 mm [%]
Figure 3. External specific surface area as a function of the
size of the clay fraction.
proved that the ratio of m o n o - to bivalent ions was
80:20. AP + or Fe 3 were not present. About 75% of the
ions were Na +. The mean value of the ion concentration was 10-2 m m o l e / c m 3. The water content o f the
saturated swelling specimens varied between 6.3 and
8.3% of the dry sample weight. The external specific
surface area varied between 22 and 37 mVg. Because
the composition of the clay fraction of the opalinum
shale was quite homogeneous, the specific surface area
depended only on the size of the clay fraction (< 0.002
mm), as shown in Figure 3. The carbonate content
varied between 7 and 18%, and the clay fraction, calculated for the original sample composition, was between 37 and 59% of the dry sample weight.
Besides clay minerals and carbonate, the opalinum
shale consists mainly of quartz (~35~
with small
amounts (< 5%) of feldspar, pyrite, and organics. According to the nomenclature of Fiichtbauer (1959), the
opalinum shale is a sandy or carbonitic sandy claystone. Figure 4 shows the X R D pattern of the clay
fraction. On the left is the air-dried specimen with
mixed-layer peaks at 12 and 35 ,~. I n the middle is the
same specimen after treatment with glycerol. According to Brown (1961) and Brindley (1966), the mixedlayer is an illite/montmorillonitewith about 30% swelling layers. The righthand diagram shows a specimen
after treatment with hydrazine, The 001 peak of kaolinite thus moved from 7 to about 10 A. The clay
fraction therefore consists of mixed-layer illite/montmorillonite, illite, kaolinite, and chlorite.
The internal specific surface area of the clay fraction
was about 75 m2/g for all specimens. These data mean
that the clay fraction contains about 35% mixed-layer
illite/montmorillonite with 30% swelling layers. The
total specific surface area of the clay fraction (external
plus internal) for all samples was between 130 and 140
m2/g (mean value 135 m//g). The cation-exchange capacity of the clay fraction varied from 28 to 34 meq/
100 g with a mean value of 31 meq/100 g.
Swelling data
The swelling test took about 14 days for each sample.
After this time, no further swelling strain was observed,
and equilibrium was assumed to have been established
between the pore water in the sample and the water in
the cell of the apparatus. The incremental values of
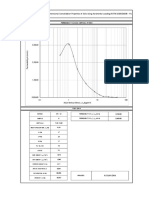
stress, Acq, and observed strain, A~i, are represented as
a summation curve for one sample in Figure 5. The
value of stress a* at which no further swelling strain
was observed was designated the swelling pressure. The
swelling pressure of the 19 investigated cores was between 0.7 and 2.2 N / r a m 2 (Table 1). (1 N e w t o n / m m 2 =
142 lb/sq in.)
DOUBLE LAYER CALCULATIONS
The following data were chosen for the calculation
of the swelling pressure function p(d): valence, v = 1;
ion concentration, n = 10 -z m m o l e / c m 3 = 6.02 x l 0 is
ions/cm3; specific surface area o f clay fraction, 135 mV
g; and cation-exchange capacity, 31 meq/100 g. The
constants are: k = Boltzmann constant = 1.38 x 10 -'6
o*
0.8-
tie i
O.4-
005
0.10
0.15
a20
Z COMPENSATED SWELLING STRAINS [~
Figure 5. Determination of the swelling pressure (r* at conslant volume of sample.
506
Clays and Clay Minerals
Madsen and Miiller-Vonmoos
erg/~ E = dielectric constant o f water = 80; e = elem e n t a r y charge = 4.80 x 10 ~o esu; and T = absolute
t e m p e r a t u r e = 293~ (20~
T h e m e a n value o f the specific surface charge, D, o f
the clay fraction was c o m p u t e d f r o m the specific surface area a n d the cation-exchange capacity (CEC) as
follows:
area per ion =
area per ion =
specific surface area
CEC(Avogadro's number) '
135 x 104 cm2/g
31 x 10 -5 6.02 1023 ions/g
2 exp -
2
3
3.5
4
4.5
5
0.7358
0.4463
0.3475
0.2707
0.2108
0.1642
F2
xd
d(A)
1.5788
1.5725
1.5718
1.5714
1.5712
1.5710
0.07122
0.11707
0.14988
0.1915
0.2440
0.3095
1.1093
0.6496
0.4941
0.3735
0.2798
0.2071
33.8
19.8
15.1
11.4
8.5
6.3
Values o f p(d) for the values o f u(d) were calculated
according to:
p = 2nkT(cosh u - 1)
= 72 x 10 -16 cm2/ion,
D - charge
area
4.80 x 10 -10 esu
72 x 10 -16 c m 2
= 6.7 x 104 e s u / c m 2.
T h e v a l u e u(d) was calculated from:
X
F~
= (8~rnCv2'/~
\ ekT ]
= 328 x 1 0 4 c m -1
and
d(~) _ 4freuD
o
~kT-----~- 38.1.
T h e v a l u e o f z for chosen values o f u was calculated
from:
__u
p ( d y n / c m 2)
d (A)
2
3
3.5
4
4.5
5
0.134
0.441
0.758
1.28
2.14
3.56
33.8
19.8
15.1
11.4
8.5
6.3
10 7
107
107
107
107
107
N o r m a l l y , double layer calculations are m a d e in the
cm-g-sec-system, and the swelling pressure p is rep o r t e d in d y n / c m 2. A c c o r d i n g to the n e w international
system o f units (SI), a force is m e a s u r e d in N e w t o n s
where 1 N = 1 m . k g / s e C , and the swelling pressure
therefore is given in N / m m 2.
1 N / m m 2 = 107
dyn/cm
2 =
142 lb/sq in.
The function p(d), as calculated according to the d o u b l e
layer theory with the mineralogical parameters o f the
o p a l i n u m shale, is plotted in Figure 6 in the terms o f
N / m m 2.
and
cosh z = 0.5
T h e swelling pressure p was n o t calculated for each
sample, but o b t a i n e d for values o f h a l f distance d f r o m
the plot o f p(d). T h e h a l f distance d is c o m p u t e d for
each sample according to
+ cosh u,
0
which yielded the following values:
u
cosh u
cosh z
2
3
3.5
4
4.5
5
3.7622
10.0677
16.5728
27.3082
45.0141
74.2099
729.5672
735.8727
742.3768
753.1132
770.8191
800.0149
7.2856
7.2942
7.3030
7.3174
7.3406
7.3778
Values o f d for values o f z, and u were calculated
as follows:
x d = 2 exp
d---
specific water v o l u m e o f the sample
specific surface area o f the sample
T h e specific water v o l u m e used was the water c o n t e n t
o f the sample after the swelling test, expressed in c m 3
water/g dry sample. It was a s s u m e d that 1 g water =
1 c m 3 water. In the calculation o f d, no difference was
a s s u m e d between the external and internal surfaces o f
the clay minerals. T h e specific surface area o f the sample was calculated f r o m the specific surface area o f the
clay fraction and the a m o u n t o f clay fraction. T h e dev i a t i o n in the d e t e r m i n a t i o n s o f the a m o u n t o f clay
was a b o u t + 1% and in the specific surface area o f the
clay, a b o u t + 5 m2/g. T h e s e d e v i a t i o n s are included in
the values o f the surface area o f the sample (Table 1)
and thus also in the h a l f distance d. F o r a k n o w n range
o f d, the m a x i m u m and m i n i m u m calculated swelling pressure p was d e d u c e d f r o m the function p(d) in
Figure 6.
Vol. 33, No. 6, 1985
Calculation of swelling pressure from mineralogical properties
507
Table 1. Mineralogical data and swelling pressure for the lower Dogger opalinum shale from northern Switzerland.
Sample
H20t
(%)
Carbonaw
con~nt
(%)
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
8.3
8.3
7.5
8.3
7.6
7.0
7.9
6.6
7.2
6.8
7.1
7.8
6.3
6.7
6.9
6.7
8.2
7.1
7.2
13
12
12
12
12
11
7
10
9
11
9
17
18
10
11
7
8
9
16
Clay fraction
(%)
45
52
51
46
45
45
49
57
56
59
58
43
38
46
51
55
48
51
37
+ 1
+ 1
+ 1
+ 1
+ 1
+ !
+ 1
+ 1
+ 1
+ 1
+_ 1
+ 1
___ 1
+ 1
+ 1
+ 1
+ 1
+ 1
+ 1
External
surface
area 2
(m2/g)
29
32
29
31
29
25
30
36
34
37
35
27
23
28
32
33
28
31
22
Surface area of
clay fraction 3
(m2/g)
135
135
135
135
135
135
135
135
135
135
135
135
135
135
135
135
135
135
135
_+ 5
+ 5
+ 5
+ 5
+ 5
___5
+ 5
+ 5
+ 5
+ 5
+ 5
+ 5
+ 5
+ 5
+ 5
+ 5
_+ 5
+ 5
__+5
Surface area
of sample
(m2/g)
Half distance
(A)
Swelling
pressure
calculated
(N/mm 2)
Swelling
pressure
measured
(N/mm 2)
64.4-57.2
74.2--66.3
72.8--65.0
65.8-58.5
64.4-57.2
64.4-57.2
70.0-62.4
81.2-72.8
79.8-71.5
84.0-75.4
82.6-74.1
61.6-54.6
54.6-48.1
65.8-58.5
72.8--65.0
78.4-70.2
68.6-61.1
72.8-65.0
53.2--46.8
12.9-14.5
11.2-12.5
10.3-11.5
12.6-14.2
11.8-13.3
10.9-12.2
11.3-12.7
8.1-9.1
9.0-10.1
8.1-9.0
8.6--9.6
12.7-14.3
11.5-13.1
10.2-11.5
9.5-10.6
8.5-9.5
12.0-13.4
9.8-10.9
13.5-15.4
1.0--0.8
1.3-1.1
1.6-1.3
1.1--0.8
1.2-1.0
1.4-1.1
1.3-1.1
2.3-1.9
2.0-1.6
2.3-1.9
2.1-1.7
1.1--0.8
1.3-1.0
1.6-1.3
1.8-1.5
2.1-1.7
1.2-0.9
1.7-1.4
1.0-0.7
0.7
1.3
1.6
0.8
1.2
1.4
1.3
1.3
2.0
1.7
1.0
0.8
1.2
1.5
1.7
2.0
0.8
2.2
0.7
' Water content after swelling t e s t .
2 External specific surface area of sample by BET.
3 External plus internal specific surface area by glycerol.
F o r s a m p l e 1 ( T a b l e 1), t h e clay f r a c t i o n was f o u n d
to b e 4 5 % _ 1% o f t h e s a m p l e ; t h e specific surface a r e a
o f t h e clay f r a c t i o n was 135 ___ 5 m2/g. T h e s e d a t a give
m a x i m u m a n d m i n i m u m specific surface a r e a s 64.4
m2/g a n d 57.2 m2/g, respectively. T h e m i n i m u m h a l f
d i s t a n c e , d, is, w i t h a w a t e r c o n t e n t a f t e r swelling test
o f 8.3%, e q u a l to
d =
=
0.083 c m a / g
64.4 x 104 cm2/g
12.9
10 - s c m
= 12.9/~.
T h e m a x i m u m d was c a l c u l a t e d to b e 14.5 ~ . T h e s e
v a l u e s give a r a n g e o f t h e swelling p r e s s u r e p (Figure
6) o f 1.0 to 0.8 N / m m 2 (142 to 114 l b / s q in.). Figure
6 also s u m m a r i z e s t h e v a l u e s o f t h e m e a s u r e d swelling
p r e s s u r e ~* as a f u n c t i o n o f t h e c a l c u l a t e d h a l f d i s t a n c e
d. T h e v a l u e s o f d are d r a w n as s m a l l b a r s a c c o r d i n g
to t h e a b o v e - m e n t i o n e d r a n g e o f u n c e r t a i n t y . T h e values are also listed i n T a b l e 1.
DISCUSSION
T h e G o u y t h e o r y was u s e d i n t h e u n c o r r e c t e d f o r m
to calculate t h e swelling p r e s s u r e f u n c t i o n p(d). Bolt
(1955) p r e s e n t e d a c o r r e c t e d f o r m o f t h e t h e o r y b y
a d d i n g several s e c o n d a r y energy t e r m s to t h e p r i m a r y
p o t e n t i a l energy t e r m for t h e i o n i n t h e electric field o f
t h e d o u b l e layer. T h e u n c o r r e c t e d f o r m , h o w e v e r , gives
results a l m o s t i d e n t i c a l w i t h t h o s e o f t h e c o r r e c t e d t h e o r y w h e n a p p l i e d t o t h e p r o b l e m o f d o u b l e layer in-
teraction. T h e greatest influence o n t h e v a l u e o f p(d)
was t h e c h o i c e o f t h e v a l e n c e to b e + 1 d e s p i t e t h e fact
t h a t o n l y 80~ o f t h e i o n s were m o n o v a l e n t . T h e influe n c e o f t h e v a l e n c e m a y b e s e e n f r o m t h e fact t h a t a
f u n c t i o n p(d) c a l c u l a t e d for d i v a l e n t i o n s w o u l d give
swelling p r e s s u r e s a b o u t f o u r t i m e s s m a l l e r t h a n for
m o n o v a l e n t ions. T h e influence o f t h e c h o i c e o f t h e
v a l e n c e was p a r t l y c o r r e c t e d b y t h e c a l c u l a t i o n o f t h e
i o n c o n c e n t r a t i o n , n. H i g h e r v a l u e s o f n result i n l o w e r
v a l u e s o f swelling pressure. T h e c o r r e c t i o n was m a d e
b y c o u n t i n g o n e d i v a l e n t i o n as t w o m o n o v a l e n t i o n s
to get t h e h i g h e r i o n c o n c e n t r a t i o n . O n t h e whole, t h e
f u n c t i o n p(d) as s h o w n in F i g u r e 6 g a v e v a l u e s w h i c h
are slightly t o o high.
By c a l c u l a t i n g t h e h a l f d i s t a n c e d all w a t e r i n t h e
s a t u r a t e d s a m p l e was a s s u m e d to b e connected to t h e
clay surfaces; h o w e v e r , t h e p o r e v o l u m e d i s t r i b u t i o n
of some specimens, determined by mercury intrusion
p o r o s i m e t r y at 3 0 0 0 a t m , i n d i c a t e d t h a t a b o u t 10 to
15% o f t h e p o r e s were o f r a d i u s > 1 0 0 ~ . I n t h e calc u l a t i o n o f d, t h e w a t e r f r o m t h e s e p o r e s was also dist r i b u t e d o n t h e clay surface a n d t h u s r e s u l t e d i n a calc u l a t e d h a l f d i s t a n c e t h a t was t o o large. L a r g e r d v a l u e s
m e a n s s m a l l e r swelling p r e s s u r e p as r e a d f r o m p(d) i n
F i g u r e 6. T h e fact t h a t t h e f u n c t i o n p(d) r e s u l t e d i n
excessively large v a l u e s was t h u s , u p t o a c e r t a i n p o i n t ,
c o m p e n s a t e d b y t h e excessively large v a l u e s o f d.
T h e effect o f t h e s i m i l a r i t y o f t h e e x t e r n a l a n d t h e
i n t e r n a l surface o f t h e clay o n t h e c a l c u l a t i o n o f d was
difficult t o e s t i m a t e . F o r h a l f d i s t a n c e s o f t h e ascert a i n e d o r d e r o r m a x i m u m 15 ,~ a n d a clay w i t h m a i n l y
508
Madsen and Miiller-Vonrnoos
U*
2.5
normally are made in the preconstrnction phase of the
tunneling work, time is not a serious problem.
The real advantage of the mineralogical method is
that once the p(d)-function for a swelling rock is known,
it is very easy to determine the swelling pressure of a
specimen by calculating the half distance d from the
water content and the specific surface area of the sample. Because of the homogeneous composition of the
clay fraction of the opalinum shale, the specific surface
area of the sample depended only on the size of the
clay fraction. The size of the clay fraction can, with a
sufficient accuracy, be ascertained from Figure 3 by
determining the external specific surface area. This routine method allows eight samples a day to be tested.
Therefore, for the opalinum shale, the swelling pressures which were determined from the drilling cores,
can be checked in an easy way during tunnel construction by investigating undisturbed fresh samples. This
will be an important control, inasmuch as drilling cores
always are disturbed (partly swelled) to some extent,
due to the use of water as drilling fluid, and therefore
tend to give swelling pressures that are erroneously
small.
REFERENCES
RESF? p
2.0
t~
c~
w
~ 1.5"
t-
-- ~_
CALCULATED
~
1.0w
m
0.5
10
1;
2'0
HALF DISTANCE [~,1
2'5
Clays and Clay Minerals
30"-
Figure 6. Calculated function p(d) with measured swelling
pressure a* as a function of the half distance d.
Na as counter-ions in the double layer, the influence
was probably not very important. On the whole, the
influence of the different assumptions seemed to compensate each other up to a certain value and thus gave
an acceptable fit of the calculated with the measured
swelling pressure.
CONCLUSION
The investigation showed the possibility of calculating the swelling pressure of the opalinum shale from
the mineralogical parameters using the Gouy theory.
As the mean value of the calculated swelling pressures
(1.39 N / m m 2) is of the same order of magnitude as the
mean value of the measured swelling pressures (1.33
N/mm2), the method was sufficiently accurate for tunnel-construction purposes. The advantage of the mineralogical method is that the swelling pressure can be
obtained for samples of any shape, whereas the direct
measurement of the swelling pressure requires samples
in form of cylindrical or rectangular discs. The disadvantage of the mineralogical method is that it is
rather complicated and in most cases takes more time
than the direct measurement. As these investigations
Bolt, G. H. (1955) Analysis of the validity of the GouyChapman theory of the electric double layer: J. Colloid Sci.
1O, 206-219.
Bolt, G. H. (1956) Physico-chemical analysis of the compressibility of pure days: Geotechnique 6, 86-93.
Brindley, G. W. (1966) Ethylene glycol and glycerol complexes of smectites and vermiculites: Clay Miner. 6, 237259.
Brown, G., ed. (1961) The X-ray Identification and Crystal
Structures of Clay Minerals: Mineralogical Society, London, 393-445.
Chapman, D. L. (1913) A contribution to the theory of
electrocapillarity: Phil, Mag. 25, 475-481.
Einstein, H. H. (1979) Tunneling in swelling rock: Underground Space 4, 51-61.
Fiichthauer, H. (1959) Zur Nomenklatur der Sedimentgesteine: Erdtl Kohle 8, 605-613.
Gouy, G. (1910) Sur la constitution de la charge 61ectrique
&la surface d'un 61ectrolyte: J. Physique 9, 457-468.
Gouy, G. (1917) Sur la fonction 61ectrocapillaire:Ann. Phys.
(Paris), S#rie 9 7, 129-184.
Grob, H. (1976) Swelling and heave in Swiss tunnels: Bull.
Int. Ass. Engng. Geol. 13, 55-60.
International Society for Rock Mechanics (1979) Commission on Standardizationof Laboratory and Field Tests: Suggested methods for determining swelling and slake-durability index properties: Int. J. Rock Mech. Min. Sci. 16,
141-156.
Jahnke, E. and Emde, F. (1933) Tables of Functions: B. G.
Teubner, Leipzig and Berlin, 124-144.
Langmuir, I. (1938) The role of attractive and repulsive
forces in the formation oftactoids, thixotropic gels, protein
crystals, and coacervates: J. Chem. Phys. 6, 873-896.
Mackenzie, R.C. (1951) A micromethod for determination
of cation-exchange capacity of clay: J. Colloid Sci. 6, 219222.
Madsen, F. T. (1977) Surface area measurements of clay
minerals by glycerol sorption on a thermobalance: Thermochimica Acta 21, 89-93.
Vol. 33, No. 6, 1985
Calculation of swelling pressure from mineralogical properties
Mehra, O. P. and Jackson, M.L. (1960) Iron oxide removal
from soils and clays by a dithionite-eitrate-system buffered
with sodium bicarbonate: in Clays and Clay Minerals, Proc.
7th NatL Conf., Washington, D.C., 1958, Ada Swineford,
ed., Pergamon Press, New York, 317-327.
Mtiller-Vonmoos, M. (1971) Zur Korngr6ssenfraktionierung tonreicher Sedimente: Beitr. GeoL Schweiz 54, 245257.
509
Piper, C. S. (1944) Soil and Plant Analysis: Interscience
Publishers, New York, 128-136.
van Olphen, H. (1977) An Introduction to Clay Colloid
Chemistry: Interscience Publishers, New York, 260-293.
Verwey, E. J. W. and Overbeek, J. Th. G. (1948) Theory of
the Stability of Lyophobic Colloids," Elsevier, Amsterdam,
22-76.
(Received 15 May 1984; accepted 5 May 1985; Ms. 1372)
S-ar putea să vă placă și
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Aircraft Steel Data SheetDocument4 paginiAircraft Steel Data Sheetrodryguo74Încă nu există evaluări
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- USGS Lithology SymbolsDocument3 paginiUSGS Lithology Symbolsgoulagh100% (2)
- Diamond Bit Design HandoutDocument19 paginiDiamond Bit Design Handoutamin peyvand100% (1)
- ASTM C131/C131M 14: Standard Test Method For Resistance To Degradation of Small-Size Coarse Aggregate by Abrasion and Impact in The Los Angeles MachineDocument5 paginiASTM C131/C131M 14: Standard Test Method For Resistance To Degradation of Small-Size Coarse Aggregate by Abrasion and Impact in The Los Angeles MachineShaheer Ahmad100% (1)
- FIU Geological Dictionary English/Spanish & Spanish/EnglishDocument108 paginiFIU Geological Dictionary English/Spanish & Spanish/EnglishAndrew Mcdonald100% (2)
- Astm d5873 EsclerometroDocument4 paginiAstm d5873 EsclerometroNabucodonosor1980Încă nu există evaluări
- Hydrogen Reformer Furnace Failure PDFDocument10 paginiHydrogen Reformer Furnace Failure PDFJacekÎncă nu există evaluări
- Storage and Conveying of Bulk SolidsDocument5 paginiStorage and Conveying of Bulk SolidsjijiakdkclsikfdsÎncă nu există evaluări
- KUNG, LEE y TSAI (2011) Examination of DMT-based Methods For Evaluating The Liquefaction Potential of SoilsDocument12 paginiKUNG, LEE y TSAI (2011) Examination of DMT-based Methods For Evaluating The Liquefaction Potential of SoilsNabucodonosor1980Încă nu există evaluări
- Comparison of DMT, CPT, SPT, and VS Based Liquefaction PDFDocument8 paginiComparison of DMT, CPT, SPT, and VS Based Liquefaction PDFNabucodonosor1980Încă nu există evaluări
- KUNG, LEE y TSAI (2011) Examination of DMT-based Methods For Evaluating The Liquefaction Potential of SoilsDocument12 paginiKUNG, LEE y TSAI (2011) Examination of DMT-based Methods For Evaluating The Liquefaction Potential of SoilsNabucodonosor1980Încă nu există evaluări
- Learning From The Past: The Ancient Egyptians and Geotechnical EngineeringDocument33 paginiLearning From The Past: The Ancient Egyptians and Geotechnical EngineeringNabucodonosor1980Încă nu există evaluări
- Lee, M.-J., Hong, S.-J., Choi, Y.-M., & Lee, W. (2010) - Evaluation of Deformation Modulus of Cemented Sand Using CPT and DMT. Engineering Geology, 115 (1-2), 28-35.Document8 paginiLee, M.-J., Hong, S.-J., Choi, Y.-M., & Lee, W. (2010) - Evaluation of Deformation Modulus of Cemented Sand Using CPT and DMT. Engineering Geology, 115 (1-2), 28-35.Nabucodonosor1980Încă nu există evaluări
- 2002 HussonMoretti PDFDocument28 pagini2002 HussonMoretti PDFNabucodonosor1980Încă nu există evaluări
- Correlation Between Flat Dilatometer (DMT) Index With Insitu Bearing Strength For Subgrade MaterialDocument5 paginiCorrelation Between Flat Dilatometer (DMT) Index With Insitu Bearing Strength For Subgrade MaterialNabucodonosor1980Încă nu există evaluări
- Comparison of DMT, CPT, SPT, and VS Based Liquefaction PDFDocument8 paginiComparison of DMT, CPT, SPT, and VS Based Liquefaction PDFNabucodonosor1980Încă nu există evaluări
- RAS-24 Digital Seismograph SystemDocument2 paginiRAS-24 Digital Seismograph SystemNabucodonosor1980Încă nu există evaluări
- KUNG, LEE y TSAI (2011) Examination of DMT-based Methods For Evaluating The Liquefaction Potential of SoilsDocument12 paginiKUNG, LEE y TSAI (2011) Examination of DMT-based Methods For Evaluating The Liquefaction Potential of SoilsNabucodonosor1980Încă nu există evaluări
- RAS-24 Digital Seismograph SystemDocument2 paginiRAS-24 Digital Seismograph SystemNabucodonosor1980Încă nu există evaluări
- Manual de Fundaciones en Suelos Expansivos PDFDocument69 paginiManual de Fundaciones en Suelos Expansivos PDFEderEnzoÎncă nu există evaluări
- Difractometro USGSDocument2 paginiDifractometro USGSNabucodonosor1980Încă nu există evaluări
- SWELLING CHLORITE EXPANDS ON GLYCEROL TREATMENTDocument2 paginiSWELLING CHLORITE EXPANDS ON GLYCEROL TREATMENTNabucodonosor1980Încă nu există evaluări
- Application of Advanced Geophysical Technologies To Landslides and Unstable SlopesDocument5 paginiApplication of Advanced Geophysical Technologies To Landslides and Unstable SlopesNabucodonosor1980Încă nu există evaluări
- Design of the Misicuni hydroelectric project in BoliviaDocument7 paginiDesign of the Misicuni hydroelectric project in BoliviaNabucodonosor1980Încă nu există evaluări
- Minerales Pesados VZ PDFDocument10 paginiMinerales Pesados VZ PDFNabucodonosor1980Încă nu există evaluări
- Structure and Cenozoic Tectonics of The Falcon Basin, Venezuela, and Adjacent AreasDocument14 paginiStructure and Cenozoic Tectonics of The Falcon Basin, Venezuela, and Adjacent AreasNabucodonosor1980Încă nu există evaluări
- SWELLING CHLORITE EXPANDS ON GLYCEROL TREATMENTDocument2 paginiSWELLING CHLORITE EXPANDS ON GLYCEROL TREATMENTNabucodonosor1980Încă nu există evaluări
- Geotechnical Investigations On The Zagrad Location in Rijeka, CroatiaDocument6 paginiGeotechnical Investigations On The Zagrad Location in Rijeka, CroatiaNabucodonosor1980Încă nu există evaluări
- ASTM D2435/2435M - 11 Soil Permeability Test ResultsDocument1 paginăASTM D2435/2435M - 11 Soil Permeability Test ResultsNabucodonosor1980Încă nu există evaluări
- Laboratory Determination of The Coefficient of Electro-Osmotic Permeability of A SoilDocument8 paginiLaboratory Determination of The Coefficient of Electro-Osmotic Permeability of A SoilNabucodonosor1980Încă nu există evaluări
- Vane Inspection Kit: Product ManualDocument4 paginiVane Inspection Kit: Product ManualNabucodonosor1980Încă nu există evaluări
- 029 PDFDocument9 pagini029 PDFNabucodonosor1980Încă nu există evaluări
- Liquid Limit and Surface Area of ClaysDocument4 paginiLiquid Limit and Surface Area of ClaysNabucodonosor1980Încă nu există evaluări
- Tabakovic 2018Document36 paginiTabakovic 2018hayet debbichÎncă nu există evaluări
- OTC-29287-MS Advances in Chemical EOR Technologies: New Development in Field-Scale Chemical Flooding SimulationDocument28 paginiOTC-29287-MS Advances in Chemical EOR Technologies: New Development in Field-Scale Chemical Flooding SimulationVeronicaÎncă nu există evaluări
- Gomes, GabrielDocument12 paginiGomes, GabrielVálter LúcioÎncă nu există evaluări
- Product Data: DescriptionDocument2 paginiProduct Data: Descriptionjhon vargasÎncă nu există evaluări
- Dhio Corporate Brochure 2023 FordistributionDocument12 paginiDhio Corporate Brochure 2023 FordistributionsrashmiiiscÎncă nu există evaluări
- En 10164-1993Document8 paginiEn 10164-1993Marija IvanovskaÎncă nu există evaluări
- 1st Presentation - Analyzing Dynamic Line Rating of Distribution Tower LineDocument19 pagini1st Presentation - Analyzing Dynamic Line Rating of Distribution Tower LineMohamed Nihaj100% (1)
- Introduction to CSAMT: A Guide to Controlled Source Audio-Frequency MagnetotelluricsDocument4 paginiIntroduction to CSAMT: A Guide to Controlled Source Audio-Frequency MagnetotelluricsAndy KurniyantoÎncă nu există evaluări
- CE 616 Structural Dynamics Homework Problems 1-3Document1 paginăCE 616 Structural Dynamics Homework Problems 1-3Kaushal KumarÎncă nu există evaluări
- Theory of Relativity and Analytic Dynamics Handwritten NotesDocument132 paginiTheory of Relativity and Analytic Dynamics Handwritten NotesAnees Ur RehmanÎncă nu există evaluări
- Physical Metallurgy of Aluminium AlloysDocument13 paginiPhysical Metallurgy of Aluminium AlloysKaanMertÎncă nu există evaluări
- THE CORROSION RATE OF Pb-Ca ALLOYS IN SULFURIC ACID SOLUTIONSDocument7 paginiTHE CORROSION RATE OF Pb-Ca ALLOYS IN SULFURIC ACID SOLUTIONScekmilanÎncă nu există evaluări
- Eae EngDocument625 paginiEae EngWahyu SantosoÎncă nu există evaluări
- Stress Analysis Using Actual Coke DrumDocument11 paginiStress Analysis Using Actual Coke Drumash1968Încă nu există evaluări
- Curie Temperature: Electronic StructureDocument21 paginiCurie Temperature: Electronic Structurejose carlos julca eleraÎncă nu există evaluări
- 4.0 Radiometry PhotometryDocument25 pagini4.0 Radiometry PhotometrynidharshanÎncă nu există evaluări
- High Performance Fiber Reinforced Concrete - OverviewDocument14 paginiHigh Performance Fiber Reinforced Concrete - Overviewhaoude9Încă nu există evaluări
- Youngs ModulusDocument10 paginiYoungs Modulusconnectrix.gohetrixÎncă nu există evaluări
- Tropospheric ducting propagation and its impact on radio performanceDocument18 paginiTropospheric ducting propagation and its impact on radio performanceRamanuj SinghÎncă nu există evaluări
- Precipitation Titration Methods for Determining HalidesDocument9 paginiPrecipitation Titration Methods for Determining Halidesحسين عمار محسن سالمÎncă nu există evaluări
- Atmospheric CO2 Concentration Variations by Location and SeasonDocument4 paginiAtmospheric CO2 Concentration Variations by Location and SeasonErick AmâncioÎncă nu există evaluări
- Thermodynamics Lab Report - Application of The Perfect Gas Laws in The Determination of Adiabatic Index of AirDocument9 paginiThermodynamics Lab Report - Application of The Perfect Gas Laws in The Determination of Adiabatic Index of Airqiaunus69% (13)
- AWS STANDARDS LIBRARY ORDER FORMDocument3 paginiAWS STANDARDS LIBRARY ORDER FORMRajan SteeveÎncă nu există evaluări
- 2014 WINTER Cryoquip Installation Tips and Considerations For Ambient Vaporizers1Document2 pagini2014 WINTER Cryoquip Installation Tips and Considerations For Ambient Vaporizers1Kom NakÎncă nu există evaluări
- C StructDesign Bendapudi Feb10 (1) Part 1Document0 paginiC StructDesign Bendapudi Feb10 (1) Part 1Rubén MenaÎncă nu există evaluări