Documente Academic
Documente Profesional
Documente Cultură
E2SEM1MT2TD06102012
Încărcat de
anmol6237Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
E2SEM1MT2TD06102012
Încărcat de
anmol6237Drepturi de autor:
Formate disponibile
E2 SEM1 ME TD MT2 QP
1) A paddle wheel provides 200 kJ of work to the air contained in a 0.2-m rigid volume,
A)
B)
C)
D)
initially at 400 kPa and 40C. Determine the entropy change if the volume is insulated.
0.504 kJ/K
0.443 kJ/K
0.312 kJ/K
0.231 kJ/K
2) Five kilograms of ice at 20C are mixed with 10 kg of water initially at 20C. If there is no
significant heat transfer from the container, determine the net entropy change. It takes 330 kJ to
melt a kg of ice.
(A) 0.064 kJ/K
(B) 0.084 kJ/K
(C) 1.04 kJ/K
(D) 1.24 kJ/K
3) The p,v,T relation of a real gas is represented with reasonable accuracy by the relation
V=RT/p + B- A/RT
Where A and B are constants. for this gas find the change in enthalphy and entropy along the
isothermal path between pressures p1 and p2
A)
B)
C)
D)
E)
(b-2A/RT)(P2 P1) and( R ln (P2 / P1) - A( P2 P1)/RT2)
(b-2A/RT)(P2 P1) and( R ln (P2 / P1) + A( P2 P1)/RT2)
(b-2A/RT)(P1 P2) and( R ln (P2 / P1) - A( P2 P1)/RT2)
(b-2A/RT)(P2 P1) and( R ln (P1/ P2) - A( P2 P1)/RT)
NONE
4) A refrigerator storage is supplied with 3600 kg of fish at a temperature of 27C. The fish has
to be cooled to 23C for preserving it for a long period without deterioration. The cooling
takes place in 10 hours. The specific heat of fish is 2.0 kJ/kg K above freezing point of fish and
0.5 kJ/kg-K below freezing point of fish, which is -3C. The latent heat of freezing is 230 kJ/kg.
What is the power to drive the plant if the actual COP is half that of
the ideal COP?
A) 30 kW
B) 15 kW
C) 12 kW
D) 6 kW
5) In the T-S diagram shown in the figure, which one of the following is represented
by the area under the curve?
E2 SEM1 ME TD MT2 QP
A) . Total work done during the process
B) Total heat absorbed during the process
C). Total heat rejected during the process
D). Degree of irreversibility
6) A 100 resistor carrying a constant current of 0.5 A is kept at a constant temperature of 300
K by a stream of cooling water. In a time interval of 30 minutes, what are the changes in entropy
for the resistor and that of the universe, respectively?
A) 0 and 150 J/K
B) 150 J/K and 0
C) 300 J/K and 0
D)0 and 300 J/K
7) According to the Maxwell relation, which of the following is/are correct?
A) (v/T)P =- (s/P)T
B) (s/v)T = -(p/T)V
c) (p/T)V = (s/v)T
D) all the above
8) Calculate the entropy change of a 10-kg block of copper if the pressure changes from 100 kPa
to 50 MPa while the temperature remains constant. Use = 5 X 10 -5 K-1 and = 8770 kg/m3
A) -0.285 J/kg K
B) -0.385 J/kg K
C) -0.485 J/kg K
D) -0.585 J/kg K
9) Find an expression for Cp - Cv. if P = RT/v - a / v2
A) TR2P/(Pv2- a )
B) TR2v/(Pv2- a )
C) TRv/(Pv2- a )
D) TR2v/(Pv -2- a )
E)none
10) What is (s/P)T for an ideal gas?
A) - 1 / P
B) - R / P
C) - 1 / T
D) -R/ T
E2 SEM1 ME TD MT2 QP
Descriptive
Answer any one from the following question
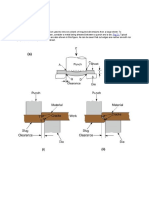
1) Air at 101.35Kpa, 270C is cooled to a lower temperature, by exchanging heat nitrogen at
103.42kpa,166K,in a steady state heat exchanger as shown in the below fig. asuming air
and nitrogen to behave ideally with constant specific heats of CP =1.006KJ/kg K and 1.08
KJ/kg K respectively, calculate the mass flow rate of nitrogen, and the temperature of the
air at the exit.
2) In a process industry hot gases are delivered by different units.one unit delivers a gas at 1
bar and 1000K at a rate of 1kmol/s while a second unit delivers a gas at 1bar and 800 K at
a rate of 2 kmol/s.These hot gases are usually cooled to 300K in heat exchangers.The
ambient atmosphere is at 300K An engineer plans to use the hot gases as source and
ambient atmosphere as sink to operate a heat engine and thus obtain some power.
Calculate the maximum power that can be obtained if
a) Gases A and B are used as separate sources, and
b) Gases A and B are mixed and the mixture is used as a source
Assume both the gases A and B are ideal with =1.4
KEY
1) A
2) ADD 1 MARK TO ALL
3) A
4) C
5) B
6) A
7) A OR C
8) A
9) B
10) B
S-ar putea să vă placă și
- Ansys Training Book.Document15 paginiAnsys Training Book.Sarath Babu SÎncă nu există evaluări
- Damping Calculations for Race Vehicle SuspensionDocument99 paginiDamping Calculations for Race Vehicle SuspensionManu PantÎncă nu există evaluări
- SHEARINGDocument6 paginiSHEARINGanmol6237Încă nu există evaluări
- Section-1: Material-Independent Property Relations: AnswersDocument20 paginiSection-1: Material-Independent Property Relations: Answersnkosana2Încă nu există evaluări
- Parametric Modeling With Creo ParametricDocument41 paginiParametric Modeling With Creo Parametricfrancesca050168Încă nu există evaluări
- Tem-290 Process Validation Protocol Template SampleDocument5 paginiTem-290 Process Validation Protocol Template SampleJonatan Dominguez Perez100% (2)
- Calculation of Induction Motor Starting Parameters Using MatlabDocument6 paginiCalculation of Induction Motor Starting Parameters Using MatlabДејан ПејовскиÎncă nu există evaluări
- Con-011 Instruction For Painting Work Rev.0Document11 paginiCon-011 Instruction For Painting Work Rev.0monchai_626Încă nu există evaluări
- Module 7: Solved ProblemsDocument15 paginiModule 7: Solved Problemscaptainhass67% (6)
- AMB AMC Machine DescriptionDocument87 paginiAMB AMC Machine DescriptionEduard Nurmetov0% (1)
- M.TECH. DEGREE EXAMINATION Model Question PapersDocument4 paginiM.TECH. DEGREE EXAMINATION Model Question PapersTantai RakthaijungÎncă nu există evaluări
- Surface Prepairation STD For PC ASTM D6386Document4 paginiSurface Prepairation STD For PC ASTM D6386Rohit SangwanÎncă nu există evaluări
- Specialty Chemicals for Corrosion Inhibition and Water TreatmentDocument27 paginiSpecialty Chemicals for Corrosion Inhibition and Water TreatmentMehman NasibovÎncă nu există evaluări
- Ragone Solution Manual From Nanyang UniversityDocument67 paginiRagone Solution Manual From Nanyang UniversityRijalCok71% (14)
- Antibacterial For Plastics PDFDocument24 paginiAntibacterial For Plastics PDFRiaÎncă nu există evaluări
- NTS GAT Test Sample Paper of PharmacyDocument12 paginiNTS GAT Test Sample Paper of PharmacyNational Testing Service77% (13)
- 05-1-Collection of Problems PDFDocument15 pagini05-1-Collection of Problems PDFFistia MaulinaÎncă nu există evaluări
- List of over 150 Solidworks clientsDocument4 paginiList of over 150 Solidworks clientsanmol6237Încă nu există evaluări
- 1 s2.0 S0048969704004279 Main PDFDocument22 pagini1 s2.0 S0048969704004279 Main PDFnavri_nalhadÎncă nu există evaluări
- CH 4 Assignment PDFDocument2 paginiCH 4 Assignment PDFAftab57.Încă nu există evaluări
- Example CH 4Document4 paginiExample CH 4Uday Prakash SahuÎncă nu există evaluări
- CL 253 Tutorial Set 3Document2 paginiCL 253 Tutorial Set 3Alexander Salado IbrahimÎncă nu există evaluări
- Solutions Set 6Document19 paginiSolutions Set 6Augustine BrockÎncă nu există evaluări
- T1 Ug 90Document6 paginiT1 Ug 90germangsilvaÎncă nu există evaluări
- Indian Institute of Technology Tirupati: Roll No: NameDocument27 paginiIndian Institute of Technology Tirupati: Roll No: NameKriti SharmaÎncă nu există evaluări
- HW 10Document2 paginiHW 10hangman001Încă nu există evaluări
- Thermo QualsDocument26 paginiThermo QualsLuc LeÎncă nu există evaluări
- Old-Exam-Questions-Ch.#20-082 (Dr. Naqvi-Phys102.04-06Document3 paginiOld-Exam-Questions-Ch.#20-082 (Dr. Naqvi-Phys102.04-06czds6594Încă nu există evaluări
- Assignment 1 - 14-09-2021Document2 paginiAssignment 1 - 14-09-2021Abhishek GuptaÎncă nu există evaluări
- Second Law Thermodynamics Problems SolvedDocument2 paginiSecond Law Thermodynamics Problems SolvedNikki ByrneÎncă nu există evaluări
- Xe e Gate 2015Document3 paginiXe e Gate 2015Ankit Kumar AJÎncă nu există evaluări
- We M7 PDFDocument13 paginiWe M7 PDFMirza MesanovicÎncă nu există evaluări
- Che 501 - TutorialsDocument7 paginiChe 501 - TutorialsIgnatius Setiadi PrabowoÎncă nu există evaluări
- EMCDocument1 paginăEMCAditya GurunathanÎncă nu există evaluări
- Module 7: Solved ProblemsDocument13 paginiModule 7: Solved ProblemsahmadkamalÎncă nu există evaluări
- EXAM - (M) 2018: Mechanical Engineering Paper - IIDocument12 paginiEXAM - (M) 2018: Mechanical Engineering Paper - IISandeep PrajapatiÎncă nu există evaluări
- Ass 6Document2 paginiAss 6MayankÎncă nu există evaluări
- Thermodynamics Exam Questions and AnswersDocument8 paginiThermodynamics Exam Questions and AnswersAshok DaraÎncă nu există evaluări
- Thermodynamics of Materials Equilibrium CalculationsDocument2 paginiThermodynamics of Materials Equilibrium CalculationsChayon MondalÎncă nu există evaluări
- Tutorial 2 Spring 2018-19Document3 paginiTutorial 2 Spring 2018-19ANMOLÎncă nu există evaluări
- Gas compression and expansion processesDocument1 paginăGas compression and expansion processesdpurnimaÎncă nu există evaluări
- FTFS Chap23 P095Document26 paginiFTFS Chap23 P095AbdulAbdulÎncă nu există evaluări
- Question 1. During An Experiment Conducted in A Room at 25Document11 paginiQuestion 1. During An Experiment Conducted in A Room at 25fivos_rgÎncă nu există evaluări
- Tutorial 1 (Lecture 1-3)Document2 paginiTutorial 1 (Lecture 1-3)eja70Încă nu există evaluări
- A1 Batch NKC Sir Heat Thermodynamics KTG 1637843224143Document57 paginiA1 Batch NKC Sir Heat Thermodynamics KTG 1637843224143Harsh SinghÎncă nu există evaluări
- 13A03302122016Document2 pagini13A03302122016EhteshTubeÎncă nu există evaluări
- CHFEN 3553 Chemical Reaction Engineering: April 7, 2004 12:55 PM - 1:45 PM Answer All QuestionsDocument3 paginiCHFEN 3553 Chemical Reaction Engineering: April 7, 2004 12:55 PM - 1:45 PM Answer All QuestionsHungDoÎncă nu există evaluări
- Assume Ideal Behavior Unless Stated Otherwise. 1.: CY11001 (Physical Chemistry) Tutorial 3Document2 paginiAssume Ideal Behavior Unless Stated Otherwise. 1.: CY11001 (Physical Chemistry) Tutorial 3Krityapriya BhaumikÎncă nu există evaluări
- Homework 8 CHE 251ADocument2 paginiHomework 8 CHE 251AAbhishek AryaÎncă nu există evaluări
- Thermodynamics and Applications - Test-1Document6 paginiThermodynamics and Applications - Test-1sap2279Încă nu există evaluări
- Tutorial 1 - QuestionsDocument5 paginiTutorial 1 - Questions2200851Încă nu există evaluări
- Ihw 2Document6 paginiIhw 2LogoÎncă nu există evaluări
- Entropy Problems PDFDocument8 paginiEntropy Problems PDFEdgar HernandezÎncă nu există evaluări
- Entropy ProblemsDocument8 paginiEntropy ProblemsTravis BickleÎncă nu există evaluări
- AlıştırmaDocument3 paginiAlıştırmaJanet Baker100% (1)
- HW02Document1 paginăHW02suresh2104Încă nu există evaluări
- Mpcte1010 Advanced Engg ThermodynamicsDocument2 paginiMpcte1010 Advanced Engg Thermodynamicsalimm raaffÎncă nu există evaluări
- 10.213 Chemical Engineering Thermodynamics Spring 2002 Problem Set DDocument2 pagini10.213 Chemical Engineering Thermodynamics Spring 2002 Problem Set DPM SHÎncă nu există evaluări
- Tutorial Chapter 2Document2 paginiTutorial Chapter 2Nur KamiliaÎncă nu există evaluări
- 082 CH 20Document3 pagini082 CH 20Jagdip ShethÎncă nu există evaluări
- Termo Isı 1011 Örnek4Document13 paginiTermo Isı 1011 Örnek4Şafak MeçoÎncă nu există evaluări
- 2304kJ/kg. H 355K, T: Bars) 0.51 (P - / 4178 C:) 310 (Document5 pagini2304kJ/kg. H 355K, T: Bars) 0.51 (P - / 4178 C:) 310 (suresh singhÎncă nu există evaluări
- Me8391 - EtdDocument3 paginiMe8391 - Etdsyed1188Încă nu există evaluări
- TD Second Law Problem SolutionsDocument10 paginiTD Second Law Problem SolutionsAditya KumarÎncă nu există evaluări
- chp1 AtdDocument3 paginichp1 AtdNivesh JalanÎncă nu există evaluări
- Solution Tutorial 06-Fall-09Document5 paginiSolution Tutorial 06-Fall-09Amaliza90Încă nu există evaluări
- ThermodynamicsDocument58 paginiThermodynamicsSushil Kumar SinghÎncă nu există evaluări
- 8-101 Hot Exhaust Gases Leaving An Internal Combustion Engine Is To Be Used To Obtain Saturated Steam in An Adiabatic HeatDocument1 pagină8-101 Hot Exhaust Gases Leaving An Internal Combustion Engine Is To Be Used To Obtain Saturated Steam in An Adiabatic HeatDedy ManurungÎncă nu există evaluări
- Ambedkar Nagar: Prime Classes For IIT-JEE/PMT, Ambedkar NagarDocument2 paginiAmbedkar Nagar: Prime Classes For IIT-JEE/PMT, Ambedkar NagarUday Prakash SahuÎncă nu există evaluări
- Heat Engines, Entropy, and the Second LawDocument11 paginiHeat Engines, Entropy, and the Second LawJose FÎncă nu există evaluări
- A Modern Course in Statistical PhysicsDe la EverandA Modern Course in Statistical PhysicsEvaluare: 3.5 din 5 stele3.5/5 (2)
- Design and Fabrication of Automated Manual Gear Transmission in Motor BikesDocument57 paginiDesign and Fabrication of Automated Manual Gear Transmission in Motor Bikesanmol6237Încă nu există evaluări
- Sri Ganapathi Industries Kushaiguda Title: Scale Size DRWG No: Sheet NoDocument1 paginăSri Ganapathi Industries Kushaiguda Title: Scale Size DRWG No: Sheet Noanmol6237Încă nu există evaluări
- Nissan Walkin Chennai 1Document2 paginiNissan Walkin Chennai 1anmol6237Încă nu există evaluări
- Nissan Walkin ChennaiDocument2 paginiNissan Walkin Chennaianmol6237Încă nu există evaluări
- ME 443/643: Introduction to HyperMeshDocument37 paginiME 443/643: Introduction to HyperMeshanmol6237Încă nu există evaluări
- Industrial EnclosuresDocument56 paginiIndustrial Enclosuresanmol6237Încă nu există evaluări
- HW Starter Manual March HresolutionDocument23 paginiHW Starter Manual March HresolutionCharan KumarÎncă nu există evaluări
- RGB Color TableDocument5 paginiRGB Color Tableanmol6237Încă nu există evaluări
- Nissan Walkin ChennaiDocument2 paginiNissan Walkin Chennaianmol6237Încă nu există evaluări
- Sri Ganapathi Industries Kushaiguda Title: Scale Size DRWG No: Sheet NoDocument2 paginiSri Ganapathi Industries Kushaiguda Title: Scale Size DRWG No: Sheet Noanmol6237Încă nu există evaluări
- Creo2 Adv PrimerDocument174 paginiCreo2 Adv PrimerAmit JhaÎncă nu există evaluări
- Nissan Walkin ChennaiDocument2 paginiNissan Walkin Chennaianmol6237Încă nu există evaluări
- M Tech Project List 2015 NewDocument5 paginiM Tech Project List 2015 Newanmol6237Încă nu există evaluări
- Tool Design TerminologyDocument5 paginiTool Design Terminologyanmol6237Încă nu există evaluări
- Press Tool Cutting ForceDocument1 paginăPress Tool Cutting Forceanmol6237Încă nu există evaluări
- GD AdvantagesDocument1 paginăGD Advantagesanmol6237Încă nu există evaluări
- GDDocument2 paginiGDanmol6237Încă nu există evaluări
- Term - 1 - Class - X Communicative English - 2010Document27 paginiTerm - 1 - Class - X Communicative English - 2010Nitin GargÎncă nu există evaluări
- Train Schedule: From Bhopal To Indore JN BG (INBD) BPL - INBD PassengerDocument1 paginăTrain Schedule: From Bhopal To Indore JN BG (INBD) BPL - INBD Passengeranmol6237Încă nu există evaluări
- Ceed Model Question PaperDocument21 paginiCeed Model Question PaperSatvender SinghÎncă nu există evaluări
- Gate Syllabus For Mech EnggDocument4 paginiGate Syllabus For Mech Engganmol6237Încă nu există evaluări
- More Than 100 Keyboard Shortcuts Must ReadDocument3 paginiMore Than 100 Keyboard Shortcuts Must ReadChenna Keshav100% (1)
- Train Schedule: From Bhopal To Indore JN BG (INBD) BPL - INBD PassengerDocument1 paginăTrain Schedule: From Bhopal To Indore JN BG (INBD) BPL - INBD Passengeranmol6237Încă nu există evaluări
- Intro To CFD ProblemDocument1 paginăIntro To CFD ProblemlinoÎncă nu există evaluări
- Problem Set 3 Simulation ActivityDocument12 paginiProblem Set 3 Simulation Activityapi-182809945Încă nu există evaluări
- Experiment - 5 Raymond Classifier: Name: Aman Agrawal Roll No:18CH30003Document6 paginiExperiment - 5 Raymond Classifier: Name: Aman Agrawal Roll No:18CH30003akshay agrawalÎncă nu există evaluări
- SR 6898 1 Tevi de Otel PDFDocument1 paginăSR 6898 1 Tevi de Otel PDFCRISTIAN SILVIU IANUCÎncă nu există evaluări
- Sasolwax SP30 TDSDocument2 paginiSasolwax SP30 TDSLaboratorio Inkctech0% (1)
- Coulombs Law PowerpointDocument48 paginiCoulombs Law PowerpointOmar JeCkÎncă nu există evaluări
- Army Public School Bhopal: TOPIC:-" "Document20 paginiArmy Public School Bhopal: TOPIC:-" "Gourav Pathariya100% (1)
- U1 MAgneticPropDocument19 paginiU1 MAgneticPropAbinash PandaÎncă nu există evaluări
- Paper 2Document8 paginiPaper 2Prateek MalhotraÎncă nu există evaluări
- 11th Chemistry Full Book MCQs SQsDocument2 pagini11th Chemistry Full Book MCQs SQsSalman AhmedÎncă nu există evaluări
- Tool Wear MechanismDocument9 paginiTool Wear MechanismDevansh AgrawalÎncă nu există evaluări
- Lighting in VRayDocument13 paginiLighting in VRayTon AlvesÎncă nu există evaluări
- KinetikaDocument8 paginiKinetikaDian Puspita SariÎncă nu există evaluări
- Design of Machine Elements GateDocument4 paginiDesign of Machine Elements GateshashankÎncă nu există evaluări
- Comet Assay: From Wikipedia, The Free EncyclopediaDocument11 paginiComet Assay: From Wikipedia, The Free EncyclopediasuryasivÎncă nu există evaluări
- Lesson Plan: LP-LP Rev. No: 0 Date: 27-12-2012 Page 1 of 5 VIDocument6 paginiLesson Plan: LP-LP Rev. No: 0 Date: 27-12-2012 Page 1 of 5 VIDhileepan KumarasamyÎncă nu există evaluări
- Class X Science Question PaperDocument24 paginiClass X Science Question PaperKalpna RaniÎncă nu există evaluări
- Stoichiometry Basics NotesDocument5 paginiStoichiometry Basics NoteswardaÎncă nu există evaluări
- Centrifuge Moisture Equivalent of Soils: Standard Test Method ForDocument5 paginiCentrifuge Moisture Equivalent of Soils: Standard Test Method ForCarlos CmbbÎncă nu există evaluări
- ChemDocument3 paginiChemLeeanne CabalticaÎncă nu există evaluări
- Sikadur 53Document2 paginiSikadur 53the pilotÎncă nu există evaluări