Documente Academic
Documente Profesional
Documente Cultură
Acrylic1 PC
Încărcat de
Thanh NguyenDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Acrylic1 PC
Încărcat de
Thanh NguyenDrepturi de autor:
Formate disponibile
PROTECTIVE COATINGS
initiator used, the more free radicals generated. The more
chains initiated, the less monomer available for growth of
each chain, and the shorter the chains. The concentration
of monomer in the total reactor charge also has an effect.
For the most part, low concentrations produce lower molecular weight. In this case, however, the final non-volatile
content of the solution will be low. Unless solvent is
removed after completion of the reaction, this method will
not result in higher solids.
One of the most effective ways of controlling molecular
weight is by chain transfer. Small additions (less than 0.5
percent of the monomer weight) of chemicals such as tbutyl mercaptan are used effectively. The chemicals cap the
ends of the growing chain by giving up an unstable (labile)
hydrogen atom, which transfers to the growing polymer
chain. The residue of the chain transfer agent, now bearing
the free radical, is then able to act as an initiator and begins
a brand new chain. Certain solvents may also react in this
manner, although they are not so effective. For this reason,
the choice of solvent for the reaction can be important.
The molecular j &ighLjpf a traditional thermoplastic
acrylic resin may range^JromjQ^OO to 150,000 Thp rpsins
are pnrrnftlly sold as 40 percent to 60 percent weight soHds
solutions (sometimes free of solvent as clear, solid beads of
polymer, known as prills). These molecular weights prevent
the formulation of applicable lacquers at much more than
40 percent by volume. (VOCs are in the range of 550 to 600
g/L [4.5 to 5 lbs/gal.].)
Utility
Traditional thermoplastic acrylics were originally used as
nitrocellulose replacements. They have f>jfp)ten\ ^s^taD
tn nlf-ravinlpf light and can be blended to provide formulations with a range of properties, depending upon monomer
selection. 1 In maintenance applications, the acrylics are
still used to modify vinvl chloride/acetate resi&s to upgrade
their resistance to ultraviolet light attack. In the extreme
case, they were used as maintenance finish coats over highbuild vinyl mid-coats. Acrylic lacquers have been used for
numerous applications. In aiitornotmeJknshing and particularly jyfinUhing applications, they were widely used
because of their ability to reflow on heating after sanding
operations. They have also been used as clears in original
equipment manufacture (OEM) applications for floor and
Methylol groups may be built into an acrylic by taking an acry-lamide or
methacrylamide copolymer and reacting the pen-' dant amide groups
with formaldehyde.
The resultant methylol groups may be etherified with butanol
(under acid conditions) to give a butoxy terminated polymer.
This and/or the methylol terminated moiety can then be used to
react with aminoplasts, epoxy or carboxy terminated resins or can be
homopolymerized.
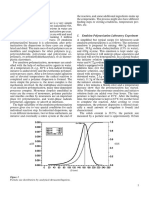
Fig. 8-4
Formation of methylol and butoxy methyl acrylics
100
S-ar putea să vă placă și
- Plastic Piping HandbookDocument359 paginiPlastic Piping Handbookludwing_ulloa100% (13)
- (Plastics Design Library) Carlos Federico Jasso-Gastinel, José M. Kenny - Modification of Polymer Properties-William Andrew (2017)Document222 pagini(Plastics Design Library) Carlos Federico Jasso-Gastinel, José M. Kenny - Modification of Polymer Properties-William Andrew (2017)Monique BarretoÎncă nu există evaluări
- Acrylamide PolymerizationDocument19 paginiAcrylamide Polymerizationrmramsundar100% (2)
- Acrylic ResinDocument14 paginiAcrylic ResinKrishna YeoleÎncă nu există evaluări
- Ion Exchange Resins and Adsorbents in Chemical Processing: Second EditionDe la EverandIon Exchange Resins and Adsorbents in Chemical Processing: Second EditionEvaluare: 5 din 5 stele5/5 (1)
- Modified Polymers, Their Preparation and Properties: Main Lectures Presented at the Fourth Bratislava Conference on Polymers, Bratislava, Czechoslovakia, 1-4 July 1975De la EverandModified Polymers, Their Preparation and Properties: Main Lectures Presented at the Fourth Bratislava Conference on Polymers, Bratislava, Czechoslovakia, 1-4 July 1975A. RomanovEvaluare: 5 din 5 stele5/5 (1)
- Living Radical Polymerization A ReviewDocument36 paginiLiving Radical Polymerization A ReviewAris SusantoÎncă nu există evaluări
- Production of Organic Fertilizer From Local WasteDocument9 paginiProduction of Organic Fertilizer From Local WasteEvans Irabor0% (1)
- Bulk and Suspenshion Polynerization of MMA Into PMMADocument5 paginiBulk and Suspenshion Polynerization of MMA Into PMMADavid Meza CarbajalÎncă nu există evaluări
- Pharmacognosy AnswersDocument22 paginiPharmacognosy AnswersRaghu Srujann100% (4)
- TODO Data Sheet PDFDocument70 paginiTODO Data Sheet PDFJake ZyrusÎncă nu există evaluări
- Siemens Transformers Prototype 420kv Power Transformer PDFDocument18 paginiSiemens Transformers Prototype 420kv Power Transformer PDFbergo197676Încă nu există evaluări
- Guide to Selecting Powder Coatings for SubstratesDocument29 paginiGuide to Selecting Powder Coatings for SubstratesThanh Nguyen100% (1)
- Advances in Blocked Isocyanates and Curatives for PolyurethanesDocument23 paginiAdvances in Blocked Isocyanates and Curatives for PolyurethanesMohammad Doost MohammadiÎncă nu există evaluări
- Astm C582 (1995) PDFDocument7 paginiAstm C582 (1995) PDFamusumuseeÎncă nu există evaluări
- How To Formulate UVDocument16 paginiHow To Formulate UVtobass82100% (3)
- Chemical Recycling of Textile PolymersDocument8 paginiChemical Recycling of Textile PolymersVaishali RaneÎncă nu există evaluări
- Four Polymerization Techniques (Bulk, Solution, Suspension and Emulsion)Document6 paginiFour Polymerization Techniques (Bulk, Solution, Suspension and Emulsion)Ashokkumar Parthipan96% (48)
- IodometryDocument16 paginiIodometryRichelle Lencioco100% (1)
- ACRYLICS: PROPERTIES AND PROCESSING OF POLY(METHYL METHACRYLATEDocument3 paginiACRYLICS: PROPERTIES AND PROCESSING OF POLY(METHYL METHACRYLATESdkmega Hh100% (1)
- How To Formulate UV PDFDocument16 paginiHow To Formulate UV PDFMOHAMEDÎncă nu există evaluări
- TCHOBANOGLOUS Et Al. 2003 Wastewater Engineering PDFDocument25 paginiTCHOBANOGLOUS Et Al. 2003 Wastewater Engineering PDFRusyadi Wicahyo 'Tyo' Aulyanurr33% (3)
- Polymerization of Acrylic EsterDocument7 paginiPolymerization of Acrylic EsterMichelleYapÎncă nu există evaluări
- 3 s2.0 B0080431526004939 MainDocument6 pagini3 s2.0 B0080431526004939 MainMohammed JamaliÎncă nu există evaluări
- Poly Acrylonitrile FiberDocument18 paginiPoly Acrylonitrile FiberSourabh Singh ChauhanÎncă nu există evaluări
- Chakrabarti 1993 Reactive-PolymersDocument45 paginiChakrabarti 1993 Reactive-PolymersPriyam NayakÎncă nu există evaluări
- Les Structures Des RésinesDocument8 paginiLes Structures Des RésinesJuan José Quijano MendozaÎncă nu există evaluări
- Assignment - 1 Chemical Process Technology: 1. Petrochemical's End Product: Polymers & Polymerization TechniquesDocument16 paginiAssignment - 1 Chemical Process Technology: 1. Petrochemical's End Product: Polymers & Polymerization TechniquesAnik MondalÎncă nu există evaluări
- Module 1Document19 paginiModule 1ABIGAIL OLAJUMOKE JOSEPHÎncă nu există evaluări
- Research ProposalDocument5 paginiResearch ProposalmuhammadsaifiÎncă nu există evaluări
- 1B EngbergDocument12 pagini1B EngbergPeter LichterveldÎncă nu există evaluări
- Introduction To Polymer ScienceDocument19 paginiIntroduction To Polymer ScienceAnshul GautampurkarÎncă nu există evaluări
- Research On ChemicalsDocument11 paginiResearch On Chemicalstahera aqeelÎncă nu există evaluări
- PolymersDocument106 paginiPolymersmambouhpriscaÎncă nu există evaluări
- Polymer: Manasa Shetty, Venkata A. Kothapalli, Christopher E. HobbsDocument3 paginiPolymer: Manasa Shetty, Venkata A. Kothapalli, Christopher E. HobbsLutfiana Miftahul JannahÎncă nu există evaluări
- Inverse-Emulsion Copolymerization of Acrylamide and Quaternary Ammonium CationicDocument10 paginiInverse-Emulsion Copolymerization of Acrylamide and Quaternary Ammonium CationicSkolastika ErnaÎncă nu există evaluări
- Vinyl Ester ThesisDocument7 paginiVinyl Ester Thesisbethhalloverlandpark100% (2)
- Acrylamide PolymerizationDocument16 paginiAcrylamide PolymerizationUmakanth Reddy Vudumula100% (1)
- Lecture 8Document29 paginiLecture 8Nagarjuna MalempatiÎncă nu există evaluări
- Chemical AdmixtureDocument19 paginiChemical AdmixturerajasekharÎncă nu există evaluări
- Catherine Lefay, Bernadette Charleux, Maud Save, Christophe Chassenieux, Olivier Guerret, Ste Phanie MagnetDocument11 paginiCatherine Lefay, Bernadette Charleux, Maud Save, Christophe Chassenieux, Olivier Guerret, Ste Phanie MagnetMohammad Doost MohammadiÎncă nu există evaluări
- mLLDPE Metallocene Catalyst Film PropertiesDocument2 paginimLLDPE Metallocene Catalyst Film PropertiesRishikesh ThakurÎncă nu există evaluări
- Enhanced Oil Recovery Techniques: VFSTR Iv Year B.Tech Petroleum Engineering I-SemisterDocument58 paginiEnhanced Oil Recovery Techniques: VFSTR Iv Year B.Tech Petroleum Engineering I-Semisterjaahnavi choudaryÎncă nu există evaluări
- PolymeDocument6 paginiPolymeXuân ĐứcÎncă nu există evaluări
- Tetrahedron Report Number 489Document92 paginiTetrahedron Report Number 489SachidanandanÎncă nu există evaluări
- Ftir On Epoxy ResinsDocument24 paginiFtir On Epoxy ResinsLintang EfendiÎncă nu există evaluări
- New Raw Materials For Cationic UvebDocument8 paginiNew Raw Materials For Cationic UvebArturo RamirezÎncă nu există evaluări
- Miniemulsion Polymerization ThesisDocument5 paginiMiniemulsion Polymerization Thesiserikamorrisfortlauderdale100% (2)
- Publication 12 13688 164Document4 paginiPublication 12 13688 164rajeev ranjanÎncă nu există evaluări
- Four Polymerization Techniques Bulk Solution Suspension and Emulsion PDFDocument6 paginiFour Polymerization Techniques Bulk Solution Suspension and Emulsion PDFGosa harikrishnaÎncă nu există evaluări
- Denture Base ResinsDocument70 paginiDenture Base ResinsNiveditha niranjanÎncă nu există evaluări
- Abbas2013e UnlockedDocument9 paginiAbbas2013e UnlockedIhsan ArifÎncă nu există evaluări
- Industrial Process of Copolymerization and Terpolymerization of Acrylonitrile Experimental Approach and Statistic ModelingDocument10 paginiIndustrial Process of Copolymerization and Terpolymerization of Acrylonitrile Experimental Approach and Statistic ModelingNIKOLEE LIZETH TORRES ZUÑIGAÎncă nu există evaluări
- Acrylic Fibre Dyeing: Scheme: Free Radical Polymerisation of AcrylonitrileDocument6 paginiAcrylic Fibre Dyeing: Scheme: Free Radical Polymerisation of AcrylonitrileCimaÎncă nu există evaluări
- Acrylic Polymers: Versatile Water-Based Polymers for Paints, Coatings & MoreDocument8 paginiAcrylic Polymers: Versatile Water-Based Polymers for Paints, Coatings & MoreShivani UpadhyayÎncă nu există evaluări
- New Applications of Catalytic Chain Transfer PolymerizationDocument11 paginiNew Applications of Catalytic Chain Transfer PolymerizationQuan Nguyen HaiÎncă nu există evaluări
- 1 and 2 Lecture, رابع بوليمرات - د.زينبDocument30 pagini1 and 2 Lecture, رابع بوليمرات - د.زينبMr. nobodyÎncă nu există evaluări
- Use of Organometallic compounds in homogeneous catalysisDocument8 paginiUse of Organometallic compounds in homogeneous catalysisiram ranaÎncă nu există evaluări
- 813201112706Document12 pagini813201112706DrRamesh RedrouthuÎncă nu există evaluări
- DWR WFH3Document3 paginiDWR WFH3barbie.monster89Încă nu există evaluări
- Emulsion Polymerization Process for VeoVa Monomer Latex ProductionDocument7 paginiEmulsion Polymerization Process for VeoVa Monomer Latex ProductionA MahmoodÎncă nu există evaluări
- Photopolymerization of Butyl Acrylate-In-Water Microemulsions: Polymer Molecular Weight and End-GroupsDocument6 paginiPhotopolymerization of Butyl Acrylate-In-Water Microemulsions: Polymer Molecular Weight and End-GroupsJoherbson DeividÎncă nu există evaluări
- Co CheDocument14 paginiCo Chemeocon234Încă nu există evaluări
- Lecture Notes - Polymer Reaction EngineeringDocument8 paginiLecture Notes - Polymer Reaction EngineeringTenson SichoneÎncă nu există evaluări
- Teoria Emulsion PolymerDocument7 paginiTeoria Emulsion PolymerSantos de PradosÎncă nu există evaluări
- Ii. Polymer Synthesis: Part C - Polymerization Techniques: Che El 10: Introduction To Polymer EngineeringDocument35 paginiIi. Polymer Synthesis: Part C - Polymerization Techniques: Che El 10: Introduction To Polymer EngineeringSarah SanchezÎncă nu există evaluări
- Polymer Class 2Document17 paginiPolymer Class 2Ñojib Ëasar ProttoyÎncă nu există evaluări
- Anionic PolymerizationDocument20 paginiAnionic PolymerizationMUHAMMAD AKRAMÎncă nu există evaluări
- Uralkyd OCDocument3 paginiUralkyd OCThanh NguyenÎncă nu există evaluări
- TP PhenalkamineDocument3 paginiTP PhenalkamineThanh Nguyen100% (1)
- D281Document2 paginiD281Thanh NguyenÎncă nu există evaluări
- Fig. 2.2.10 Principles Ofmethods For Milling To Get Small ParticleDocument1 paginăFig. 2.2.10 Principles Ofmethods For Milling To Get Small ParticleThanh NguyenÎncă nu există evaluări
- D281Document2 paginiD281Thanh NguyenÎncă nu există evaluări
- Reactions of Rosin. It Will Be Evident From The Previous DiscussionDocument2 paginiReactions of Rosin. It Will Be Evident From The Previous DiscussionThanh NguyenÎncă nu există evaluări
- PigmentDocument1 paginăPigmentThanh NguyenÎncă nu există evaluări
- Vegetable Oils: The Properties of Raw OilsDocument1 paginăVegetable Oils: The Properties of Raw OilsThanh NguyenÎncă nu există evaluări
- Styrofan ECO 7623 - ENDocument1 paginăStyrofan ECO 7623 - ENLong An ĐỗÎncă nu există evaluări
- Swiss Target PredictionDocument5 paginiSwiss Target PredictionramdaniÎncă nu există evaluări
- Manufacturing Restricted Substances List (MRSL)Document21 paginiManufacturing Restricted Substances List (MRSL)Sajjad AhmedÎncă nu există evaluări
- Understanding key chemistry conceptsDocument9 paginiUnderstanding key chemistry conceptsAwais AliÎncă nu există evaluări
- English DR Soil Sericulture PDFDocument2 paginiEnglish DR Soil Sericulture PDFChathur ChathraÎncă nu există evaluări
- Fat Substitutes and Replacers in Food Product, PDFDocument31 paginiFat Substitutes and Replacers in Food Product, PDFYustinAyuÎncă nu există evaluări
- Chemical TrainingDocument68 paginiChemical Traininghi2lathaÎncă nu există evaluări
- Antioxidant Activity: An OverviewDocument11 paginiAntioxidant Activity: An Overviewsia1811Încă nu există evaluări
- Tender Coconut WaterDocument10 paginiTender Coconut WaterJakka V ReddyÎncă nu există evaluări
- A Comparative Evaluation of Mono Di Triglycerides of Medium Chain Fatty Acids by Lipid-Surfactant-Water Phase Diagram PDFDocument21 paginiA Comparative Evaluation of Mono Di Triglycerides of Medium Chain Fatty Acids by Lipid-Surfactant-Water Phase Diagram PDFpatchris36Încă nu există evaluări
- Meselson Stahl ExperimentDocument12 paginiMeselson Stahl ExperimentLuca Ra BiavatiÎncă nu există evaluări
- Istilah Istilah CoatingDocument63 paginiIstilah Istilah CoatingxajoÎncă nu există evaluări
- TextileDocument18 paginiTextilemadÎncă nu există evaluări
- 2002 Demirbas - Relationships Between Heating Value and Lignin, Moisture, Ash and Extractive Contents of Biomass Fuels PDFDocument7 pagini2002 Demirbas - Relationships Between Heating Value and Lignin, Moisture, Ash and Extractive Contents of Biomass Fuels PDFskakindÎncă nu există evaluări
- Biang Barang Supplier Listing and DescriptionsDocument196 paginiBiang Barang Supplier Listing and DescriptionsNUR CAHYONOÎncă nu există evaluări
- HPMC Experimental ProcedureDocument2 paginiHPMC Experimental ProcedureMae Florizel FalculanÎncă nu există evaluări
- Complete List of Inorganic AcidsDocument2 paginiComplete List of Inorganic AcidsNormina AboÎncă nu există evaluări
- Organic Halides Live Class-3 Teacher NotesDocument40 paginiOrganic Halides Live Class-3 Teacher Notesmardarchod 123Încă nu există evaluări
- EFFECTS OF RADIATION ON PLANTSDocument35 paginiEFFECTS OF RADIATION ON PLANTSOkaroFrankÎncă nu există evaluări
- Liquid-liquid extraction optimizationDocument6 paginiLiquid-liquid extraction optimizationPaula RinconÎncă nu există evaluări
- CA MechanismDocument2 paginiCA MechanismREGINE COELI LANSANGANÎncă nu există evaluări
- Bl-Optigear Synthetic 800 220Document10 paginiBl-Optigear Synthetic 800 220Emin MešićÎncă nu există evaluări