Documente Academic
Documente Profesional
Documente Cultură
tmpA7C TMP
Încărcat de
FrontiersTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
tmpA7C TMP
Încărcat de
FrontiersDrepturi de autor:
Formate disponibile
News and Thoughts
www.microbialcell.com
Non-genetic impact factors on chronological lifespan and
stress resistance of bakers yeast
Michael Sauer and Diethard Mattanovich*
Department of Biotechnology, BOKU VIBT, University of Natural Resources and Life Sciences, Vienna, Muthgasse 18, 1190 Vienna,
Austria.
Austrian Centre of Industrial Biotechnology, Muthgasse 11, 1190 Vienna, Austria.
* Corresponding Author:
Prof. Diethard Mattanovich, Department of Biotechnology, University of Natural Resources and Life Sciences, Muthgasse 18, 1190
Vienna, Austria; Tel: +43 1 47654 6569; E-mail: diethard.mattanovich@boku.ac.at
Survival under nutrient limitation is an essential feature
of microbial cells, and it is defined by the chronological
lifespan. We summarize recent findings, illustrating how
crucial the choice of the experimental setup is for the
interpretation of data in this field. Especially the impact
of oxygen supply differs depending on the culture type,
highlighting the differences of alternatives like the retentostat to classical batch cultures. Finally the importance
of culture conditions on cell aging and survival in biotechnological processes is highlighted.
INTRODUCTION
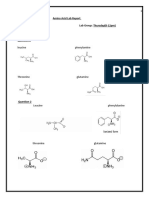
Chronological aging defines the survival of cells in
stationary phase after nutrients become limiting and cells
no longer divide [1] (Figure 1). Cells in this phase of nondivision are interesting because they are known to be more
stress resistant than fast growing cells. However, while the
cells are more resistant to sudden detrimental changes in
their environment, such as oxidative stress or heat shock,
they nevertheless die after a while also under ideal
conditions - due to the aforementioned chronological
aging. The measure for this is the chronological lifespan
(CLS). These cells are furthermore interesting as this state
of non-division is generally important for biology. While
the natural habitat of many yeast species remains a
question for debate, it can be speculated that the natural
state of yeasts is non-dividing under nutrient scarcity for
long periods of time, wherever they are found.
Furthermore, many industrial applications involving bakers
yeast, such as wine making or ethanol production, take
place at least partially under conditions of no-growth.
The ability to survive a period of famine and reproduce
again upon re-feeding is influenced by a variety of internal
and external parameters. While some light has been shed
on the genetic program of aging, less is known about the
environmental impact on chronological aging in bakers
yeast, particularly because the experiments were often not
designed to obtain this knowledge.
FACTORS INFLUENCING CHRONOLOGICAL LIFESPAN
Caloric restriction, that is nutrient limitation without malnutrition, is up to now the most prominent non-genetic
intervention known to prolong the CLS [1]. In fact, the CLS
of yeast cells exposed to glucose under experimental conditions, which do not allow cell growth (such as lack of an
essential amino acid) is dramatically reduced, when compared to cells which are arrested due to the absence of a
carbon source. However, not all carbon sources have the
same effect. Exposure of yeast cells to glycerol under nongrowth conditions has been shown to have no detrimental
effect on CLS [2]. An explanation for this behavior has been
suggested by Burtner et al., who could show that the one
major cause for CLS analyzed under standard conditions, is
the presence of acetic acid at low pH [3]. Experiments to
determine the CLS of yeast cells are usually done in defined,
unbuffered medium. Bakers yeast growing on glucose
accumulates organic acids, among them acetic acid, thereby lowering the pH of the medium significantly (to 3 or
even less). However, it was shown that the presence of
acetic acid at low pH kills the cells rapidly. Buffering the pH
or suspending the starving cells in water instead of spent
medium significantly increases the CLS. Coming back to the
carbon source, it becomes clear why growth on glycerol
has a beneficial effect on CLS, as no acetic acid is produced
under these conditions. The connection of CLS to acetic
________________________
Comment on Bisschops MM, Vos T, Martnez-Moreno R, de la Torre Corts P, Pronk JT, and Daran-Lapujade P (2015). Oxygen availability
strongly affects chronological lifespan and thermotolerance in batch cultures of Saccharomyces cerevisiae. Microbial Cell 2(11): 429-444.
doi: 10.15698/mic2016.06.504
Received originally: 18.01.2016; in revised form: 06.03.2016, Accepted 06.03.2016, Published 12.04.2016.
Keywords: Yeast, chronological lifespan, aging, stationary culture, oxygen.
OPEN ACCESS | www.microbialcell.com
232
Microbial Cell | June 2016 | Vol. 3 No. 6
M. Sauer and D. Mattanovich (2016)
Chronological lifespan of yeast
FIGURE 1: Schematic representation of growth and chronological aging of yeast cells with an indication of factors influencing the chronological
lifespan.
acid at low pH also brings along the explanation why stress
resistance and CLS are tightly interconnected. Many stress
reactions overlap. For example, it has been shown that
stress resistance against high osmolarity at least partially
leads to tolerance against organic acids at low pH. It makes
therefore sense, that preconditioning of bakers yeast cells
at high osmolarity leads to a significantly prolonged CLS
under the standard conditions. However, the stress caused
directly by acetic acid might not be the only metabolic
cause for a reduced CLS, as the acetate metabolism proceeds via the central metabolite acetyl-CoA, which is in
turn a signaling molecule influencing autophagy and thereby interfering with another mechanism, which is essential
for cell survival under nutrient depletion [4]. A further
connection between stationary phase and stress resistance
is the cell cycle control. It has been proposed that tight
control of the cell cycle progression is fundamental to prolonging lifespan [5]. At the same time nutrient scarcity has
a direct impact on cell cycle control, with nutrient sensing
pathways as master regulators thereby closing the connection.
OPEN ACCESS | www.microbialcell.com
233
WHAT HAPPENS TO CELLS UPON NUTRIENT DEPLETION?
The interesting question is, what exactly happens when
cells enter a phase of nutrient scarcity. The obvious and
visible response is the cessation of cell division. However,
much more must happen in order to guarantee the survival
of the culture as long as possible. Stationary-phase cells
must actively respond to these environmental changes and
must remain metabolically and biosynthetically active at
least at reduced levels [6]. Endocytosis, autophagy, and
respiration play a crucial role for the mobilization of intracellular reserves and energy provision for survival. In fact, it
is relatively simple to find correlations of certain biochemical traits with aging and lifespan, however it is extremely
difficult to obtain proof for causal relationships.
It is emerging that new technologies and new approaches need to be applied to understand the underlying
processes. First of all, it has to be acknowledged that a
yeast culture in stationary phase is not made of uniform
cells, which are stationary. At least two populations can
be identified quiescent and non-quiescent cells, which
behave entirely different. A proper description of such
Microbial Cell | June 2016 | Vol. 3 No. 6
M. Sauer and D. Mattanovich (2016)
Chronological lifespan of yeast
cultures must take this fact into account [7]. Furthermore,
choice of the strain and definition of the experimental setup are crucial for obtaining relevant results. Natural
bakers yeast strains are diploid and prototroph, while the
typical laboratory yeast strain is haploid and auxotroph.
The genetic setup directly interferes with the nutrient
sensing pathways, thereby compromising the results
depending on what should be the conclusion. It has been
shown for example, that certain genetic factors have opposite effects on the CLS under laboratory and winemaking
conditions, pointing in a direction that not the actual nutrient concentration might be important, but the ratio in
which nutrients are supplemented [8]. The influence of the
different auxotrophies of laboratory yeast strains on CLS
has not been sufficiently analyzed yet, although it is evident that such an influence exists [9]. First of all, the involved nutrient sensing pathways react not only to carbon
sources but also to nitrogen sources such as amino acids,
thereby dictating a connection. Another aspect is that
yeast cells will take up amino acids when available, and
repress their respective biosynthetic pathways. This clearly
leads to a reprogrammed metabolic network with all its
implications. More directly, it has been shown, that the
concentration and type of amino acids added to the
growth medium are decisive for a toxic effect of ammonium (another typical ingredient of laboratory media) on CLS
[10]. Which amino acids are present, finally depends on the
strain used, which in turn is more often dictated by laboratory habit rather than rational choice. On top of all, CLS
analyses in unbuffered media always include weak acid
stress a fact that needs to be taken into account when
interpreting the results.
However, not only conditions, but also methods need
to be scrutinized. It has been shown only recently using
live-imaging technologies that active actin remodeling is an
essential factor influencing CLS [11]. Active actin points
into the direction that energy availability is a crucial factor
for survival also for resting cells. The most efficient way of
energy provision is respiration and it has been shown that
mitochondrial fractionation is a sign of cellular degeneration leading to death in stationary phase [12]. It appears
therefore also plausible that oxygen availability is beneficial for survival. However, the mitochondria and more particularly reactive oxygen species produced by these respiring organelles have also a central (negative) role in the
aging of cells.
OXYGEN AND CHRONOLOGICAL LIFESPAN
It has been reported that enhancing respiration and mitochondrial gene expression promotes chronological longevity [13]. Under conditions of high glucose availability,
bakers yeast produces energy primarily via fermentation
to ethanol. Only when glucose availability is low, yeast
switches to a respiratory metabolism. Thus, a metabolic
shift from fermentation to respiration is expected under
conditions of caloric restriction, pointing to a connection
between caloric restriction and use of oxygen. It has also
OPEN ACCESS | www.microbialcell.com
234
been shown that a certain pre-adaptation to respiratory
growth promotes a longer CLS [14].
Bisschops et al. looked into more detail of the transition from the growth phase into the stationary phase in
presence and absence of oxygen to understand why the
chronological lifespan of cells grown in anaerobiosis is reduced [15]. In fact, the data presented point into the direction that oxygen availability itself is not the driving force
for better survival (and increased heat stress tolerance) of
aerobically grown cells of bakers yeast. The key-point
seems to be kind of a caloric restriction of these cells,
which grow diauxic first on glucose, then on ethanol. The
growth rate on ethanol is significantly lower as compared
to growth on glucose and the transcriptome is already remodeled during growth on ethanol into the direction of the
stationary phase. This remodeling does not take place in
anaerobic cells. They grow with high growth rate until the
glucose is consumed then they suddenly stop growing,
since ethanol is a non-fermentable carbon source. This
leaves the cells somehow unprepared when glucose is consumed. This result has direct impact on industrial processes
relying on yeast survival in a late fermentation phase, such
as winemaking or bioethanol production. We conclude that
microaeration would allow for the required slow growth in
this phase and lead to proper adaptation for the stationary
phase.
Interestingly, Bisschops et al. show that cells kept in a
retentostat cultivation under anaerobiosis exhibit a CLS
comparable to cells grown in simple batch under aerobic
conditions, proving that the preparation of the cells by
gradually slowing down the growth rate is a key-factor and
this is hardly influenced by the availability of oxygen per se.
However, it shall be underlined that a retentostat culture
looks at cells with a growth rate approaching zero, which is
not exactly the same as a stationary culture of cells. Still
some exogenous energy is fed, even if at extremely low
uptake rate. This means that maintenance energy is provided from outside even when zero growth is achieved,
which separates this state from the true stationary phase
of a culture, illustrating again the importance of a clear
definition of the experimental setup.
CONCLUSION
Particularly for biotechnology, but also to understand the
ecology of yeasts, their ageing behavior in response to
environmental factors is of eminent importance. However,
the techniques used and the experimental setup employed
to study yeast aging have to be appropriately designed to
get the desired results. So was life imaging inevitable to
determine the role of actin even if microscopy with fixed
cells is the standard procedure. The retentostat is necessary to unveil the true potential of bakers yeast to survive
in anaerobiosis even if batch cultures are standard for
physiological analyses. Heterogeneity of cultures needs to
be taken into account even if microbiologists traditionally
tend to describe cultures as a number of uniform cells.
Finally, the choice of the strain and the precise definition of
the experimental setup might have to be re-evaluated,
Microbial Cell | June 2016 | Vol. 3 No. 6
M. Sauer and D. Mattanovich (2016)
Chronological lifespan of yeast
when the aim of the study is fundamental biological understanding.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
COPYRIGHT
2016 Sauer and Mattanovich. This is an open-access
article released under the terms of the Creative Commons
Attribution (CC BY) license, which allows the unrestricted
use, distribution, and reproduction in any medium, provided the original author and source are acknowledged.
Please cite this article as: Michael Sauer and Diethard
Mattanovich (2016). Non-genetic impact factors on chronological
lifespan and stress resistance of bakers yeast Microbial Cell 3(6):
232-235. doi: 10.15698/mic2016.06.504
REFERENCE
1. Longo VD and Fabrizio P (2012). Chronological aging in Saccharomyces cerevisiae. Subcell Biochem 57:101-21.
2. Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li, LM, and Longo VD
(2009). Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet
5:e1000467.
3. Burtner CR, Murakami CJ, Kennedy BK, and Kaeberlein M
(2009). A molecular mechanism of chronological aging in yeast.
Cell Cycle 8:12561270.
4. Schroeder S, Zimmermann A, Carmona-Gutierrez D, Eisenberg T,
Ruckenstuhl C, Andryushkova A, Pendl T, Harger A, and Madeo F
(2015). Metabolites in aging and autophagy. Microbial Cell
1(4):110-114.
5. Jimnez J, Bru S, Ribeiro MPC, and Clotet J (2015). Live fast, die
soon: cell cycle progression and lifespan in yeast cells. Microbial
Cell 2(3):62-67.
6. Gray JV, Petsko GA, Johnston GC, Ringe D, Singer RA, and Werner-Washburne M (2004). Sleeping beauty: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 68:187206.
7. Werner-Washburne M, Roy S, and Davidson GS (2012). Aging
and the survival of quiescent and non-quiescent cells in yeast
stationary-phase cultures. Subcellular Biochemistry 57:123-143.
8. Picazo C, Orozco H, Matallana E, and Aranda A (2015). Interplay
among Gcn5, Sch9 and mitochondria during chronological aging of
wine yeast is dependent on growth conditions. PLoS One
10(2):e0117267.
10. Santos J, Leo C, and Sousa MJ. (2013). Ammoniumdependent shortening of CLS in yeast cells starved for essential
amino acids is determined by the specific amino acid deprived,
through different signaling pathways. Oxid Med Cell Longev
2013:161986.
11. Vasicova P, Lejskova R, Malcova I, and Hasek J (2015). The
Stationary-Phase Cells of Saccharomyces cerevisiae Display Dynamic Actin Filaments Required for Processes Extending Chronological Life Span. Mol Cell Biol 35(22):3892-908.
12. Breitenbach M, Rinnerthaler M, Hartl J, Stincone A, Vowinckel
J, Breitenbach-Koller H, and Ralser M (2014). Mitochondria in
ageing: there is metabolism beyond the ROS. FEMS Yeast Res
14(1):198-212.
13. Bonawitz ND, Chatenay-Lapointe M, Pan Y, and Shadel GS
(2007). Reduced TOR signaling extends chronological life span via
increased respiration and upregulation of mitochondrial gene
expression. Cell Metab 5:26577.
14. Piper PW, Harris NL, and MacLean M (2006). Preadaptation to
efficient respiratory maintenance is essential both for maximal
longevity and the retention of replicative potential in chronologically ageing yeast. Mech Ageing Dev 127:73340.
15. Bisschops MM, Vos T, Martnez-Moreno R, de la Torre Corts
P, Pronk JT, and Daran-Lapujade P (2015). Oxygen availability
strongly affects chronological lifespan and thermotolerance in
batch cultures of Saccharomyces cerevisiae. Microbial Cell 2(11):
429-444.
9. Mlleder M, Capuano F, Pir P, Christen S, Sauer U, Oliver SG,
and Ralser M. (2012). A prototrophic deletion mutant collection
for yeast metabolomics and systems biology. Nat Biotechnol
30:1176-8.
OPEN ACCESS | www.microbialcell.com
235
Microbial Cell | June 2016 | Vol. 3 No. 6
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Sample SOP For Biotechnology in CanadaDocument3 paginiSample SOP For Biotechnology in CanadaAlbert MarreddyÎncă nu există evaluări
- tmpF178 TMPDocument15 paginitmpF178 TMPFrontiersÎncă nu există evaluări
- tmpE3C0 TMPDocument17 paginitmpE3C0 TMPFrontiersÎncă nu există evaluări
- Tmp1a96 TMPDocument80 paginiTmp1a96 TMPFrontiersÎncă nu există evaluări
- Tmpa077 TMPDocument15 paginiTmpa077 TMPFrontiersÎncă nu există evaluări
- tmp998 TMPDocument9 paginitmp998 TMPFrontiersÎncă nu există evaluări
- tmp3656 TMPDocument14 paginitmp3656 TMPFrontiersÎncă nu există evaluări
- tmp27C1 TMPDocument5 paginitmp27C1 TMPFrontiersÎncă nu există evaluări
- tmp96F2 TMPDocument4 paginitmp96F2 TMPFrontiersÎncă nu există evaluări
- tmpA7D0 TMPDocument9 paginitmpA7D0 TMPFrontiersÎncă nu există evaluări
- tmp97C8 TMPDocument9 paginitmp97C8 TMPFrontiersÎncă nu există evaluări
- Baileys 13th Ed Chapter QuestionsDocument79 paginiBaileys 13th Ed Chapter QuestionsrhymeÎncă nu există evaluări
- KARYA ILMIAH KERIPIK MAWAR SalinanDocument13 paginiKARYA ILMIAH KERIPIK MAWAR SalinanNanda BasÎncă nu există evaluări
- AbD Serotec Antibodies France - rEDDocument1.323 paginiAbD Serotec Antibodies France - rEDrdLuis1Încă nu există evaluări
- Exosomes For Medicine: Babak NamiDocument50 paginiExosomes For Medicine: Babak NamiBabak NamiÎncă nu există evaluări
- Clinical Pharmachology MUDocument489 paginiClinical Pharmachology MUسلام شاكر حميد جميل 6506Încă nu există evaluări
- Crossword-9 Lrat MVRPDocument2 paginiCrossword-9 Lrat MVRPFrancis ValdezÎncă nu există evaluări
- Classification of Nutrients: Nutrition (Unit 2)Document7 paginiClassification of Nutrients: Nutrition (Unit 2)thakshiÎncă nu există evaluări
- Alkaloids: December 2010Document33 paginiAlkaloids: December 2010Jessica Asitimbay ZuritaÎncă nu există evaluări
- PHD Pre-Defence Presentation - Final - 1Document50 paginiPHD Pre-Defence Presentation - Final - 1Ink OfficeÎncă nu există evaluări
- Cell DivisionDocument17 paginiCell DivisionRaj AgrawalÎncă nu există evaluări
- Singh 2019 Lulu and Nana Open Pandoras Box FarDocument2 paginiSingh 2019 Lulu and Nana Open Pandoras Box FarPetru CernatÎncă nu există evaluări
- Dna Fingerprinting2Document12 paginiDna Fingerprinting2lindayanaÎncă nu există evaluări
- 7 Points: Clear SelectionDocument7 pagini7 Points: Clear SelectionCristine EchaveÎncă nu există evaluări
- Chapter 8 - Molecular Basis of InheritanceDocument20 paginiChapter 8 - Molecular Basis of Inheritancejhela18Încă nu există evaluări
- Amino Acid Lab ReportDocument5 paginiAmino Acid Lab ReportShanya ShayalÎncă nu există evaluări
- Kher 2014Document4 paginiKher 2014Chinar PatelÎncă nu există evaluări
- Breast Cancer Cell LineDocument34 paginiBreast Cancer Cell LineCatherine RajanÎncă nu există evaluări
- Insect Metamorphosis PresentationDocument10 paginiInsect Metamorphosis PresentationTomi ObohÎncă nu există evaluări
- The Physiology of Potassium in Crop ProductionDocument31 paginiThe Physiology of Potassium in Crop ProductionSumanth KumarÎncă nu există evaluări
- Estequiometría Del Crecimiento Microbiano y Formación de ProductoDocument9 paginiEstequiometría Del Crecimiento Microbiano y Formación de ProductoDaniela NavasÎncă nu există evaluări
- Native Page Note v3Document2 paginiNative Page Note v3Ramakrishnan KumarÎncă nu există evaluări
- Cri Du Chat SyndromeDocument20 paginiCri Du Chat Syndromehadiqa aimen100% (1)
- Water Saturated Phenol ADocument2 paginiWater Saturated Phenol Aوائل عبدهÎncă nu există evaluări
- Efek Dimethyl Sulfoxide (DMSO) Terhadap Karakteristik Sel Punca Limbal (SPL) TikusDocument6 paginiEfek Dimethyl Sulfoxide (DMSO) Terhadap Karakteristik Sel Punca Limbal (SPL) Tikusdindashezaria7Încă nu există evaluări
- MCAT Topic Focus Biology Electrophoresis and Blotting FSQ DrillDocument1 paginăMCAT Topic Focus Biology Electrophoresis and Blotting FSQ DrillAnjalie GulatiÎncă nu există evaluări
- Pharmacokinetic and Pharmacodynamic Drug Interactions Associated With Antiretroviral DrugsDocument136 paginiPharmacokinetic and Pharmacodynamic Drug Interactions Associated With Antiretroviral DrugsNenad PanzalovićÎncă nu există evaluări
- TRIGLISERIDADocument28 paginiTRIGLISERIDAAstika RahayuÎncă nu există evaluări
- Fluvoxamine A Review of Its Mechanism of Action and Its Role in Covid19 - Sukhatme2021Document9 paginiFluvoxamine A Review of Its Mechanism of Action and Its Role in Covid19 - Sukhatme2021nienminhÎncă nu există evaluări
- 2009-Transient Expression of Red and Yellow Fluorescent Protein Vectors in HCT-8 Cells Infected With Cryptosporidium ParvumDocument7 pagini2009-Transient Expression of Red and Yellow Fluorescent Protein Vectors in HCT-8 Cells Infected With Cryptosporidium ParvumwiwienÎncă nu există evaluări