Documente Academic
Documente Profesional
Documente Cultură
Water & PH
Încărcat de
TomLeahTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Water & PH
Încărcat de
TomLeahDrepturi de autor:
Formate disponibile
water
H-O-H covalently bonded. 104.5 degrees. van der waals radius of O=1.4A H=1.2A covalent=0.96A
highly polar molecule: H +ve and oxygen -ve form a dipole bond called a hydrogen bond.
this makes water a liquid not a gas at room temp. the bonds are strong but not stable.
they are constantly breaking and reforming so structure is chaotic.
structure of ice is organised and spacious so less dense than water i.e. ice floats on water
water is a good solvent for charge/polar substances but a bad solvent for non polar substances.
water dissolves salt due to the polarity.
hydrogen bonds
hydrogen bonds form in many other molecules than water.
requires prsesence of hydrogen covalently bonded to an electronegative molecule like oxygen or
nitrogen (donor) and in presence of another polar electronegative molecule (acceptor)
bond is stronger when straight aligned with the compound hydrogen is bonded to
bond strengths: covalent > ionic > hydrogen bond > van der waals
ionic is much weaker in water
acid base buffers pH
water self ionises: H20 H+ + OH- H+ is in the form of hydronium ion H3O+
a mole contains 6.02x1023 molecules
molecular mass is the number of g in one mole (given on periodic table)
concentration is measured in MOLES per LITRE (M)
e.g. 1L = 1000ml = 1000g
1 mole of water has a mass of 18g
so mol/L is 1000/18 = 55.6 M or moles of water in a litre of water
equilibrium constant is the concentration of products / reactants
Keq = [H+] . [OH-] / [H2O]
Kw is the ion product of water and is always constant at 1x10-14 M2
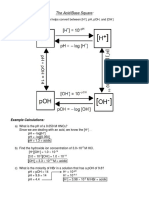
pH = -log[H+] as pH is determined by conc of H+ (H3O+)
remember pH measures occurence of H+ (H3O+) i n water i.e. 1 in 100,000
pH and pOH always adds to 14 e.g. in neutral solution [H+] = [OH-] and so pH is 7
pH scale is logarithmic so pH 5 is 10 times more acidic than pH 6
acids are proton donors.
bases are proton acceptors.
strong acids and bases will completely dissociate in water
whereas weak acids and bases only partially dissociate
each acid has a conjugate base and vice versa that forms in dissociation
HCl is a strong acid so it fully dissociates
1M of HCl gives 1M of H+ pH = -log(1) = 0
NaOH is a strong base
1M NaOH gives 1M of OH[OH-] . [H+] = 10-14 pH = -log(10-14) = 14
weak acid dissociation is determined by the acid dissociation constant: Ka
can be used to calculate pH
log(Ka) = pKa
-log(H+) = pH
pH = pKa + log [proton acceptor] / [proton donor] or [base] / [acid]
known as henderson-hasselbach equation
when [A-] = [HA] then pH = pKa solution can be used as a buffer
buffers resist the change of pH and are most efficient at pH = pKa
they are mixtures of weak acid and their conjugate base
buffering region is 1 pH above and below. after this pH changes rapidly.
different weak acids have different pKas and buffer regions so can limit to different pHs
the Ka of a weak acid is constant
Ka = [A-].[H+] / [HA]
when strong acid is added (H+ added) then [A-] must go down (by combining with the H+) in order to
keep the equilibrium constant
pI is the isoelectric point and is the pH at which the molecule has no net charge
pI = 1/2 (pK1 + pK2) where 1 and 2 are either side of the neutral molecule
in panic attacks, hyperventilation increases CO2 conc so blood pH drops below 7.35 (acidemia)
different enzymes have different optimal pHs and ranges at which they function
S-ar putea să vă placă și
- Nerve CellsDocument112 paginiNerve CellsTomLeahÎncă nu există evaluări
- ImmunologyDocument4 paginiImmunologyTomLeahÎncă nu există evaluări
- Abhishek 0713 Worms - JJJJJJJJJJJJJJJJJJJJJJJJJDocument34 paginiAbhishek 0713 Worms - JJJJJJJJJJJJJJJJJJJJJJJJJatti174Încă nu există evaluări
- MND NotesDocument1 paginăMND NotesTomLeahÎncă nu există evaluări
- NotesDocument2 paginiNotesTomLeahÎncă nu există evaluări
- NoneDocument1 paginăNoneTomLeahÎncă nu există evaluări
- TransposonsDocument1 paginăTransposonsTomLeahÎncă nu există evaluări
- StructureDocument2 paginiStructureTomLeahÎncă nu există evaluări
- Antibiotic NotesDocument1 paginăAntibiotic NotesTomLeahÎncă nu există evaluări
- BiochemistryDocument1 paginăBiochemistryTomLeahÎncă nu există evaluări
- Amino AcidsDocument1 paginăAmino AcidsTomLeahÎncă nu există evaluări
- PolypeptidesDocument1 paginăPolypeptidesTomLeahÎncă nu există evaluări
- Enzymology DataDocument6 paginiEnzymology DataTomLeahÎncă nu există evaluări
- Translation 7Document1 paginăTranslation 7TomLeahÎncă nu există evaluări
- An Understanding About Why Some Chilli Peppers Are Hotter Than OthersDocument1 paginăAn Understanding About Why Some Chilli Peppers Are Hotter Than OthersTomLeahÎncă nu există evaluări
- Origin & Diversity PlantsDocument1 paginăOrigin & Diversity PlantsTomLeahÎncă nu există evaluări
- Grand ChallengeDocument1 paginăGrand ChallengeTomLeahÎncă nu există evaluări
- Water & PHDocument2 paginiWater & PHTomLeahÎncă nu există evaluări
- GlycolysisDocument2 paginiGlycolysisTomLeahÎncă nu există evaluări
- Chi SquaredDocument1 paginăChi SquaredTomLeahÎncă nu există evaluări
- 914 BbsDocument2 pagini914 BbsjamesyuÎncă nu există evaluări
- HelloDocument1 paginăHelloTomLeahÎncă nu există evaluări
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5782)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (72)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Cap 3Document12 paginiCap 3Milton OrtegaÎncă nu există evaluări
- Simultaneous Spectrophotometric Determination of Valsartan and Ezetimibe in PharmaceuticalsDocument7 paginiSimultaneous Spectrophotometric Determination of Valsartan and Ezetimibe in PharmaceuticalsEllie satrianiÎncă nu există evaluări
- Stability Indicating HPLC Method for Mesalamine AnalysisDocument5 paginiStability Indicating HPLC Method for Mesalamine Analysiscavalo2080Încă nu există evaluări
- Chemical Equilibrium - Lecture NotesDocument46 paginiChemical Equilibrium - Lecture NotespokeyballÎncă nu există evaluări
- Liquinox Cleaning ValidationDocument12 paginiLiquinox Cleaning Validationdnagent007Încă nu există evaluări
- Determination of The Equilibrium Constant For The Triiodide Ion Formation Using Distribution Coefficient and Kinetics MeasurementsDocument7 paginiDetermination of The Equilibrium Constant For The Triiodide Ion Formation Using Distribution Coefficient and Kinetics MeasurementsUsama MaoudÎncă nu există evaluări
- Crystal Systems Nomenclature and Conventions ExplainedDocument8 paginiCrystal Systems Nomenclature and Conventions ExplainedArley GonzálezÎncă nu există evaluări
- AP Unit9 Worksheet AnswersDocument5 paginiAP Unit9 Worksheet AnswersAAVANIÎncă nu există evaluări
- NCM 103 Final ExamDocument5 paginiNCM 103 Final ExamRichmond LacadenÎncă nu există evaluări
- Tutorial 6 2016 - 496Document12 paginiTutorial 6 2016 - 496EdcelPerlacioÎncă nu există evaluări
- BM 501 Essentials of BiophysicsDocument2 paginiBM 501 Essentials of BiophysicssampotÎncă nu există evaluări
- Column Chromatography: Lab ReportDocument7 paginiColumn Chromatography: Lab ReportChiÎncă nu există evaluări
- QC FINAL EXAM PART 1 KEY TERMSDocument2 paginiQC FINAL EXAM PART 1 KEY TERMSSunshine_Bacla_4275Încă nu există evaluări
- Acids, Bases and SaltsDocument8 paginiAcids, Bases and SaltsAnonymous TX2OckgiZÎncă nu există evaluări
- Esomeprazole Mag. Trihydrate Pellets 22.5%Document5 paginiEsomeprazole Mag. Trihydrate Pellets 22.5%Life Pearl100% (1)
- Marking Scheme Chemistry Perfect Score Module Form 4 Set 4Document11 paginiMarking Scheme Chemistry Perfect Score Module Form 4 Set 4Nurhanani Mohd NazariÎncă nu există evaluări
- Chapter 14 Equilibrium AnnotatedDocument81 paginiChapter 14 Equilibrium Annotatedyour mamaÎncă nu există evaluări
- Tutorial HPLCDocument34 paginiTutorial HPLCMauricio CamarenaÎncă nu există evaluări
- No Name SubmitionDocument7 paginiNo Name SubmitionANIME SOLOÎncă nu există evaluări
- Acidity and Basicity of DrugsDocument52 paginiAcidity and Basicity of DrugsMichelle PatriciaÎncă nu există evaluări
- Instrumental Chromatographic Techniques (Part 3Document24 paginiInstrumental Chromatographic Techniques (Part 3Hannah Jean EstebanÎncă nu există evaluări
- CHEM 1101 Introduction To Solid State ChemistryDocument7 paginiCHEM 1101 Introduction To Solid State ChemistryNicholas OwÎncă nu există evaluări
- Calculating PH PogilDocument6 paginiCalculating PH PogilLily StantonÎncă nu există evaluări
- Development and Validation of New RP HPLC Method For Analysis of Capecitabine in Pharmaceutical Dosage Form - Ijsit - 2.1.3Document10 paginiDevelopment and Validation of New RP HPLC Method For Analysis of Capecitabine in Pharmaceutical Dosage Form - Ijsit - 2.1.3International Journal of Science Inventions TodayÎncă nu există evaluări
- Protein Purity and Molecular WeightDocument6 paginiProtein Purity and Molecular WeightAbg Khairul Hannan Bin Abg AbdillahÎncă nu există evaluări
- Acid Base PK PHDocument58 paginiAcid Base PK PHsimon njorogeÎncă nu există evaluări
- IS 3025 (PART 23) : 1986 Metiiods of Sampling and Test (Physical Chemical) For Wa"FerDocument4 paginiIS 3025 (PART 23) : 1986 Metiiods of Sampling and Test (Physical Chemical) For Wa"FerRaghav TiwaryÎncă nu există evaluări
- Analysis of Hydrocarbons in Common Fuels by Solid-Phase Microextraction (Spme) and Gas Chromatography - Mass Spectrometry (GC-MS)Document6 paginiAnalysis of Hydrocarbons in Common Fuels by Solid-Phase Microextraction (Spme) and Gas Chromatography - Mass Spectrometry (GC-MS)Amirul Azhar100% (11)
- Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption SpectrometryDocument7 paginiAnalysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption SpectrometryaaÎncă nu există evaluări
- 7.1 Dynamic EquilibriumDocument26 pagini7.1 Dynamic EquilibriumScotrraaj GopalÎncă nu există evaluări