Documente Academic
Documente Profesional
Documente Cultură
Difference Between Osmolarity, Osmolality and Tonicity - Deranged Physiology
Încărcat de
Shruti YennamDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Difference Between Osmolarity, Osmolality and Tonicity - Deranged Physiology
Încărcat de
Shruti YennamDrepturi de autor:
Formate disponibile
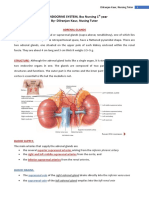
Difference between osmolarity, osmolality and tonicity - Deranged Physiology http://www.derangedphysiology.com/main/core-topics-intensive-care/ma...
1 of 2
Deranged Physiology Home Core Topics in Intensive Care Manipulation of Fluids and Electrolytes Chapter 0.1.2
Di#erence Between Osmolarity, Osmolality and Tonicity
Osmolarity
Osmolarity is the measure of solute concentration per unit VOLUME of solvent.
Its not the same as tonicity! Osmolarity takes into account ALL of the solute concentrations, not just the ones that cant cross the semipermeable
membrane.
Osmolality
Osmolality is the measure of solute concentration per unit MASS of solvent.
You never measure osmolarity in practice, because water changes its volume according to temperature (but mass remains the same, and so it is
more convenient and consistent)
Osmolality is the same in the ICF and the ECF.
Both inside and outside, the osmolality is 285-290 mOsm/Kg.
Stubbornly, in spite of the membrane being water-impermeable lipid, water moves across the cell membrane well enough; more on this later.
Tonicity
Tonicity is the measure of the osmotic pressure gradient between two solutions.
Unlike osmolarity, tonicity is only in uenced by solutes that cannot cross this semipermeable membrane, because these are the only solutes
in6uencing the osmotic pressure gradient.
7us, you can have iso-osmolar solutions which are not isotonic.
Iso-osmolar solutions which are not isotonic: 5% dextrose and intracellular uid
5% dextrose, when infused, is iso-osmolar with the body 6uid compartments. Its osmolality is the same as the osmolality of the cellular contents
(about 300mOsm/L) However, because dextrose penetrates the cells so easily, it cannot contribute to tonicity. 7us, the infused dextrose is
iso-osmolar but hypotonic.
7us, a solution can be iso-osmolar and hypotonic, when the solute contributing to its osmolality is not a solute which can contribute to its
tonicity. 7ese osmoles, which don't contribute to the tonicity, are derisively termed "ine>ective osmoles" by Brandis. Dextrose and urea are the
main ine>ective osmoles. In a diabetic patient, dextrose can still be an e>ective osmole.
Dextrose and urea are the main ine ective osmoles.
10/4/2016 9:57 PM
Difference between osmolarity, osmolality and tonicity - Deranged Physiology http://www.derangedphysiology.com/main/core-topics-intensive-care/ma...
2 of 2
In a diabetic patient, dextrose can still be an e>ective osmole.
7e major "e>ective" osmole is SODIUM.
Sodium and its anion harem contribute to 86% of the osmolality and 92% of the tonicity.
References
References
anaesthesiamcq.org, as always;
My source for some of this information has been Ohs Manual (6th edition), speciDcally chapter 84 by Simon Finfer and Anthony
Delaney.
Most of this information derives from easily accessible physiology textbooks, such as Ganongs Review of Medical Physiology 23rd
edition.
10/4/2016 9:57 PM
S-ar putea să vă placă și
- Appendicitis: The Etiology, Hygenic and Dietetic TreatmentDe la EverandAppendicitis: The Etiology, Hygenic and Dietetic TreatmentEvaluare: 3 din 5 stele3/5 (2)
- CVS Lect 6 Blood Pressure, PathophysiologyDocument13 paginiCVS Lect 6 Blood Pressure, PathophysiologySherwan R Shal100% (5)
- Genetics Unit OutineDocument2 paginiGenetics Unit Outinejmunozbio@yahoo.comÎncă nu există evaluări
- Thalamus and HypothalamusDocument19 paginiThalamus and Hypothalamusapi-266034924Încă nu există evaluări
- Introduction To General AnatomyDocument7 paginiIntroduction To General AnatomyPia Abila100% (1)
- Cutaneous Circulation: DR Pushpa Lata Sachan Associate Professor CIMS&H, LucknowDocument19 paginiCutaneous Circulation: DR Pushpa Lata Sachan Associate Professor CIMS&H, LucknowNishankumar JhaÎncă nu există evaluări
- BLOOD PresentationDocument33 paginiBLOOD PresentationLezlie Jane SahaliÎncă nu există evaluări
- Plasma ProteinDocument46 paginiPlasma ProteinM.PRASAD NAIDUÎncă nu există evaluări
- 1 Prof Chauvins Instructions For Bingham CH 4Document35 pagini1 Prof Chauvins Instructions For Bingham CH 4Danielle Baldwin100% (2)
- Human Anatomy,: First Edition Mckinley & O'LoughlinDocument40 paginiHuman Anatomy,: First Edition Mckinley & O'LoughlinAgung PurmanaÎncă nu există evaluări
- Asthma: A. Practice EssentialsDocument8 paginiAsthma: A. Practice EssentialsCandha NurcahyaÎncă nu există evaluări
- Arterial Blood Gas - ppt1Document53 paginiArterial Blood Gas - ppt1Madhuri100% (1)
- Anatomy and Physiology of Blood VesselDocument3 paginiAnatomy and Physiology of Blood Vesselneleh grayÎncă nu există evaluări
- 11-Acid-Base BalanceDocument28 pagini11-Acid-Base BalanceKathlene BarasiÎncă nu există evaluări
- Cardiovascular System: K. Hariharan Iv Eee - 'B'Document33 paginiCardiovascular System: K. Hariharan Iv Eee - 'B'Hari Haran100% (1)
- Types of ParalysisDocument6 paginiTypes of ParalysisLoh Wei ChiehÎncă nu există evaluări
- Chapter 24 - The Solar SystemDocument36 paginiChapter 24 - The Solar SystemHeather Blackwell100% (1)
- Composition and Function of Blood ComponentsDocument17 paginiComposition and Function of Blood ComponentsPrakash Kumar Nayak100% (1)
- Administering Ear DropDocument2 paginiAdministering Ear DropJenny WongÎncă nu există evaluări
- Blood Transfusion: Etiology of Blood CellDocument4 paginiBlood Transfusion: Etiology of Blood Cellbunso padillaÎncă nu există evaluări
- Urinary SystemDocument10 paginiUrinary SystemElyka Alivan Valdez PolonioÎncă nu există evaluări
- The Cockroach (Periplaneta Americana, L.): An Introduction to Entomology for Students of Science and MedicineDe la EverandThe Cockroach (Periplaneta Americana, L.): An Introduction to Entomology for Students of Science and MedicineEvaluare: 4.5 din 5 stele4.5/5 (2)
- Vasodilators: A B C D XDocument1 paginăVasodilators: A B C D XPatricia Marie BuenafeÎncă nu există evaluări
- List of Modern Equipment and Farm ToolsDocument15 paginiList of Modern Equipment and Farm ToolsCarl Johnrich Quitain100% (2)
- BloodDocument38 paginiBloodchukwukerechimezirimÎncă nu există evaluări
- Body Fluid Compartment and Formation of Edema 1Document4 paginiBody Fluid Compartment and Formation of Edema 1JayricDepalobosÎncă nu există evaluări
- Body Fluids Fluid Physiology NotesDocument19 paginiBody Fluids Fluid Physiology NotesBrianÎncă nu există evaluări
- Fluid ElectrolyteDocument115 paginiFluid ElectrolytePaul EbenezerÎncă nu există evaluări
- Ebola VirusDocument16 paginiEbola VirusSisilia AlfinaÎncă nu există evaluări
- Basic of Fluid Therapy ImaDocument69 paginiBasic of Fluid Therapy Imal Made ArtawanÎncă nu există evaluări
- GastrointestinalDocument39 paginiGastrointestinalالمسوول الاعلاميÎncă nu există evaluări
- Pulmonary CirculationDocument16 paginiPulmonary Circulationsajid_saiyad0% (1)
- Five Minute Personality Test PDFDocument1 paginăFive Minute Personality Test PDFSekar MuruganÎncă nu există evaluări
- Anatomy and Physiology of Adrenal GlandsDocument4 paginiAnatomy and Physiology of Adrenal Glandsxxxcamzxxx100% (3)
- The Circulatory System BookletDocument30 paginiThe Circulatory System BookletTroy CampbellÎncă nu există evaluări
- Myasthenia Gravis: An Autoimmune Neurologic DisorderDocument16 paginiMyasthenia Gravis: An Autoimmune Neurologic DisorderHibba NasserÎncă nu există evaluări
- Physiology of RespirationDocument2 paginiPhysiology of RespirationIOSRjournalÎncă nu există evaluări
- Circulatory System SummaryDocument3 paginiCirculatory System SummaryNiel S. DefensorÎncă nu există evaluări
- 411 419Document9 pagini411 419Afif MansorÎncă nu există evaluări
- Endo 3 Notes PDFDocument9 paginiEndo 3 Notes PDFDilÎncă nu există evaluări
- AerosolsDocument9 paginiAerosolsTubaÎncă nu există evaluări
- Phalanx FractureDocument46 paginiPhalanx FractureBagas WidhiarsoÎncă nu există evaluări
- Sickle Cell DiseaseDocument29 paginiSickle Cell DiseaseAzzam FaridÎncă nu există evaluări
- Medical Terminology of Anatomical Positions - FK Undana Lsit 2016Document127 paginiMedical Terminology of Anatomical Positions - FK Undana Lsit 2016Maria JozilynÎncă nu există evaluări
- Intro Head and NeckDocument74 paginiIntro Head and NeckAuza Moses Ibrahim100% (2)
- Fracture Treatment ProtocolsDocument14 paginiFracture Treatment ProtocolsZikrul IdyaÎncă nu există evaluări
- Nursing Care - TraditionalDocument38 paginiNursing Care - Traditionalchishimba louisÎncă nu există evaluări
- General Principles of Gastrointestinal Motility: Alimentary TractDocument6 paginiGeneral Principles of Gastrointestinal Motility: Alimentary TractKC White Dela Rosa100% (1)
- Concept Map 5Document2 paginiConcept Map 5api-354331689Încă nu există evaluări
- Case Study: MYXEDEMATOUS COMADocument5 paginiCase Study: MYXEDEMATOUS COMAjisooÎncă nu există evaluări
- Health and IllnessDocument38 paginiHealth and IllnessKathrina AlfonsoÎncă nu există evaluări
- Action Potential ECGDocument11 paginiAction Potential ECGAndrei ManeaÎncă nu există evaluări
- NotesDocument14 paginiNotesJan Rey L. TejereroÎncă nu există evaluări
- Cardiac OutputDocument31 paginiCardiac OutputanojÎncă nu există evaluări
- Systemic CirculationDocument3 paginiSystemic CirculationWira SentanuÎncă nu există evaluări
- Properties of BloodDocument3 paginiProperties of BloodRaj SinghÎncă nu există evaluări
- 20 Questions On AtherosclerosisDocument5 pagini20 Questions On AtherosclerosisPaul WestonÎncă nu există evaluări
- Principles of Anatomy and Physiology: 14 EditionDocument62 paginiPrinciples of Anatomy and Physiology: 14 EditionWilliam C Chisha100% (1)
- CT KubDocument2 paginiCT KubKumail KhandwalaÎncă nu există evaluări
- Ana Phisio Lab Report.Document4 paginiAna Phisio Lab Report.Diana Amor100% (1)
- Introduction in Human Anatomy and PhysiologyDocument35 paginiIntroduction in Human Anatomy and Physiologyraul nino MoranÎncă nu există evaluări
- Diabetes Insipidus (Agu Presentation)Document15 paginiDiabetes Insipidus (Agu Presentation)Um HamoOdÎncă nu există evaluări
- Osmolarity: Osmolarity Is The Measure of Solute Concentration Per Unit VOLUME of SolventDocument4 paginiOsmolarity: Osmolarity Is The Measure of Solute Concentration Per Unit VOLUME of SolventCoy BroÎncă nu există evaluări
- R15 Understanding Business CyclesDocument33 paginiR15 Understanding Business CyclesUmar FarooqÎncă nu există evaluări
- Sanskrit Lessons: �丘��恆� � by Bhikshuni Heng HsienDocument4 paginiSanskrit Lessons: �丘��恆� � by Bhikshuni Heng HsiendysphunctionalÎncă nu există evaluări
- Trading Journal TDA Branded.v3.5 - W - Total - Transaction - Cost - BlankDocument49 paginiTrading Journal TDA Branded.v3.5 - W - Total - Transaction - Cost - BlankChristyann LojaÎncă nu există evaluări
- Assembly InstructionsDocument4 paginiAssembly InstructionsAghzuiÎncă nu există evaluări
- Electromagnetism WorksheetDocument3 paginiElectromagnetism WorksheetGuan Jie KhooÎncă nu există evaluări
- SAP HR - Legacy System Migration Workbench (LSMW)Document5 paginiSAP HR - Legacy System Migration Workbench (LSMW)Bharathk KldÎncă nu există evaluări
- MQXUSBDEVAPIDocument32 paginiMQXUSBDEVAPIwonderxÎncă nu există evaluări
- SKF Shaft Alignment Tool TKSA 41Document2 paginiSKF Shaft Alignment Tool TKSA 41Dwiki RamadhaniÎncă nu există evaluări
- Marieb ch3dDocument20 paginiMarieb ch3dapi-229554503Încă nu există evaluări
- Chunking Chunking Chunking: Stator Service IssuesDocument1 paginăChunking Chunking Chunking: Stator Service IssuesGina Vanessa Quintero CruzÎncă nu există evaluări
- Channel & Lomolino 2000 Ranges and ExtinctionDocument3 paginiChannel & Lomolino 2000 Ranges and ExtinctionKellyta RodriguezÎncă nu există evaluări
- AAR Shell ProgrammingDocument13 paginiAAR Shell ProgrammingMarimuthu MuthaiyanÎncă nu există evaluări
- Energy-Roles-In-Ecosystems-Notes-7 12bDocument10 paginiEnergy-Roles-In-Ecosystems-Notes-7 12bapi-218158367Încă nu există evaluări
- Corporate Tax Planning AY 2020-21 Sem V B.ComH - Naveen MittalDocument76 paginiCorporate Tax Planning AY 2020-21 Sem V B.ComH - Naveen MittalNidhi LathÎncă nu există evaluări
- Icici PrudentialDocument52 paginiIcici PrudentialDeepak DevaniÎncă nu există evaluări
- Bustax Midtem Quiz 1 Answer Key Problem SolvingDocument2 paginiBustax Midtem Quiz 1 Answer Key Problem Solvingralph anthony macahiligÎncă nu există evaluări
- Saif Powertec Limited Project "Standard Operating Process" As-Is DocumentDocument7 paginiSaif Powertec Limited Project "Standard Operating Process" As-Is DocumentAbhishekChowdhuryÎncă nu există evaluări
- Community Resource MobilizationDocument17 paginiCommunity Resource Mobilizationerikka june forosueloÎncă nu există evaluări
- USDA List of Active Licensees and RegistrantsDocument972 paginiUSDA List of Active Licensees and Registrantswamu885Încă nu există evaluări
- Annex To ED Decision 2013-015-RDocument18 paginiAnnex To ED Decision 2013-015-RBurse LeeÎncă nu există evaluări
- Agm 1602W-818Document23 paginiAgm 1602W-818Daniel BauerÎncă nu există evaluări
- Junos ErrorsDocument2 paginiJunos ErrorsrashidsharafatÎncă nu există evaluări
- Pontevedra 1 Ok Action PlanDocument5 paginiPontevedra 1 Ok Action PlanGemma Carnecer Mongcal50% (2)
- Empanelled Hospitals List Updated - 06-12-2022 - 1670482933145Document19 paginiEmpanelled Hospitals List Updated - 06-12-2022 - 1670482933145mechmaster4uÎncă nu există evaluări
- Atlascopco XAHS 175 DD ASL Parts ListDocument141 paginiAtlascopco XAHS 175 DD ASL Parts ListMoataz SamiÎncă nu există evaluări
- MN Rules Chapter 5208 DLIDocument24 paginiMN Rules Chapter 5208 DLIMichael DoyleÎncă nu există evaluări
- MSC ACFN2 RD4 ClassDocument25 paginiMSC ACFN2 RD4 Classmengistu jiloÎncă nu există evaluări