Documente Academic
Documente Profesional
Documente Cultură
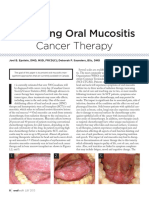
EKSTRAVASASI
Încărcat de
Sasha Hidayat FullDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
EKSTRAVASASI
Încărcat de
Sasha Hidayat FullDrepturi de autor:
Formate disponibile
Newborn & Infant Nursing Reviews 13 (2013) 189195
Contents lists available at ScienceDirect
Newborn & Infant Nursing Reviews
journal homepage: www.nainr.com
Neonatal Extravasation: An Overview and Algorithm for Evidence-based Treatment
Victoria Beall, BSN, CWOCN, RN a,, Brent Hall, PharmD c,
James T. Mulholland, BSN, RN b, Sheila M. Gephart, PhD, RN b
a
b
c
Neonatal/Pediatric Wound, Ostomy and Continence Nurse, Diamond Childrens Medical Center at the University of Arizona, Tucson, AZ
The University of Arizona, College of Nursing, Tucson, AZ
Diamond Children's Medical Center at The University of Arizona, Tucson, AZ
a r t i c l e
i n f o
Keywords:
Neonatal
Inltration
Extravasation
Vesicant
Peripheral intravenous (PIV) therapy
Algorithm
a b s t r a c t
The peripheral intravenous (PIV) catheter is the most used vascular access device for the administration of
medications in hospitalized neonates, however 95% of PIV catheters are removed due to complications.
Inltration and extravasation are one of the most destructive complications to the neonate's fragile skin. This
article reviews multiple aspects of inltration and extravasation injury. First, starting at the cellular level the
role of vesicants in vascular injury and its role triggering inammation will be discussed, followed by a
comprehensive review of vesicants and their mechanism of injury, by pH, osmolality or chemical composition,
then an overview of the NICU nurses knowledge and actions to prevent inltration and ending with the use of
an evidence-based algorithm that was developed at one children's hospital to minimize injury caused by
extravasations through targeted, prompt treatment.
2013 Elsevier Inc. All rights reserved.
Many medications given to neonates have the potential to injure
when an extravasation occurs. An extravasation is described by the
Infusion Nurses Society (INS) as the inadvertent administration of a
vesicant solution or medication into the surrounding tissues. 1 A
vesicant is dened as a solution or medication that causes the
formation of blisters leading to tissue necrosis and sloughing.
Extravasation can result in varying degrees of localized tissue injury
and can cause pain, infection, and partial to full thickness tissue loss
involving muscles and nerves. If extravasation is severe and depending on the site, skin grafts, long hospitalization and high costs result.
Not surprisingly, with disgurement and loss of function, parents may
try to recover payment for their loss by initiating lawsuits. 2,3
Inltration Is Common Among Neonates
The peripheral intravenous (PIV) catheter is the most used vascular
access device for the administration of medications in hospitalized
neonates; however 95% of PIV catheters are removed due to complications such as leaking, occlusion and inltration.4 Inltration rates
among neonates are as high as 57%70% with extravasation occurring in
1123%.5 Both inltration and extravasation are destructive, causing
localized injury to the neonate's fragile skin.6 Inltration of nonvesicants can cause considerable tissue damage from pressure on inter Address correspondence to Victoria Beall, RN, BSN, CWOCN, Neonatal/Pediatric
Wound, Ostomy and Continence Nurse, Diamond Childrens Medical Center at the
University of Arizona, 1501 N. Campbell Ave, P.O. Box 245116 Tucson, AZ 85724.
Tel.: +1 520 694 7328.
E-mail addresses: victoria.beall@uahealth.com (V. Beall), brent.hall@uahealth.com
(B. Hall), mulhollj@email.arizona.edu (J.T. Mulholland), gepharts@arizona.edu
(S.M. Gephart).
1527-3369/1304-0528$36.00/0 see front matter 2013 Elsevier Inc. All rights reserved.
http://dx.doi.org/10.1053/j.nainr.2013.09.001
nal structures as additional uid collects around the vein, and in severe
cases can result in compartment syndrome.6 Extravasations have the
potential to cause peripheral tissue injury depending on the type of
vesicant, concentration of the vesicant, location, amount, and duration of
exposure to the vesicant. Damage from a vesicant may progress over
time and become evident 4872 hours after the extravasation occurs.7,8
Neonatal Vulnerability to Vascular Injury
The preterm and sick neonate is more susceptible to skin injury
and complications from extravasation injury than their mature,
healthy counterparts. Their immature skin structures, exible subcutaneous tissue, small blood vessels and poor venous integrity increase
the risk of complication from venipuncture and IV infusions. 5,8 The
goal in neonatal care is to prevent skin breakdown whenever possible.
Similarly, attention to thermoregulation, pain and stress that infants
endure as a result of repeated IV attempts or restarts, and inltrations
and extravasations must be considered and managed. 9,10 Multiple
tools are available to score pain responses and enable the NICU
(neonatal intensive care unit) nurses to manage this appropriately.
Nonpharmacologic measures to decrease pain include the use of a
pacier, swaddling, or administration of sucrose during the insertion
of an IV or for inltration and extravasation injury. 10 Aside from nonpharmacologic interventions, treating inltration and extravasation
pain with analgesics should be considered. 8
Inammation in the Premature Infant
The neonatal immune system is poorly regulated compared to
adults and dysregulation is magnied when neonates are born
190
V. Beall et al. / Newborn & Infant Nursing Reviews 13 (2013) 189195
early. 1113 While intravenous therapy is necessary in this population,
it is not without its risks. Vesicants can harm the endothelial lining of
the blood vessel, triggering production and release of oxygen
free radicals that spur inammation. 14,15 The release of the locally
induced free radicals into the circulatory system when not controlled
can trigger a system-wide response to the stressor and further free
radical release. In a normal response, the infant's body mounts
an anti-inammatory release of free radical scavengers. When this
anti-inammatory response is inadequate, which is common in
prematurity, or the inammatory assault is severe, endothelial
dysfunction leads to programmed cell death (apoptosis). 16 The load
of oxidative stress in premature infants is especially of concern as
it has been linked to various neonatal morbidities including
necrotizing enterocolitis, 16,17 retinopathy of prematurity, 1820 and
chronic lung disease. 16,2123
The Neonatal Intensive Care Unit (NICU) Nurse's Role
NICU nurses monitor the PIV site with vigilance to aid in early
identication of inltration and extravasation and prevent this type of
injury whenever possible. Identifying an inltration may be difcult,
even for the most experienced nurse. 10 The NICU nurse is aware of the
subtle changes in heart rate, oxygen saturations, apnea, and the more
obvious change in behavior such crying and agitation that may
indicate problems with the PIV therapy. 4 Neonatal nursing entails not
only basic knowledge of the anatomy and physiology of neonatal skin
and how to prevent iatrogenic injury from routine nursing care but
also knowledge of vascular anatomy and infection control for the safe
delivery of infusion therapy. 4,8,10 Several organizations have developed standards and guidelines that assist nurses with the best practice
in infusion therapy. 10 These evidence-based guidelines and grading
scales for inltrations can aid with standardizing documentation and
protocols that guide nursing care and improve patient outcomes. 5,6
Pathophysiology of Vascular Injury
In the human body, vascular injury, oxidative stress, and
inammation are intricately related. Inltration and extravasation
are both symptoms of vascular injury. When vascular injury occurs,
the release of free radicals is stimulated and the energy producing
ability of the cell becomes dysfunctional as mitochondria are
damaged. As free radicals are released and the capacity of the
damaged mitochondria to produce energy is limited, the balance
between nitric oxide and superoxide is disrupted. This reactive
oxygen species excess extends the damage. 2426 A continual cycle of
free radical production and endothelial apoptosis occurs leading to
injury of cell membranes and vessels. Symptoms of this process
include redness, swelling (inammation with increasing vascular
permeability), and visible tissue injury. 25 The inammatory process
involved in extravasation is depicted in Fig 1. Note how the
interaction of the biological response to chemical and non-chemical
risk factors when exposed to the harmful drug directly affects healthy
endothelium, leading to vascular injury. The framework is a helpful
tool in understanding the biological aspects of extravasation and
action targets to prevent injury.
Potential Origins of Inltration
There is a supposition that an inltration or extravasation is caused
by IV catheter dislodgement or puncture of the vein during insertion
or during handling of the infant. Chemical composition of medications
also impacts risk of vein rupture. 5 The vein's tolerance to an infusion is
affected by the osmolality and pH of the vesicant, the duration of the
exposure, and irritation to the endothelial cells. 4 An additional factor
in causing a cannulated vessel to rupture and leak is the pressure in
which the medication is being delivered by the infusion pump. 3,5
Janet Pettit describes the following 3 theories of the mechanism of
inltration and extravasations. The rst theory states that the
irritation of the venous endothelium from the infusate causes
vasoconstriction and diminished blood ow. 4 The second theory has
been conrmed by dye studies. It shows inltration of the infusate
through the catheter insertion hole created with IV placement; this
can occur when the ow proximal to the catheter tip becomes
obstructed. 4 The third theory is that irritation of the venous
endothelium caused by the osmolality, pH, or chemical composition
Fig 1. Model of vascular injury.
V. Beall et al. / Newborn & Infant Nursing Reviews 13 (2013) 189195
of the infusate damages the lining of the vein and allows transmission
of the infusate into the tissue, without creating a puncture in the
vein. 4 These three theories demonstrate how inltration and
extravasation can occur; the remainder of the damage is thought to
be caused by the irritants and vesicants.
Irritants and Vesicants Given to Neonates
Intravenous medications can be divided into three major subcategories: 1) non-vesicants, 2) irritants, and 3) vesicants. In order for
an inltration to be a true extravasation, the offending agent, by
denition, must be a vesicant. There are a number of different
qualities that affect the potential for a medication to result in tissue
damage. These include, but are not limited to: osmolarity, pH, direct
medication effects and solubility. 27 See Table 1 for medications
categorized by their mechanism of injury.
Osmolarity describes the number of particles which are suspended
in solution. Normal serum osmolarity of a neonate is approximately
280 mOsm. When a solution has a higher osmolarity, it is considered
to be hyperosmolar. The effect causes cells to shrink as uid shifts
from inside to outside of the cell. This response occurs in order to
increase the osmolarity inside the cell to equilibrate with the elevated
osmolarity of the serum. The opposite of this effect would be a
solution that has a lower osmolarity or is hypoosmolar. In this
situation, uid would enter the cell in order to decrease the
osmolarity of the cell to equal that outside. At the most extreme,
this can result in cell lysis as the volume of the cell exceeds its
capacity. Osmolarity may be altered by how a medication is diluted.
Ampicillin is a good example of this. If 50 mg of ampicillin is mixed
with 1 mL of sterile water, the osmolarity is 243. If 50 mg of ampicillin
is mixed with 1 mL of normal saline, the osmolarity would be
191
increased to 493520 and thus increase potential damage to tissue. 4 It
is generally felt that solutions with an osmolarity b900 mOsm can be
administered peripherally. This is based on an American Academy of
Pediatrics Committee on Nutrition recommendation governing the
use of peripheral parenteral nutrition (PPN). 4 There is some
controversy with this recommendation due to a lack of supporting
evidence. The Infusion Nurses Society recommends limiting the
osmolarity of peripherally administered solutions to b 500 mOsm.
This is based on evidence that more concentrated solutions are more
likely to result in chemical phlebitis. 4
The measurement of the concentration of hydrogen ions, pH, is
another important consideration when evaluating a medication's risk
of inltration or extravasation. A normal pH of blood is between 7.35
and 7.45 (arterially). As the pH of a medication moves away from this
normal range, the risk of tissue injury is higher. As the number of
hydrogen ions increase, the solution will become more acidic (pH
b7.35). The majority of medications have an acidic to neutral pH. Once
medications move out of the range of 59, risk of inammation and
vascular injury increases signicantly. Vancomycin, for example, has a
pH of 2.54.5, which helps to explain its potential for extravasation.
Medications with alkalotic pH (N7.45) can be just as damaging to
tissue. One example is phenytoin with a pH of 1012, approximating
that of household bleach.
Medications have specic mechanisms of action that may result in
cellular damage when an inltration/extravasation occurs. Vasoactive
medications such as dopamine have effects on alpha-receptors. If this
product is introduced into tissue, this alpha stimulation will result in
constriction of capillary beds, which subsequently will decrease local
blood ow. Local tissue will be deprived of oxygen and ischemic injury
will ensue. 4 Another group of medications that may have direct effects
on tissues are electrolytes. Calcium is necessary for depolarization and
contraction of smooth muscle. If a concentrated calcium solution is
Table 1
Medication Mechanisms of Vascular Injury.
Inciting Agent
Vesicants
(phenylephrine)
Irritants
Hypo-osmolar
Hyperosmolar
(N10% dextrose,
mannitol,
potassium, propofol
and sodium
bicarbonate)
Alkalotic Medication
Vasoactive
Medication
Highly-Lipophilic
Medications
Denition
Medications capable
of causing tissue
damage9
e.g. Calcium
stimulates smooth
muscle to contract
capillaries, leading
to hypo-perfusion
and ischemic injury
Causes pain within
the vessel or
surrounding tissue
but does not result
in tissue necrosis
Osmolarity
b280 mOsm
causing cells to
swell as uid shifts
into the cell. As the
intracellular volume
exceeds its capacity,
cells can lyse.
Osmolarity
N280 mOsm,
causing cells to
shrink as uid shifts
outside of the cell
High concentration
of hydrogen ions
(high pH) causes
inammation and
can lead to vascular
injury
Alpha-receptor
stimulation
constricts capillary
beds, decreases local
blood ow and
deprives local tissue
of oxygen leading to
ischemic injury5
Medications do not
dilute well in water,
making it difcult to
ush out or wash
off, damage results
from the high
concentration of the
medication in the
tissue
Medication
Examples
Acyclovir
Aminophylline
Calcium Chloride
Dobutamine
Dopamine
Epinephrine
Nafcillin
Norepinephrine
Oxacillin
Penicillin
Phenytoin,
Potassium Salts
Total parenteral
nutrition (TPN)
Peripheral
parenteral nutrition
(PPN)
Vancomycin3
Aminophylline
Calcium Gluconate
Digoxin,
Erythromycin,
Gentamicin,
Theophylline3
0.2 % NaCl
Sterile water5
3% sodium chloride
Calcium chloride
Contrast media
Total parenteral
nutrition5
Sodium Bicarbonate
Phenobarbital
Sodium thiopental
Phenytoin
Dobutamine
Epinephrine
Norepinephrine
Vasopressin
Diazepam
Digoxin
Nitroglycerine
Phenytoin
192
V. Beall et al. / Newborn & Infant Nursing Reviews 13 (2013) 189195
infused into tissue, this may result in capillary constriction via
stimulation of smooth muscle, which will also result in ischemic
injury to hypoperfused tissue. 4,7 Finally the solubility of a medication
can inuence its contribution to local tissue damage. The more
lipophilic a medication, the less water-soluble it is. This makes
washing out the site impractical and leads to high concentrations of
medications located in relatively small areas of tissue that can lead to
concentration dependent direct tissue damage.
Nursing Actions to Prevent Vascular Injury
The best method to decrease complications of PIV therapy is to
prevent them in the rst place.2 Serious complications are not entirely
preventable, but following recommended standards of IV therapy is the
best approach for avoiding complications.3 The decision to place a
peripherally inserted central catheters (PICC) or central venous lines
(CVL) might be needed if vascular access is difcult or long-term
parenteral therapy is planned. However, this may be difcult in low birth
weight (LBW) neonates when access for a CVL is limited, central line
infection prevention initiatives are paramount, or a variety of incompatible infusions are indicated.2,6 The risks and benets of PICC and CVL are
evaluated by the medical and surgical teams. If the risks are reasoned to
be too great for PICC or CVL, responsibility will be on the nurse to manage
the PIV and when vesicants are being infused vigilance needs to heighten.
Evidence-based guidelines for infusion therapy start with the insertion.
See Table 2 for recommendations to prevent peripheral vascular injury.
On insertion, attention needs to be paid to the selection of the appropriate
catheter, site selection, skin antisepsis and stabilization once the device is
in place.10 NICU nurses learn to leave the neonates' extremity out of the
swaddling or sleep sacks for easy visualization of the PIV site; so that
hourly assessments (and more frequent assessments if a known vesicant
is infusing) do not interrupt the neonate's developmental sleep. Warning
signs of possible complication warranting further investigation include a
PIV that is difcult to ush or an infant who responds to the ush with
crying or inching.3 Assessments might reveal swelling, redness, and
coolness of the skin. Most inltrates resolve spontaneously after the IV
catheter is removed, however in severe cases blistering and skin necrosis,
prolonged capillary rell and decreased pulses and movement of the
extremities can result. Inltration scales can be useful to nurses in
identifying the severity of the assessment ndings.3
Key Actions to Minimize Injury When Extravasation Occurs
Once an inltration or extravasation is discovered, immediate
treatment is the key to preventing progressive damage from the
vesicant. 2 Treatment decisions are based on the size and appearance
of the injury, type of IV infusing, duration of exposure and location. 8
Protocols and algorithms can be used to assist nurses in the steps
needed to minimize the potential damage and start the treatment
process. Stopping the infusion and elevation of the extremity is the
rst actions followed by placement of a saline soaked gauze or
prepackaged normal saline pad. The saline draws out the vesicant, and
impedes a scab from forming to allow uid to leak out. Gently
squeezing the uid from the open insertion site can also help to
remove the offensive agent. 8 While the saline soaks are held in place,
assistance with the various tasks that are required for treatment may
require additional personnel. A new PIV may need to be inserted
immediately, the physician or nurse practitioner (NP) will be called to
assess the site and plan for pharmacologic treatment if indicated. For
an extravasation, treatment should be determined prior to catheter
removal. 8 The administration of antidotes or enzymes such as
hyaluronidase is the method of choice to manage IV extravasation. 6
Treatment that improves tissue perfusion and prevents progressive
tissue necrosis has been shown to be most effective if initiated within
1 hour. 8 Ongoing assessment of the neonate and measures to improve
comfort or alleviate pain may be indicated. Compassionately reporting
the situation to the family as soon as possible and keeping them up to
date on the plan of action are required. 8 Prior exposure to standards of
care, protocols and algorithms and staff education could aid the
process of administering antidotes, as this could be time consuming
on-the-job-training for an inexperienced NICU nurse, when timely
treatment provides better patient outcomes. 8 An algorithm based on
the work of Sawatzky-Dickson and colleagues (2006) (see Fig 2) was
developed to streamline the described process and improved patient
outcomes. 2
The quality improvement initiatives implementing the algorithm
have not been formally evaluated. However, physicians, pharmacists
and nurses are managing inltration and extravasations promptly as a
team. The administration of hyaluronidase or the appropriate antidote
without the WOCN's assistance, as well as a decline in referrals for
long-term wound management for extravasation injuries are now the
NICU's norm.
Pharmacologic Options to Treat Extravasations
Currently there are multiple medications that are used to treat
extravasations. These include hyaluronidase, phentolamine, and
topical nitroglycerin 2% ointment. 8 Sodium thiosulfate, dimethyl
sulfoxide (DMSO) and dexrazoxane are antidotes primarily associated
with treatment of chemotherapy extravasations. As the scope of this
article is directed at the neonatal population, it will focus on the rst
three non-chemotherapeutic extravasation medications: hyaluronidase, phentolamine and nitroglycerin. 7,8,26,27
Hyaluronidase
Table 2
Recommendations for Practice to Prevent Vascular Injury.
Peripheral IV Insertion and Maintenance
Use small enough plastic/silicone catheter to avoid restriction of blood ow
Avoid repeated use of a vein
Avoid placing a PIV in an areas difcult to immobilize
Use transparent tape to secure
Cover the site with a sterile semi-permeable transparent dressing that will permit
ongoing visualization of the insertion site
Upper extremities less likely to inltrate or leak compared with peripheral IV in
lower extremities or scalp veins
Place tape loosely over boney prominences to avoid restricting blood ow to the
extremity
Infusion Maintenance
Limit PIV glucose to 12.5%
Dilute medications as much as possible before administration are other solutions
to preventing extravasation
PIV= peripheral intravenous.
Hyaluronidase is an enzyme that breaks down hyaluronic acid, a
compound best described as the glue which holds cells together.
When this extracellular glue is dissolved, cells are separated. This is
helpful in extravasations as it allows for the medication to distribute
through a larger area by breaking down the walls that keep it
localized. This not only helps to decrease the concentration effects of
the extravasated product, but also exposes the medication to more
capillary beds that allows for reduction of edema via more rapid
reabsorption and removal of the product from the damaged area. The
overall effect is to minimize the local damage caused by the
extravasation. This change is not permanent, as hyaluronic acid will
be regenerated within a 24 to 48 hour period. Efcacy of hyaluronidase is supported by an FDA subcommittee evaluation from 2009
based on evidence from animal studies, human studies and clinical
case reports. Hyaluronidase has been used to limit extravasation
injury since 1976. One study suggested that use of hyaluronidase may
V. Beall et al. / Newborn & Infant Nursing Reviews 13 (2013) 189195
193
Fig 2. Diamond Childrens IV Inltration and Extravasation Algorithm (adapted from Sawatzky-Dickson and colleagues, [2006]). LIP = Licensed Independent Practitioner, MD =
Medical Doctor, WOCN =Wound, Ostomy, and Continence Nurse.
194
V. Beall et al. / Newborn & Infant Nursing Reviews 13 (2013) 189195
decrease the development of skin ulcers by 5060% and decrease skin
ulcer size by as much as 50%. 7,28,29
Hyaluronidase has been used in various ways and consensus is
lacking on which way is best. Most protocols advocate administration
of hyaluronidase within 1 to 2 hours of the initial injury but there are
case reports suggesting administration as far out as 10 days may be
benecial. Concentrations of hyaluronidase used in studies ranges
from 15 unit/mL to 1500 unit/mL but standard practice for neonatal
and pediatric patients is to use a concentration of 15 unit/mL. Some
centers will have the practitioner pull back the inltrated IV 1 to
2 mm to avoid IV injection and then administer 0.2 mL of a 15 unit/mL
solution through the catheter to deliver hyaluronidase directly to the
site of extravasation. The standard way to utilize hyaluronidase is to
draw up 5 syringes of 0.2 mL of the 15 unit/mL concentrations and,
after cleansing the site, inject these at 5 separate sites around the area
of extravasation. Again, the idea is to break down the barrier that is
holding the medication in that specic area and allow it to disperse to
a larger surface area. 26,28 Adverse effects are uncommon but
potentially include tachycardia, hypotension, dizziness, chills, urticarial erythema, angioedema, nausea and vomiting.
and atraumatic for the neonate with minor skin breakdown or in the
neonate with full thickness wounds requiring wound care for weeks.
Hydrogel dressings are one of the moist wound healing treatments
that have been found to be evidence-based and safe for neonates of all
gestations. 8,9 Hydrogels consist of 8090% water, which can be
soothing and gentle to skin and keeps the wound moist to facilitate
auto-debridement of wounds by rehydrating sloughing tissue and
enhancing the rate of autolysis. 8 A section of hydrogel sheet cut to
cover the wound and secured with transparent dressing reduces
dressing changes to every 3 days, resulting in decreased handling and
discomfort of the neonate, and less trauma to regenerated tissue, as
well as protects the tissue from outside oxygen tension and provides a
lower pH, which inhibits the growth of pathogens. 2,3 Full thickness
injury may require surgical debridement and skin grafting, by a
pediatric or plastic surgeon. 8 Adhesive silicone foam can be used for
shallow wounds that may only require a small daily application of
amorphous gel to maintain moisture to the site. The moisture of
hydrogels at times can macerate the periwound, in infants greater
than 30 days; an alcohol free skin barrier can be applied to protect the
fragile skin surrounding the wound. 8
Phentolamine
Conclusions
Phentolamine is an antidote that will counteract the effect of
vasoactive agents such as dopamine, epinephrine, norepinephrine
and phenylephrine. 8 These medications result in vasoconstriction via
stimulation of alpha-receptors. Phentolamine acts to block the activity
of alpha-receptors and subsequently will help relax vascular smooth
muscle. This will improve circulation in the area of the extravasation
and thus decrease ischemia and cell death. Phentolamine can also be
utilized for vasopressin or dopamine extravasation. 28 Phentolamine
should be administered within 12 hours of initial exposure but
administration should occur as soon as possible. Prepare a 0.5 to
1 mg/mL solution and administer 0.1 mg/kg (to a max of 2.5 mg in
neonates, 10 mg in older children and adults) into the existing IV
of the affected area. Neonates should be monitored closely for
potential development of hypotension, dysrhythmias and tachycardia
due to potential systemic absorption of phentolamine. 3,28 The
efcacy of phentolamine has been demonstrated in animal studies
and case reports. 3032
Preventing and detecting early damage from vesicants are a
priority of every neonatal nurse. In this article we have described the
pathophysiology of vascular injury, the medications that incite it and
recommendations to prevent and treat inltrations and extravasations. Incorporating training on critical nursing assessments, signs of
injury and key actions to treat it are an important part of every NICU's
education plan. We recommend using an algorithm, like the one
presented here, to guide actions once extravasation occurs. Using
non-pharmacologic approaches to treat the injury is a rst step in the
process of limiting damage. For certain vesicant-induced injury,
pharmacologic treatment using hyaluronidase, in particular, has been
shown to limit the injury. If ineffective or the injury is extensive,
surgical treatment may be necessary and a plastic surgeon may need
to be consulted. Along the way, openly speaking with the parents
about the treatment is necessary and important to maintain the
family-healthcare partnership.
Nitroglycerin Ointment (2%)
References
Nitroglycerin 2% is an option to treat extravasations. 8 Nitroglycerin
acts to relax smooth muscle resulting in arteriolar, arterial and venous
vasodilation that results in increased capillary blood ow, counteracting the effects of vasoactive medications. This will help to reverse
tissue ischemia and cell death. In the neonatal population, there is a
case report describing the use of 1 inch of 2% nitroglycerin ointment
for treatment of a dopamine extravasation, located in the dorsum of
the left hand, in a 1.8 kg 34 week preemie. This resulted in return of
circulation within a few minutes. Of note, treatment was started
almost 12 hours after the extravasation was initially noted and
patient had no signicant change in hemodynamics. 33
Ongoing Assessment and Wound Care
If the skin is intact, elevation of the extremity and hourly
observation for the next 12 crucial hours will aid in resolution of
the edema and early identication for the development of surface skin
injury. If there is blistering or skin breakdown; moist wound healing is
indicated. The days that these injuries were left open to dry out and
heal on their own should be gone and moist wound healing methods
took their place. A moist wound environment will facilitate epidermal
regeneration, broblast proliferation, and endothelial cell proliferation. 2 Now with advanced wound care treatments; healing can be safe
1. Alexander M, Corrigan A, Gorski L, et al. Infusion Nurses Society (INS) Infusion
nursing an evidence-based approach. St. Louis, Missouri: Saunders Elsevier; 2010:
366, 470-471, 481.
2. Sawatzky-Dickson D, Bodnaryk K. Neonatal Intravenous extravasation injuries:
evaluation of a wound care protocol. Neonatal Network. 2006;25:13-19.
3. Thigpen J. Peripheral intravenous extravasation: nursing procedure for initial
treatment. Neonatal Network. 2007;26:379-384.
4. Pettit J. Assessment of the infant with a peripheral intravenous device. Advances in
Neonatal Care. 2003;3:230-240.
5. McCullen K. A retrospective chart review of risk factors for extravasation among
neonates receiving peripheral intravascular uids. Journal of Wound, Ostomy and
Continence Nurses. 2006;33:133-139.
6. Amjad I, Murphy T, Nylander-Housholder L, Ranft A. A new approach to
management of intravenous inltration in pediatric patients. Journal of Infusion
Nursing. 2011;34:242-249.
7. Kuensting L. Treatment of intravenous inltration in a neonate. Journal of Pediatric
Health Care. 2012;24:184-188.
8. Association of Women's Health, Obstetrics, and Neonatal Nurses. Neonatal Skin
Care; Evidence-based Guidelines. 3rd Ed. Washington, DC: Association of Womens
Health, Obstetric and Neonatal Nurses; 2013. p. 57-62.
9. Cisler-Cahill L. A protocol for the use of amorphous hydrogel to support wound
healing in neonatal patients: an adjunct to nursing skin care. Neonatal Network.
2006;25:267-273.
10. Sundquist-Beauman S, Swanson A. Neonatal infusion therapy: complications and
improving outcomes. Newborn and Infant Nursing Reviews. 2006;6:193-201.
11. Jurges ES, Henderson DC. Inammatory and immunological markers in preterm
infants: correlation with disease. Clin Exp Immunol. 1996;105:551-555.
12. Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis
and developmental impact. Pediatrics. 2010;125:1031-1041.
13. Matoba N, Yu Y, Mestan K, et al. Differential patterns of 27 cord blood immune
biomarkers across gestational age. Pediatrics. 2009;123:1320-1328.
V. Beall et al. / Newborn & Infant Nursing Reviews 13 (2013) 189195
14. Ng PC, Li K, Wong RP, Chui K, Wong E, Li G, Fok TF. Proinammatory and antiinammatory cytokine responses in preterm infants with systemic infections. Arch
Dis Child Fetal Neonatal Ed. 2003;88:F209-F213.
15. Kim M, Christley S, Alverdy JC, Liu D, An G. Immature oxidative stress management
as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights
from an agent-based model. Surgical infections. 2012;13:18-32.
16. Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet
Gynecol. 2001;13:147-153.
17. Aydemir C, Dilli D, Uras N, et al. Total oxidant status and oxidative stress are
increased in infants with necrotizing enterocolitis. J Pediatr Surg. 2011;46:
2096-2100.
18. Dani C, Cecchi A, Bertini G. Role of oxidative stress as physiopathologic factor in the
preterm infant. Minerva Pediatr. 2004;56:381-394.
19. Kumar P, Sankar MJ, Deorari A, et al. Risk factors for severe retinopathy of prematurity
in preterm low birth weight neonates. Indian J Pediatr. 2011;78:812-816.
20. Lin HJ, Lin CC, Tsai SW, Lin HC, Su BH. Risk factors for retinopathy of prematurity in
very low birth-weight infants. Journal of the Chinese Medical Association: JCMA.
2003;66:662-668.
21. Dennery PA. Role of redox in fetal development and neonatal diseases. Antioxidants
& redox signaling. 2004;6:147-153.
22. Bassiouny MR, Almarsafawy H, Abdel-Hady H, Nasef N, Hammad TA, Aly H. A
randomized controlled trial on parenteral nutrition, oxidative stress, and
chronic lung diseases in preterm infants. J Pediatr Gastroenterol Nutr. 2009;48:
363-369.
23. Baird LL. Protecting TPN, and lipid infusions from light: reducing hydroperoxides in
NICU patients. Neonatal Netw. 2001;20:17-22.
195
24. Mordente A, Meucci E, Silverstrini A, Martorana GE, Giardina B. Anthracyclines and
mitochondria. In: Scantena R, Bottoni P, Giardina B, eds. Advances in Mitochondrial
Medicine. Netherlands: Springer; 2012. p. 385-419.
25. Sauerland C, Engelking C, Wickham R, Corbi D. Vesicant extravasation part 1:
Mechanisms, pathogenesis, and nursing care to reduce risk. Oncology Nursing
Forum. 2006;33:1134-1141.
26. Schulmeister L. Extravasation management: clinical update. Seminars in Oncology
Nursing. 2011;27:82-90.
27. Ricthey K, Sorenson M. Extravasation Guidelines. Childrens Oncology Group (COG)
Pharmacy Committee; 2007. p. 1-11.
28. Beauleiu M. Hyaluronidase for extravasation management. Neonatal Network.
2012;31:413-418.
29. Perez Fidalgo JA, Garcia Fabregat L, Cervantes A, Margulies A, Vidall C, Roila F, and
on behalf of the ESMO Guidelines Working Group. Management of chemotherapy
extravasation: ESMO-EONS Clinical Practice Guidelines. Annals of Oncology.
2012;23(supplement 7):vii167-vii173.
30. Bey D, El-Chaar GM, Bierman F, Valderrama E. The use of phentolamine in the
prevention of dopamine-induced tissue extravasation. Journal of Critical Care.
1998;13:13-20.
31. Subhani M, Sridhar S, DeCristofaro JD. Clinical perinatal/neonatal case presentation: Phentolamine use in a neonate for the prevention of dermal necrosis caused
by dopamine: A case report. Journal of Perinatology. 2001;21:324-326.
32. Schummer W. Extravasation injury in the perioperative setting. Anesthesia and
Analgesia. 2005;100:722-727.
33. Denkler K, Cohen B. Reversal of dopamine extravasation injury with topical
nitroglycerin ointment. Plastic and Reconstructive Surgery. 1989;84:811-813.
S-ar putea să vă placă și
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Occupational Therapy in Neonatal Services and Early Intervention PDFDocument152 paginiOccupational Therapy in Neonatal Services and Early Intervention PDFLauren100% (1)
- Hospital Business PlanDocument49 paginiHospital Business PlanJoseph QuillÎncă nu există evaluări
- Nicu NCPDocument2 paginiNicu NCPYette Polillo Conde100% (1)
- NN.03.03.D Olympic Brainz MonitorDocument10 paginiNN.03.03.D Olympic Brainz MonitorNativasrlÎncă nu există evaluări
- Case Study Olivia JonesDocument4 paginiCase Study Olivia JonesSharon Williams94% (16)
- NCM 2107 - Framework For Maternal and Child Health NursingDocument8 paginiNCM 2107 - Framework For Maternal and Child Health NursingHannah Angela Grace NeriÎncă nu există evaluări
- Ekstravasasi PDFDocument7 paginiEkstravasasi PDFSasha Hidayat FullÎncă nu există evaluări
- Genetic Journal PDFDocument12 paginiGenetic Journal PDFSasha Hidayat FullÎncă nu există evaluări
- Askep Meningitis AnakDocument10 paginiAskep Meningitis AnakSasha Hidayat FullÎncă nu există evaluări
- Revista Paulista DE PediatriaDocument6 paginiRevista Paulista DE PediatriaSasha Hidayat FullÎncă nu există evaluări
- _____ ______ __ _ ____Document6 pagini_____ ______ __ _ ____Sasha Hidayat FullÎncă nu există evaluări
- Best Evidence-Based Practices To Treat Intravenous InfiltrationDocument1 paginăBest Evidence-Based Practices To Treat Intravenous InfiltrationSasha Hidayat FullÎncă nu există evaluări
- Infiltration and Extravasation Update On.10Document9 paginiInfiltration and Extravasation Update On.10Beauty CassieÎncă nu există evaluări
- Four Perspectives On Child Care Quality: Deborah Ceglowski and Chiara BacigalupaDocument7 paginiFour Perspectives On Child Care Quality: Deborah Ceglowski and Chiara BacigalupaSasha Hidayat FullÎncă nu există evaluări
- P H & S J: The Ace Scoring SystemDocument6 paginiP H & S J: The Ace Scoring SystemSasha Hidayat FullÎncă nu există evaluări
- B - PDF PDFDocument11 paginiB - PDF PDFSasha Hidayat FullÎncă nu există evaluări
- MucositisDocument6 paginiMucositisHarry ArdiyantoÎncă nu există evaluări
- Genetic Journal PDFDocument12 paginiGenetic Journal PDFSasha Hidayat FullÎncă nu există evaluări
- Managing Oral Mucositis Oral Health July 2015Document4 paginiManaging Oral Mucositis Oral Health July 2015fiora.ladesvitaÎncă nu există evaluări
- Ok JurnalDocument6 paginiOk JurnalSasha Hidayat FullÎncă nu există evaluări
- Position 3Document5 paginiPosition 3Sasha Hidayat FullÎncă nu există evaluări
- Evid Based Nurs 2012 Meek 84 5Document3 paginiEvid Based Nurs 2012 Meek 84 5Sasha Hidayat FullÎncă nu există evaluări
- 212Document5 pagini212Sasha Hidayat FullÎncă nu există evaluări
- Overweight and Obesity Among White, Black, and Mexican American Children: Implications For When To InterveneDocument10 paginiOverweight and Obesity Among White, Black, and Mexican American Children: Implications For When To InterveneSasha Hidayat FullÎncă nu există evaluări
- Acute Respiratory Distress Syndrome in Pediatric Intensive Care UnitDocument4 paginiAcute Respiratory Distress Syndrome in Pediatric Intensive Care UnitSasha Hidayat FullÎncă nu există evaluări
- Harrison Prevention and Management of Pain and Stress in The Neonate 2015Document8 paginiHarrison Prevention and Management of Pain and Stress in The Neonate 2015Sasha Hidayat FullÎncă nu există evaluări
- Nejm CER Consent KassDocument3 paginiNejm CER Consent KassSasha Hidayat FullÎncă nu există evaluări
- Hipospadia Ej1083631 OkDocument17 paginiHipospadia Ej1083631 OkSasha Hidayat FullÎncă nu există evaluări
- Position 2Document8 paginiPosition 2Sasha Hidayat FullÎncă nu există evaluări
- JCCNC v1n4p205 enDocument6 paginiJCCNC v1n4p205 enSasha Hidayat FullÎncă nu există evaluări
- 4as To Rise Above Moral Distress PDFDocument14 pagini4as To Rise Above Moral Distress PDFOscar PerezÎncă nu există evaluări
- Gynecologic Oncology: Nozomi Donoyama, Toyomi Satoh, Tetsutaro Hamano, Norio Ohkoshi, Mamiko OnukiDocument8 paginiGynecologic Oncology: Nozomi Donoyama, Toyomi Satoh, Tetsutaro Hamano, Norio Ohkoshi, Mamiko OnukiSasha Hidayat FullÎncă nu există evaluări
- Ethical SkillDocument8 paginiEthical SkillSasha Hidayat FullÎncă nu există evaluări
- Gynecologic Oncology: Nozomi Donoyama, Toyomi Satoh, Tetsutaro Hamano, Norio Ohkoshi, Mamiko OnukiDocument8 paginiGynecologic Oncology: Nozomi Donoyama, Toyomi Satoh, Tetsutaro Hamano, Norio Ohkoshi, Mamiko OnukiSasha Hidayat FullÎncă nu există evaluări
- Massase For Cancer 2Document16 paginiMassase For Cancer 2Sasha Hidayat FullÎncă nu există evaluări
- Oral Care For Cancer PDFDocument10 paginiOral Care For Cancer PDFSasha Hidayat FullÎncă nu există evaluări
- Comparison of Kangaroo Mother Care With Conventional Method of Care in The Care of Low Birth Weight InfantsDocument58 paginiComparison of Kangaroo Mother Care With Conventional Method of Care in The Care of Low Birth Weight Infantssunny kumarÎncă nu există evaluări
- Ayantu Feked (Main Journal)Document51 paginiAyantu Feked (Main Journal)Ira g'GurlzÎncă nu există evaluări
- Nursing Diagnosis Rationale Goals/ Objectives Nursing Intervention S Rational E EvaluationDocument21 paginiNursing Diagnosis Rationale Goals/ Objectives Nursing Intervention S Rational E EvaluationJoanne Bernadette Aguilar100% (1)
- Prematurity ContentDocument2 paginiPrematurity ContentYsabel Francesca AbadÎncă nu există evaluări
- Eship Case Study Group 4 FinalDocument13 paginiEship Case Study Group 4 FinalArzoo ChaudharyÎncă nu există evaluări
- 11.objectives &clinical Rotation Plan M. Sc. Nursing Previous 2015-16Document4 pagini11.objectives &clinical Rotation Plan M. Sc. Nursing Previous 2015-16Naresh JeengarÎncă nu există evaluări
- Covid 19 Batch 5 TMSDocument860 paginiCovid 19 Batch 5 TMSMohamad Mosallam AyoubÎncă nu există evaluări
- of IncubatorDocument22 paginiof IncubatorSarah PavuÎncă nu există evaluări
- T-Piece Infant Resuscitation BQ70Document2 paginiT-Piece Infant Resuscitation BQ70Surta DevianaÎncă nu există evaluări
- NicuDocument35 paginiNicuJaya Prabha100% (2)
- Analysis of Sensory Processing in Preterm Infants PDFDocument5 paginiAnalysis of Sensory Processing in Preterm Infants PDFYESSICA MARCELA RODRIGUEZ QUECHOÎncă nu există evaluări
- NICU Sound ControlDocument3 paginiNICU Sound ControldkalatzisÎncă nu există evaluări
- Knowledge and Attitudes of Pediatric Clinicians Regarding Pediatric Pain ManagementDocument11 paginiKnowledge and Attitudes of Pediatric Clinicians Regarding Pediatric Pain ManagementIkek StanzaÎncă nu există evaluări
- NEONATAL APNOEA SMNRDocument18 paginiNEONATAL APNOEA SMNRAswathy RC100% (1)
- KMC Guide EnglishDocument70 paginiKMC Guide EnglishNewborn2013Încă nu există evaluări
- Karat Primary Hospital NICU ProposalDocument8 paginiKarat Primary Hospital NICU Proposalgirma balleÎncă nu există evaluări
- 496Document1 pagină496Suman MondalÎncă nu există evaluări
- Rsud Konawe SelatanDocument6 paginiRsud Konawe SelatanewÎncă nu există evaluări
- Update Price List All Product 2022 TerbaruDocument87 paginiUpdate Price List All Product 2022 TerbaruRatti FitrianaÎncă nu există evaluări
- Perinatal Factor Journal PediatricDocument8 paginiPerinatal Factor Journal PediatricHasya KinasihÎncă nu există evaluări
- The Fifth Vital Sign Implementation PDFDocument8 paginiThe Fifth Vital Sign Implementation PDFTeten RustendiÎncă nu există evaluări
- Standard Equipment ListDocument212 paginiStandard Equipment ListRuchi TapdiyaÎncă nu există evaluări
- Neonatal Respiratory Distress SyndromeDocument14 paginiNeonatal Respiratory Distress SyndromeDrex CuritanaÎncă nu există evaluări
- PNAA November 2022 Issue Final Web VersionDocument144 paginiPNAA November 2022 Issue Final Web Versionlolman69 wkwkwkÎncă nu există evaluări