Documente Academic
Documente Profesional
Documente Cultură
Calabar Python PDF
Încărcat de
John GamesbyTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Calabar Python PDF
Încărcat de
John GamesbyDrepturi de autor:
Formate disponibile
The CITES Nomenclature Committee does not adopt
Kluges proposal because it seems to cause more misunderstanding than understanding. Zoogeographically
speaking, it is rather strange to have a genus distributed
only in California and West Africa (HOOGMOED,
2003).
Finally, an interesting phylogenetic study based on
the sequence of portions of two mitochondrial genes
(12S and 16S ribosomal RNA) has demonstrated that
Calabaria could be more closely related to the uropeltid genus Rhinophis than to either boa or python
genera (HEISE et al., 1995). This suggests that the
family Boidae needs a thorough overall revision including molecular analysis. It is evident that the possible
relationship between the African burrowing python
and the subfamily Erycinae remains to be demonstrated, and most scientists therefore prefer to maintain the
old system.
Distribution, habitat, and climate
African Burrowing Python
Calabaria reinhardtii (Schlegel, 1851) is commonly called the African burrowing python.
Other names are frequently used as well (see
Table 1), most of them also incorporating the
word python. However, the relationship of
this species to others of the subfamily Pythoninae is currently disputed among herpetologists, as will be discussed
later in this article.
In Nigeria, the Efik people, predominant ethnic group
in the southeastern region of the country, call this snake
iwod iba. In pidgin English it is called snake with two
heads, or sometimes rainbow snake (LUISELLI, pers.
comm.). In Cameroon, this strange snake is well known,
and some people believe it causes young women to
become pregnant.
The genus name Calabaria derives from Calabar, a town
with an active port near where the Calabar River enters
the Cross River estuary in southeastern Nigeria, and currently the capital of Cross River State. Historically, Old
Calabar was an important trading state and one of the
countrys earliest contacts with Europeans, growing during the 19th century as a hub of the palm oil trade.
Description
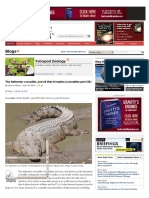
The African burrowing python is an unusual snake with
a cylindrical body, head, and tail, all of fairly uniform
diameter. The head is small and not set off from the body,
such that it surprisingly resembles the tail. The eyes are
small with vertical pupils, and have the same brown
colour as the surrounding scales. The small mouth, not
suited to large prey, is inconspicuous. There are no
heat-sensitive pits. The scales are glossy and smooth,
and a projecting rostral area aids in burrowing. The tail
is blunt and very short. The dorsum and flanks are
blackish-brown, brown, or reddish-brown, with lighter,
reddish or yellowish flecks and irregular blotches; the
head and tail are generally darker. The belly is grey or
brown, and may have some brown blotches. The
African burrowing python can reach a maximum of
80100 centimetres.
Taxonomy
Calabaria is a monotypic genus whose taxonomic position is currently unresolved. KLUGE (1993) proposed a
taxonomic review of Calabaria reinhardtii. He believes
it is more closely related to members of the subfamily
Erycinae, the sand boas, and should be assigned to the
genus Charina thus becoming Charina reinhardtii,
the African burrowing boa. But other herpetologists
think that many of the characteristics this snake shares
with the Erycinae could be simply adaptations to a similar subterranean lifestyle, and therefore the genus name
Calabaria should be retained. In fact, Kluges system has
been followed only by McDIARMID et al. (1999).
REPTILIA 66
The African burrowing python is present in western
tropical Africa, where it is widespread across the
Liberia-Congo rainforest belt. It is found in Sierra
Leone, Liberia, Ivory Coast, Ghana, Togo, Benin,
Nigeria, Cameroon, the Central African Republic,
Equatorial Guinea, Gabon, the Congo, and the
Democratic Republic of the Congo (Zaire).
It inhabits the leaf-covered ground of tropical rainforests and overgrown cultivated areas with dense
undergrowth. In a recent study in southeastern Nigeria,
ANGELICI et al. (2000) found Calabaria mainly in
thick forest, swamp forest, and also in clearings and cultivated areas, especially during the wet season it burrows into decaying leaves and soil, and also inhabits the
burrows of small mammals, seeming to prefer more
superficial rather than deep underground galleries. The
same study demonstrated that the African burrowing
python often takes shelter in termite nests, especially
near forested areas and during the dry season. It is also
found climbing among small bushes and fallen branches.
The climate in the range of Calabaria is characterized
by alternating wet and dry seasons, with a corresponding but less notable variation of temperature.
Generally, the dry season is from November through
April, and the rainy season from May through October.
Temperatures rise from October to February, and
remain at their highest from March to May. Naturally
Table I.
Common names of Calabaria reinhardtii.
English
Spanish
French
Dutch
German
Italian
Calabar python, Calabar ground python,
West African ground python, burrowing
python, West African burrowing python,
two-headed python
Pitn de Calabar, Pitn ciega
Calabare de Reinhardt, Calabaria de
Reinhardt
Aardpython
Erdpython
Calabaria, pitone di Calabar
there is some variation, and the more northern areas of
the range are generally drier than the coastal areas.
Most imported African burrowing pythons come from
Togo and Benin. It is therefore useful to know that in
northern Benin the main wet season is between June
and October, whereas in the south there are two wet
seasons: early April to mid-July, and mid-September to
late October. In the north, the temperature can reach
46C (115F); in the south, it stays between 18 and 35C
(6495F). Throughout the country, the hottest months
are March to June. Similarly, in Togo, the wet season is
from May to October, with a dry interlude in the south
between mid-July and mid-September. The hottest
period in Togo is from mid-February to mid-April.
Little-known biology
Many aspects of the biology of Calabaria reinhardtii
are still little known. This subterranean species is shy
and elusive, and mostly nocturnal. It burrows under the
moist soil, humus, and leaves, where it likes to hide.
These factors make it difficult to study the behaviour of
the African burrowing python. Nonetheless recent
research has probed into many aspects of this snakes
mysterious biology.
In southeastern Nigeria, ANGELICI et al. (2000)
used radiotracking to study the African burrowing
python, and found the snakes below ground in more
than 80% of locations, during both dry and wet seasons.
The mean daily movement rate appears to be higher in
males than in females, without evident seasonal change.
These first examined biological aspects suggest an
Table II. Relationships between reproductive activities and seasons of the year.
Jan
Feb
Mar
Apr
May
Jun
Wet season
Dry season
Mate
Eggs Laid
Hatch
REPTILIA 67
Jul
Aug
Sep
Oct
Nov
Dec
ground. Then it lifts its tail and swings it slightly to
mimic the head. Many white scales are often present on
the tail, which could help attract attention to the false
head. The other defence technique is called balling, in
which the python coils itself into a tight ball when
threatened, often keeping its head in the centre and its
tail exposed (this behaviour is also seen in other snakes
such as Python regius). In any case, the African burrowing python is quite docile, and never tries to bite or
hiss when handled or disturbed.
Terrarium
Calabaria reinhardtii. Photo: P. Martnez Carrin
interseasonal ecological homogeneity that could be
interpreted as an evolutionary response to the relative
year-round stability of habitat conditions.
In another study on the ecology of Calabaria,
LUISELLI et al. (1999) showed that, in southeastern
Nigeria, the African burrowing python mates between
November and January (dry season), lays eggs from
March to April (the end of dry season), and the eggs
hatch from June to July (wet season).
These studies also demonstrated that the African burrowing python eats mainly small mammals, especially
mice, which it usually takes from their nests. The disposition of the snake to eat small prey could be related to
its post-cranial prey transport mechanism. Recent
research (KLEY et al., 2002) indicates that cervical compression in Calabaria reinhardtii is great because of lacking palatopterygoid teeth, which could be related to its
feeding on small prey.
It seems that Calabaria kills mice by squashing them
against the walls of their burrows. In detailed dietary
analysis of regurgitated items and faecal pellets,
LUISELLI et al., (2002) have shown that the main prey
of the African burrowing python is small mammals,
especially rodents: regurgitated items included adult and
nestling Mus musculoides (mice), young Rattus rattus
(rats), snake eggs, and Mabuya (skink); faecal pellets
contained Rattus sp., Mus musculoides, and Crocidura
(shrews). According to such studies, those pythons that
feed mostly on rodents do not appear to eat shrews, and
only occasionally eat lizards or reptile eggs; on the other
hand, those that eat shrews do not appear to eat rodents.
The analysed wild African burrowing pythons also do
not seem to eat earthworms.
Although Calabaria is mainly nocturnal, some studies
have demonstrated that it can also be active and forage
during the day (GARTLAN et al., 1971).
One well-known aspect of the African burrowing
python is its defence behaviour. Two techniques have
been observed. One is a tail display in which Calabaria
presents its tail as a false head to distract predators away
from more vulnerable parts of its body. When disturbed, the snake freezes and presses its chin to the
The African burrowing python is not a common terrarium subject, and unfortunately the specimens available on the market are almost always wild-caught.
Captive-bred specimens are still very rare. Imported
snakes should therefore be carefully checked for the
usual external and internal parasites.
Although this snake is considered relatively easy to
keep, it is important that the Calabaria terrarium reproduce its natural living conditions as closely as possible.
Calabaria requires high humidity, so the enclosure
should be made of materials that withstand moisture,
such as glass or plastic (rather than wood). The enclosure need not be very large. A cage of 80 x 50 x 60 centimetres is good for one or two specimens. The species
can be housed in small groups, but more than three or
four pythons in the same tank is not recommended.
The burrowing medium can be a mix of sandy soil,
humus, moss, decaying leaves, mulch, and pieces of
bark. It should be kept lightly moistened, but there
should never be standing water because Calabaria is susceptible to fungal infections. The substrate should be
deep enough so the python can bury itself (about 1015
centimetres), and hide boxes are beneficial these
should be cramped enough that the snake touches the
sides and feels like it is in an underground tunnel.
STAUB (2001) suggests laying a large flat piece of hard
plastic over part of the substrate to retain moisture. This
gives the snake more choices of degree of moisture and
temperature while remaining hidden.
Calabaria occasionally likes to climb a little, so it is
good to provide branches in the terrarium. It also needs
a large basin of clean water. The temperature should be
2632C (7990F) during the day, and can drop to
2224C (7275F) at night. A heating pad can be used
to keep one side of the enclosure at 32C, and a lamp to
keep the other side at 2628C.
Many keepers cool the pythons down for a few
months, often to stimulate breeding, but temperatures
can be kept stable year-round without problems, in
which case the snakes continue eating. It is advisable to
spray with warm water once a week. The humidity
should be kept at 7585 percent. Adequate ventilation
should be provided with perforated plastic or metal panels. Keepers suggest never touching these very shy
snakes. If properly maintained, the captive African burrowing python can live to more than 20 years of age.
REPTILIA 68
Feeding
As already mentioned, the African burrowing python
feeds on small mammals, especially rodents. Wildcaught Calabaria, especially when already adult, may
not readily accept food in the beginning. The keeper
must be patient, and try repeatedly. The best choice of
food is rat pinkies and mouse fuzzies, before their eyes
open and they become too jumpy. Prey can be offered
either alive or pre-killed; sometimes the latter method
works best at first.
Not a typical constrictor, this species kills prey by
pressing it against the floor or sides of the cage; it is not
able to compress prey within its coils as well as other
constrictors can. This unusual behaviour has also been
noted by LOMAN (2003) in a wild-caught Calabaria
found 1020 kilometres north of Kribi (Cameroon) in
November 1974. It was collected and kept in captivity
for some years. During feeding, it tried to squeeze
small mice (sometimes several at once) against the terrarium wall before coiling around them. LOMAN
interpreted this as an adaptation to raiding small-mammal nest burrows. For this reason, some keepers
believe that the African burrowing python can be stimulated to feed by offering many small prey items at once
rather than fewer larger items, to simulate its natural
feeding conditions. Thus, it seems that offering a litter
of four or five small prey items (no larger than rat or
mouse fuzzies) is the best method to successfully feed
this python.
In addition, HARRISON (2003) and other herpetologists have described a particular behaviour called
multiple constriction response: after an African burrowing python has constricted and eaten the first prey
item, the snake is stimulated to eat others when they are
gently pressed against its body; the python tries to constrict or press them at once, and then eats all of them. If
the python is healthy and adapts well to captivity, it
quickly becomes a prodigious eater. Females often
seem to be more voracious than males.
Breeding
Recent data indicates that wild Calabaria generally
have a biennial breeding frequency (LUISELLI et al.,
2002), although there are observations that demonstrate an annual breeding cycle, especially in captivity.
Captive-born Calabaria reinhardtii are rare, and almost
always cases where females were already gravid when
wild-caught.
The main limiting factors in captive reproduction
have been the incubation of eggs and the body weight of
the females. Females seem to require a longer acclimation period than males do, probably because of the
greater energy expenditure required for egg production
(CHERNOFF, 2003).
The sexes can be identified by cloacal probing,
although this is not always simple. Males probe to a
depth of 1011 subcaudal scales; females to a depth of
about 3 (ROSS et al., 1990).
Breeding activity seems depend on certain environmental and feeding factors. In captivity, the natural
western African seasonal cycle should be simulated,
alternating between wet and dry periods as already
described. In the wild, African burrowing pythons mate
during the dry season, specifically between November
and January. STAUB (2001) also observed breeding in
captivity during these months.
Calabaria reinhardtii with eggs. Photo: P. Martnez Carrin
REPTILIA 69
Calabaria reinhardtii. Photo: P. Martnez Carrin
Another factor is food supply. It has been observed
that females ingest a lot of food during the breeding season in order to reach a minimum breeding mass, which
should be about 500 grams (STAUB, 2001). Therefore,
it seems important to offer a lot of food during the
breeding period between November and January. A
comprehensive breeding scheme for captive Calabaria
reinhardtii, is summarized in Table 3.
Other factors which can stimulate a reproductive
response include the presence of abundant water in the
basin, the presence of many hiding places, and leaving
the snakes absolutely undisturbed; also, the male should
be left with the female for sufficient time (STAUB,
2001) I think 812 weeks are reasonable. As already
mentioned, temperature fluctuation seems to contribute
to triggering a reproductive response; the photoperiod,
however, does not seem to be determinant.
It is very difficult to observe courtship and copulation.
After mating, the female is gravid for 110114 days, or in
some cases as long as 150 days, during which time her
posterior section increases in girth as the eggs develop.
The female usually stops eating 12 days before laying
her eggs. It is advisable to provide a nest box (containing a mix of peat and sphagnum moss), otherwise she
will lay her large eggs in a depression in the damp substrate. According to STAUB (2001), the female usually
lays eggs between February and June, having invested a
substantial amount of energy into the process typically 3545 percent of her pre-laying weight.
Although the eggs seem huge at slightly more than 70
grams each, hatchlings weigh only 4050 percent of the
original egg weight, suggesting that much of the egg
weight is simply water.
The female lays 15 eggs (average 3) measuring 911.5
x 35 centimetres, and weighing 6575 grams. The eggs
are very flaccid and thin, and are very susceptible to fungus and putrefaction. Maternal care such as coiling
around the clutch or body spasms to regulate incubation
temperature seems to be absent.
Incubation has been the great problem with captive
breeding of the African burrowing python. For artificial
incubation, vermiculite, pieces of sponge, or a similar
substrate material can be used. Unlike the eggs of other
snakes, Calabaria eggs require a relatively dry incubation medium. Some keepers suggest a medium-to-water
ratio of 2:1, but others consider this too wet. I recommend adding no water to the substrate. Ambient
humidity in the incubator (80-95%) already provides
enough moisture for the eggs, and if the substrate is wet
the eggs will die. The key to successful incubation of
Calabaria eggs is dry substrate and high ambient humidity. Incubation temperature should be 2931.5C (84
89F) best results are at temperatures of 3031C
(8688F) and should never drop below 26C (79F).
Under these conditions, the incubation period is generally 4048 days, although it has occasionally been
only 3234 days. After breaking the shells, babies commonly remain inside the eggs for 1848 hours, or as
long as 96 hours (STAUB, 2001). Hatchlings measure
2632 centimetres, weigh 1840 grams, and are more
colourful than adults. They soon start to eat live pinkie
mice, sometimes within 2 days of hatching, and shed for
the first time within 2 weeks. The newborn snakes
need to be kept warm with high humidity: they are not
as tolerant of dry conditions as the adults. These
pythons grow relatively fast and can reach breeding
size at 3 years of age.
Table III. Scheme for the captive breeding of Calabaria reinhardtii.
Jan
Feb
Mar
Humidity % LOW
LOW
LOW
Food
Apr
May
Jun
Jul
Aug
Sep
HIGH
HIGH
HIGH
HIGH
HIGH
MUCH MUCH
Oct
Nov
Dec
LOW
LOW
MUCH MUCH MUCH
Mate
Eggs Laid
Hatch
REPTILIA 70
Law and protection
CHERNOFF, N., 2003. Snakes The Home Page of Neil Chernoff.
Internet Web Site: <www4.ncsu.edu/~chernoff/>
Calabaria reinhardtii is regularly imported from
CHIPPAUX, J. P., 1999. Les serpents d Afrique occidentale et cenWest Africa (mainly from Ghana, Benin, and Togo)
trale. IRD Editions, Paris.
for the pet trade in Europe, and also often in the
COBORN, J. 1995. Boas & Pythons and Other Friendly Snakes.
United States. The species is listed in CITES AppenT.F.H., Neptune City.
dix II (reference A-305.004.005.001; date listed, 4
GARTLAN, J. S. and STRUHSAKER, T. T., 1971. Notes on the
February 1977).
habits of the Calabar ground python (Calabaria reinhardtii Schlegel)
Most marketed species of python are now ranched,
in Cameroon, West Africa. Br. J. Herpetol. 4: 201202.
which is defined by CITES as the
GOSSMANN, V., LTTERS, S., OBAME,
rearing in a controlled environF., and BHME, W., 2002. Zur HerpetoTable IV.
fauna Gabuns. Teil II. Herpetofauna 24:
ment of specimens taken from
Calabaria reinhardtii
1933.
the wild. Eggs or juveniles are
captive breeding data.
GRAY, J. E., 1858. Description of a new
harvested from a wild populagenus of boidae from old Calabar, and a
tion, and then raised in captivity
list of west African Reptiles. Proceedings
Gestation
period
(days)
110114
until they reach a commercially
of the Zoological Society of London 1858:
exploitable size. Most are then
Number of eggs
15 (3)
154167.
exported, but a portion of the
HARRISON, C., 2003. The Sand Boa Page:
Egg length (cm)
911.5
captive-raised juveniles are
<www.kingsnake.com/sandboa/calabar.html>
Egg weight (grams)
6575
HEISE, P. J., MAXSON, L. R., DOWLING,
released back into the wild popuH. G., and HEDGES, S. B., 1995. Higherlation. The primary objective of
Incubation period (days)
4048
level snake phylogeny inferred from mitoranching is conservation of the
chondrial DNA sequences of 12S rRNA
Incubation
humidity
(%)
8095
local population.
and 16S rRNA genes. Mol. Biol. Evol.
HOOGMOED (2003) explains
Incubation temperature (C) 3031
12(2): 259265.
that the term ranching was
HOOGMOED, M. S., 2003. Notes on the
Newborn length (cm)
2632
originally coined for CITES
nomenclature and the conservation status
Newborn weight (grams)
1840
Appendix I species (mainly crocof Calabaria reinhardtii. Personal comm.
odiles), but is now also used for
HORTENBACH, G. and HORTENBACH, H., 1998. Maintenance, behaviour and breeding of Calabaria
CITES Appendix II species that are managed in some
reinhardtii
Schlegel, 1851 under captive conditions. Salamandra 34(2).
way. In most cases, the animals exported are wild
KLEY,
N.
J. and BRAINERD, E. L., 2002. Post-cranial prey transspecimens that have not been bred in captivity, and
port mechanisms in the black pinesnake, Pituophis melanoleucus
there is a lot of confusion about the correct use of the
lodingi: an x-ray videographic study. Zoology 105: 153164.
term ranched. As far as we know, all specimens that
KLUGE, A., 1993. Calabaria and the phylogeny of erycine snakes.
are exported from West Africa as ranched and
Zool. J. Linn. Soc. 107: 293351.
farmed animals are actually wild-caught specimens,
LEHMANN, H. D., 1971. Notizen ber Nahrungsaufnahme und
nothing else.
Abwehrverhalten von Calabaria reinhardtii (Serpentes: Boidae).
Salamandra 7(2): 5560.
Finally, also the European Council Regulation No.
LOMAN, J., 2003. Notes on the captive feeding in Calabaria rein2724/2000 includes this python in Appendix B (date
harditti
. Personal communication.
listed, 18 December 2000).
Acknowledgments
The author wishes to express sincere gratitude to
Luca Luiselli for his contribution to the knowledge of
the African burowing python (Calabaria reinhardtii),
and for his help, kindness, and advice regarding this
manuscript; to Dr. Marinus S. Hoogmoed, member of
the CITES Nomenclature Committee, for valuable correspondence on nomenclature and conservation status;
to Rick Staub for providing his interesting publication;
and to Jon Loman for his personal observations of
Calabaria reinhardtii in the wild.
Bibliography
ANGELICI, F. M., INYANG, M. A., EFFAH, C., and LUISELLI,
L., 2000. Analysis of activity patterns and habitat use of radiotracked
African burrowing pythons, Calabaria reinhardtii. Israel Journal of
Zoology 46(2): 131141.
BARTLETT, D., 1998. West African burrowing pythons. Reptiles
6: 3641.
CANSDALE, G. S., 1961. West African snakes. Longmans. London.
LUISELLI, L. and AKANI, G. C., 1998 (1999). Aspects of the ecology of Calabaria reinhardtii (Serpentes: Boidae) in southeastern
Nigeria. Herpetological Natural History 6(1): 6571.
LUISELLI, L., AKANI, G. C., and CAPIZZI, C., 1998. Food
resource partitioning of a community of snakes in a swamp rainforest
of south-eastern Nigeria. J. Zool. Lond. 246(4): 125133.
LUISELLI, L., EFFAH, C., ANGELICI, F. M., ODEGBUNE, E.,
INYANG, M. A., AKANI G. C., and POLITANO, E., 2002. Female
breeding frequency, clutch size and dietary habits of a Nigerian population of Calabar Ground Python, Calabaria reinhardtii. Herpetological Journal 12(3): 127129.
McDIARMID, R. W., CAMPBELL J. A., and TOURE, T. A.,
1999. Snake Species of the World. Vol. 1. Herpetol. League, U.S.A.
RODRGUEZ, J. A., BELL, C. J., and GREENE, H. W., 1999.
Gape size and evolution of diet in snakes: feeding ecology of erycine
boas. J. Zool. Lond. 248: 4958.
ROSS, R. A. and MARZEC, G., 1990. The Reproductive
Husbandry of Pythons and Boas. Institute for Herpetological
Research, Stanford, California.
SCHLEGEL, H., 1851. Description d'une nouvelle espce du genre
Eryx, Eryx reinhardtii. Bijdragen tot de Dierkunde, Amsterdam 1: 13.
STAUB, R., 2001. The Calabar Burrowing Boa/Python. Reptile
and Amphibian Hobbyist 6(6): 3441.
REPTILIA 71
S-ar putea să vă placă și
- Quadrupeds, What They Are and Where Found: A Book of Zoology for BoysDe la EverandQuadrupeds, What They Are and Where Found: A Book of Zoology for BoysÎncă nu există evaluări
- Numer OnDocument10 paginiNumer OnHeizyl ann VelascoÎncă nu există evaluări
- Anura (: (Hide)Document4 paginiAnura (: (Hide)kbarn389Încă nu există evaluări
- Cebu FlowerpeckerDocument7 paginiCebu FlowerpeckerPushpendra KumarÎncă nu există evaluări
- History and Origin of ParrotsDocument38 paginiHistory and Origin of ParrotsShanmukha priyaÎncă nu există evaluări
- Great Tit: Jump To Navigationjump To SearchDocument8 paginiGreat Tit: Jump To Navigationjump To SearchQuin CusayÎncă nu există evaluări
- AnimalDocument22 paginiAnimalJudin BellÎncă nu există evaluări
- PilandokDocument4 paginiPilandokpatricksvdÎncă nu există evaluări
- Frogs: From Wikipedia, The Free Encyclopedia This Article Is About The Group of Amphibians. For Other Uses, SeeDocument7 paginiFrogs: From Wikipedia, The Free Encyclopedia This Article Is About The Group of Amphibians. For Other Uses, SeemcmusbixÎncă nu există evaluări
- Frogs: From Wikipedia, The Free Encyclopedia This Article Is About The Group of Amphibians. For Other Uses, SeeDocument6 paginiFrogs: From Wikipedia, The Free Encyclopedia This Article Is About The Group of Amphibians. For Other Uses, SeemcmusbixÎncă nu există evaluări
- Cap. 05 - SnakesDocument17 paginiCap. 05 - SnakesNailson JúniorÎncă nu există evaluări
- Lit Review of TilapiaDocument3 paginiLit Review of Tilapiakimarthy gordonÎncă nu există evaluări
- Journal of Natural History Series 2: To Cite This Article: Alfred R. Wallace (1854) : On The Monkeys of The AmazonDocument6 paginiJournal of Natural History Series 2: To Cite This Article: Alfred R. Wallace (1854) : On The Monkeys of The AmazonJose Manuel Villasuso AguiñagaÎncă nu există evaluări
- Philippine TarsierDocument14 paginiPhilippine TarsierSharmistha Talukder KhastagirÎncă nu există evaluări
- From Wikipedia, The Free Encyclopedia: Jump To:, For Other Uses, SeeDocument19 paginiFrom Wikipedia, The Free Encyclopedia: Jump To:, For Other Uses, Seenassamerg23Încă nu există evaluări
- INTRODUCTION TO PERISSODACTYLA (Odd-Toed Ungulates) : Ungulates (Meaning Roughly "BeingDocument5 paginiINTRODUCTION TO PERISSODACTYLA (Odd-Toed Ungulates) : Ungulates (Meaning Roughly "BeingYusni AtifahÎncă nu există evaluări
- Platypus PDFDocument2 paginiPlatypus PDFRaajeswaran BaskaranÎncă nu există evaluări
- Biology 10 Performance Task #3: Shelly Marie L. de La Cruz 10-LaurenciumDocument20 paginiBiology 10 Performance Task #3: Shelly Marie L. de La Cruz 10-LaurenciumShelly Marie De la CruzÎncă nu există evaluări
- Writing Unit: Non-Chronological ReportDocument23 paginiWriting Unit: Non-Chronological Reportanimedarkzone084Încă nu există evaluări
- GiraffeDocument3 paginiGiraffePradeep DesaiÎncă nu există evaluări
- FROGDocument16 paginiFROGRegia IlmahaniÎncă nu există evaluări
- Marabou StorkDocument4 paginiMarabou StorkOkello PaulÎncă nu există evaluări
- Pangolin Saola: VaquitaDocument5 paginiPangolin Saola: VaquitaHariharan BalasundaramÎncă nu există evaluări
- Botulism Breakthrough by SlidesgoDocument19 paginiBotulism Breakthrough by SlidesgoGameropediaÎncă nu există evaluări
- Basic Design: Peter AndrewsDocument4 paginiBasic Design: Peter AndrewsFrancisco BecerraÎncă nu există evaluări
- Dwarf Boas of The Caribbean PDFDocument5 paginiDwarf Boas of The Caribbean PDFJohn GamesbyÎncă nu există evaluări
- Birds DictionaryDocument11 paginiBirds DictionaryImran Khan0% (1)
- ReptileDocument4 paginiReptileDratonius 101Încă nu există evaluări
- Report Text About PandaDocument17 paginiReport Text About PandaAndre Andrian0% (1)
- Philippine Amphibians and Reptiles An Overview ofDocument11 paginiPhilippine Amphibians and Reptiles An Overview ofEmanuel MalacasteÎncă nu există evaluări
- Hippo ResearchDocument6 paginiHippo ResearchkeithÎncă nu există evaluări
- Company NameDocument10 paginiCompany NameRakesh DuttaÎncă nu există evaluări
- Inrtoduction: Natator From Estancia, Iloilo, Western Philippines. The Study Also Intends To IdentifyDocument23 paginiInrtoduction: Natator From Estancia, Iloilo, Western Philippines. The Study Also Intends To IdentifyRey Malvin SG PallominaÎncă nu există evaluări
- Parelhoenders in Handbook of The Birds of The World 01Document21 paginiParelhoenders in Handbook of The Birds of The World 01Alyssa LeonidoÎncă nu există evaluări
- Class Mammalia Class MarsupialaDocument17 paginiClass Mammalia Class MarsupialaLouise Margaux B. BrizÎncă nu există evaluări
- Horseshoe Crab: Limulidae Horseshoe Crabs Are Marine and Brackish Water Arthropods of TheDocument9 paginiHorseshoe Crab: Limulidae Horseshoe Crabs Are Marine and Brackish Water Arthropods of Theenzo abrahamÎncă nu există evaluări
- Boa Constrictor OccidentalisDocument9 paginiBoa Constrictor OccidentalisJ HalsteadÎncă nu există evaluări
- Neotropical Rainforest Mammals TalkDocument6 paginiNeotropical Rainforest Mammals TalkMisterJanÎncă nu există evaluări
- Inrtoduction: Natator From Estancia, Iloilo, Western Philippines. The Study Also Intends To IdentifyDocument12 paginiInrtoduction: Natator From Estancia, Iloilo, Western Philippines. The Study Also Intends To IdentifyRey Malvin SG PallominaÎncă nu există evaluări
- Old World MonkeyDocument6 paginiOld World Monkeydaitya99Încă nu există evaluări
- Mandrillus Sphinx - Sexual Dimorphism and Physical Diversity of Individuals in Relation To Social StatusDocument14 paginiMandrillus Sphinx - Sexual Dimorphism and Physical Diversity of Individuals in Relation To Social StatusJonathan Schwartz100% (2)
- Black-Footed CatDocument6 paginiBlack-Footed CatSharmistha Talukder KhastagirÎncă nu există evaluări
- Studies On Biology and Reproduction of Butterflies (Family: Papilionidae) in Nilgiris Hills, Southern Western Ghats, IndiaDocument11 paginiStudies On Biology and Reproduction of Butterflies (Family: Papilionidae) in Nilgiris Hills, Southern Western Ghats, IndiaEman SamirÎncă nu există evaluări
- The 20 Weird Animals YouDocument3 paginiThe 20 Weird Animals YouDanie AlonzoÎncă nu există evaluări
- Endemic Animals InfoDocument3 paginiEndemic Animals InfoAlessandra Denise LandichoÎncă nu există evaluări
- Quadrupeds, What They Are and Where FoundA Book of Zoology For Boys by Reid, Mayne, 1818-1883Document85 paginiQuadrupeds, What They Are and Where FoundA Book of Zoology For Boys by Reid, Mayne, 1818-1883Gutenberg.orgÎncă nu există evaluări
- Clipping Report Text (Animals) : D I S U S U N Oleh: Kelompok 6Document9 paginiClipping Report Text (Animals) : D I S U S U N Oleh: Kelompok 6masdarÎncă nu există evaluări
- Survey of AmphibiansDocument35 paginiSurvey of AmphibiansAeriel Venice VergaraÎncă nu există evaluări
- 3 Primates Classification & DiversityDocument48 pagini3 Primates Classification & DiversityArif Nur RifkiÎncă nu există evaluări
- Weird Birds BeaksDocument12 paginiWeird Birds BeaksLeonardo Molano DussanÎncă nu există evaluări
- Topic EcologyDocument5 paginiTopic EcologyJavier SmithÎncă nu există evaluări
- Amphibians - Advanced: Amphibians Nature General Conference See Also Skill Level 1 Year of Introduction: 1945Document15 paginiAmphibians - Advanced: Amphibians Nature General Conference See Also Skill Level 1 Year of Introduction: 1945Jun Siguenza AmbrocioÎncă nu există evaluări
- Servañez EndangerdDocument4 paginiServañez EndangerdrechangservanezÎncă nu există evaluări
- 8 1 Animals TextDocument7 pagini8 1 Animals Textapi-285108231Încă nu există evaluări
- White-Headed VultureDocument4 paginiWhite-Headed Vulturejosh321Încă nu există evaluări
- Presentor: Alyanna D. de Leon Beed-1EDocument36 paginiPresentor: Alyanna D. de Leon Beed-1EAly D. De LeonÎncă nu există evaluări
- Mammals Guide Book - From A to F | Mammals for Kids Encyclopedia | Children's Mammal BooksDe la EverandMammals Guide Book - From A to F | Mammals for Kids Encyclopedia | Children's Mammal BooksÎncă nu există evaluări
- ##The Journals of Raymond Brooks PDFDocument323 pagini##The Journals of Raymond Brooks PDFJohn GamesbyÎncă nu există evaluări
- Translocation of Urban Gila Monsters A P PDFDocument8 paginiTranslocation of Urban Gila Monsters A P PDFJohn GamesbyÎncă nu există evaluări
- Dark Confluence Book One of The Darkening TrilogyDocument107 paginiDark Confluence Book One of The Darkening TrilogyJohn GamesbyÎncă nu există evaluări
- HingebackTortoise PDFDocument4 paginiHingebackTortoise PDFJohn GamesbyÎncă nu există evaluări
- Pygmy Alligator Lizard of Nuevo Leon, Mexico PDFDocument3 paginiPygmy Alligator Lizard of Nuevo Leon, Mexico PDFJohn GamesbyÎncă nu există evaluări
- Saltwater Crocodile-Crocodiles Part IIIDocument21 paginiSaltwater Crocodile-Crocodiles Part IIIJohn GamesbyÎncă nu există evaluări
- Prey Envenomation Does Not Improve Digestion.Document10 paginiPrey Envenomation Does Not Improve Digestion.John GamesbyÎncă nu există evaluări
- Relating Geographic Pattern To Phylogene PDFDocument8 paginiRelating Geographic Pattern To Phylogene PDFJohn GamesbyÎncă nu există evaluări
- Origin and Phylogenetic Position of The PDFDocument6 paginiOrigin and Phylogenetic Position of The PDFJohn GamesbyÎncă nu există evaluări
- Giant Snakes of Bygone Days PDFDocument11 paginiGiant Snakes of Bygone Days PDFJohn GamesbyÎncă nu există evaluări
- Pig-Nosed Turtle PDFDocument5 paginiPig-Nosed Turtle PDFJohn GamesbyÎncă nu există evaluări
- Authors Metrics Comments Related ContentDocument15 paginiAuthors Metrics Comments Related ContentJohn GamesbyÎncă nu există evaluări
- On The Origins and Dispersal of Neotropi PDFDocument7 paginiOn The Origins and Dispersal of Neotropi PDFJohn GamesbyÎncă nu există evaluări
- Freshwater Crocodile PDFDocument9 paginiFreshwater Crocodile PDFJohn GamesbyÎncă nu există evaluări
- Asiatic Cobras Systematics and SnakebiteDocument5 paginiAsiatic Cobras Systematics and SnakebiteJohn GamesbyÎncă nu există evaluări
- Dwarf Boas of The Caribbean PDFDocument5 paginiDwarf Boas of The Caribbean PDFJohn GamesbyÎncă nu există evaluări
- Morphological Correlates of Incipient Ar PDFDocument10 paginiMorphological Correlates of Incipient Ar PDFJohn GamesbyÎncă nu există evaluări
- Deep Intraspecific Divergences in The Me PDFDocument9 paginiDeep Intraspecific Divergences in The Me PDFJohn GamesbyÎncă nu există evaluări
- Evaluating The Drivers of Indo-Pacific B PDFDocument13 paginiEvaluating The Drivers of Indo-Pacific B PDFJohn GamesbyÎncă nu există evaluări
- Keeping and Breeding BredliDocument4 paginiKeeping and Breeding BredliJohn GamesbyÎncă nu există evaluări
- Destruction of The Collection of Reptile PDFDocument3 paginiDestruction of The Collection of Reptile PDFJohn GamesbyÎncă nu există evaluări
- Crocodiles of New Guinea Philippines Crocodiles Part IV PDFDocument15 paginiCrocodiles of New Guinea Philippines Crocodiles Part IV PDFJohn GamesbyÎncă nu există evaluări
- Burmese Brown Tortoise PDFDocument5 paginiBurmese Brown Tortoise PDFJohn GamesbyÎncă nu există evaluări
- Colostethus Beebei - Golden Rocket Frog PDFDocument4 paginiColostethus Beebei - Golden Rocket Frog PDFJohn GamesbyÎncă nu există evaluări
- Crocodile Empire Crocodiles Part 1 PDFDocument21 paginiCrocodile Empire Crocodiles Part 1 PDFJohn GamesbyÎncă nu există evaluări
- Brazilian Snake-Necked TurtleDocument3 paginiBrazilian Snake-Necked TurtleJohn GamesbyÎncă nu există evaluări
- Casquehead Iguana PDFDocument3 paginiCasquehead Iguana PDFJohn GamesbyÎncă nu există evaluări
- An Introduction To Wolf SpidersDocument4 paginiAn Introduction To Wolf SpidersJohn GamesbyÎncă nu există evaluări
- Borneo Activity Cat DropDocument7 paginiBorneo Activity Cat DropBldcÎncă nu există evaluări
- RatDocument192 paginiRatsy734100% (2)
- 托福阅读功能目的题1 0Document59 pagini托福阅读功能目的题1 0jessehuang922Încă nu există evaluări
- English Listening Skills Class XIDocument5 paginiEnglish Listening Skills Class XIShashankKumarÎncă nu există evaluări
- Squeaks in The Deep-Rulebook SDVDocument160 paginiSqueaks in The Deep-Rulebook SDVMMK100% (1)
- Home Economics For Secondary Schools Book 2Document162 paginiHome Economics For Secondary Schools Book 2EleciaÎncă nu există evaluări
- Jurnal Dyan SamsaraDocument18 paginiJurnal Dyan SamsaraDaily Alman&momÎncă nu există evaluări
- Rat Blockers PDFDocument12 paginiRat Blockers PDFRatblockersÎncă nu există evaluări
- Controlling Rats and Mice LeafletDocument8 paginiControlling Rats and Mice LeafletEtooAKAmeÎncă nu există evaluări
- Rodents and Sectional Title ComplexDocument2 paginiRodents and Sectional Title ComplexFredSmith777Încă nu există evaluări
- RUBBER Pest N DiseaseDocument18 paginiRUBBER Pest N DiseaseSuriana Bakri0% (1)
- Roald Hoffmann On The Philosophy Art and Science of Chemistry - NodrmDocument354 paginiRoald Hoffmann On The Philosophy Art and Science of Chemistry - NodrmEugene Lim100% (2)
- Mock Defense Parcon 1Document19 paginiMock Defense Parcon 1Nash Gemar Braga EvangelistaÎncă nu există evaluări
- LP Mapeh6Document3 paginiLP Mapeh6jevie gonzalesÎncă nu există evaluări
- Enriched Environment Exposure Accelerates Rodent Driving SkillsDocument5 paginiEnriched Environment Exposure Accelerates Rodent Driving SkillsVanêssa CarvalhoÎncă nu există evaluări
- Popular Pet Snakes Available in South Africa EbookDocument15 paginiPopular Pet Snakes Available in South Africa EbookTimothy ZediÎncă nu există evaluări
- Bahamas Invasive Species Field GuideDocument55 paginiBahamas Invasive Species Field GuidecavrisÎncă nu există evaluări
- "His Brain, Her Brain"Document4 pagini"His Brain, Her Brain"Alfredo Rios JrÎncă nu există evaluări
- Control of Insects and RodentsDocument21 paginiControl of Insects and RodentsZam PamateÎncă nu există evaluări
- Xenopsylla Cheopisoriental Rat FleaDocument5 paginiXenopsylla Cheopisoriental Rat FleaNurul HidayatiÎncă nu există evaluări
- Rodent and Mice ControlDocument14 paginiRodent and Mice Controljuan dela cruzÎncă nu există evaluări
- Weiss 1972Document10 paginiWeiss 1972PÎncă nu există evaluări
- Rats and MiceDocument2 paginiRats and MicekaylaÎncă nu există evaluări
- NTEAdvancedUnit7 Sample Studentbook PDFDocument14 paginiNTEAdvancedUnit7 Sample Studentbook PDFMaria ProkopovychÎncă nu există evaluări
- Sudden Death and HopeDocument8 paginiSudden Death and HopemanowarÎncă nu există evaluări
- Rats - Identification and ManagementDocument25 paginiRats - Identification and ManagementRayge HarbskyÎncă nu există evaluări
- Company ProfileDocument16 paginiCompany ProfilehinzaawanÎncă nu există evaluări
- Animal HappyDocument9 paginiAnimal HappyTô Yến VyÎncă nu există evaluări
- Night Horrors - Enemy ActionDocument166 paginiNight Horrors - Enemy ActionMin 0tavr60% (5)
- Mitkadmim 2Document11 paginiMitkadmim 2Silberman MarinaÎncă nu există evaluări