Documente Academic
Documente Profesional
Documente Cultură
A Redox-Neutral Fe-Catalyzed Cycloisomerization of Enyne Acetates ACS BOM
Încărcat de
Anonymous btFsuBkbwDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
A Redox-Neutral Fe-Catalyzed Cycloisomerization of Enyne Acetates ACS BOM
Încărcat de
Anonymous btFsuBkbwDrepturi de autor:
Formate disponibile
Research Article
pubs.acs.org/acscatalysis
A Redox-Neutral Fe-Catalyzed Cycloisomerization of Enyne Acetates
Johannes Teske and Bernd Plietker*
Institut fur Organische Chemie, Universitat Stuttgart, Pfaenwaldring 55, DE-70569 Stuttgart, Germany
S Supporting Information
*
ABSTRACT: The cationic complex [(Ph3P)2Fe(CO)(NO)]BF4
catalyzes the redox-neutral cycloisomerization of enyne acetates to diastereomerically pure allenyl pyrrolidines in good to
excellent yields.
KEYWORDS: iron catalysis, allene, cycloisomerization, enyne, heterocycle, diastereoselectivity
INTRODUCTION
Generating dened molecular complexity from simple
precursor molecules is one of the major driving forces for the
development of methodology in organic chemistry. Among
the methods developed today, metal-catalyzed intramolecular
cycloadditions1 and the related cycloisomerizations2 occupy an
important place. Starting from rather simple unsaturated precursor molecules, the initial cyclization is in the case of the
cycloisomerization succeeded by a shift of functional groups, or
in case of the cycloaddition, a second cyclization takes place.
Although versatile metal catalysts and complex molecular transformations have been developed within the past years, the eld
of cycloaddition/cycloisomerization reaction is still one of the
most vigorously developing elds in catalysis. In particular,
Pd-based,3 Rh-based,4 Ir-based,5 and Ru-based6 catalysts have
been shown to promote these reactions through metal-centered
two-electron redox mechanisms.7 In the past decade, the mechanistic portfolio was broadened through the advent of novel
catalysts that act as -acids in non-redox-type cycloisomerizations/
cycloadditions. Catalysts based on Au,8 Ag,9 W,10 and Cu11 but
also on Ga12 or In13 were shown to promote these reactions.
Interestingly, depending on the catalyst employed in the reaction, dierent products arise starting from identical precursor
molecules.
Within the past 15 years, signicant progress has been made
in the eld of base metal catalysis.14 Iron as one of the most
abundant metals in the upper earth crust has attracted a great
deal of interest.15 Although a plethora of Fe-catalyzed transformations have been reported in the past years, the eld of
Fe-catalyzed atom-economic redox-neutral cycloisomerization
is comparably less well elaborated.16 To the best of our
knowledge, the only examples for this type of Fe catalysis were
reported in 2005 by the group of Furstner.17 For a couple of
years, we have been involved in the development of catalytic
transformations that rely on the use of FeNO complexes.18
Whereas initial studies concentrated on the use of the anionic
XXXX American Chemical Society
Bu4N[Fe(CO)3(NO)] in mostly allylic substitution chemistry,19 we expanded more recently our investigation to the use
of the corresponding FeH complexes of the general type
H[(phosphine)2Fe(CO)(NO)], which show good activities
in hydrosilylation reactions.20 In line with this interest in
Fe catalysis, we became interested in developing catalytic transformations that rely on the use of the corresponding cationic
Fe complexes that are derived from the FeH species by
protonation. Herein, we describe the synthesis and application
of the cationic Fe complex [(Ph3P)2Fe(CO)(NO)]BF421 as a
potent catalyst in a redox-neutral cycloisomerization of enyne
esters to allenyl pyrrolidines or tetrahydrofurans with good to
excellent diastereoselectivities and yields (Scheme 1).
Scheme 1. Fe-Catalyzed Cycloisomerizations
RESULTS AND DISCUSSION
The catalytically active species was prepared in three steps
starting from Fe(CO)5 following a procedure that was originally
Received: August 8, 2016
Revised: September 12, 2016
7148
DOI: 10.1021/acscatal.6b02260
ACS Catal. 2016, 6, 71487151
Research Article
ACS Catalysis
described by Roustan as early as 1980.21 The 16-electron
complex 6 was obtained as an amorphous powder; however,
upon treatment with CO gas or acetonitrile, we were able to
isolate the corresponding complexes 7 and 8, from which we
could conrm the structure by X-ray analysis (Scheme 2).
Scheme 3. Scope and Limitations of Fe-Catalyzed
Cycloisomerization,
Scheme 2. Preparation of Complexes 7 and 8
We employed complex 6 in the cycloisomerization of enyne
acetate 9 and were pleased to nd that 9 reacted smoothly
to the corresponding allenyl pyrrolidine 10 with perfect
diastereoselectivity using only 10 mol % Fe complex 6 in
dichloromethane at 50 C (entry 2, Table 1).22 Importantly,
Table 1. Fe-Catalyzed Cycloisomerization of Enyne 9
no.
catalyst
conversion (%)
1
2
3
4
5
6
6
7
8
HBF4
[Bu4N][BF4]
66b
decomposition
All reactions were performed on a 0.2 mmol scale using 10 mol %
catalyst 6 in dry CH2Cl2 (1 mL) at 50 C under a N2 atmosphere for
22 h. bIsolated yield.
within the substrate core did not aect the course of the
reaction. Starting from achiral propargylic acetates, we observed
a high degree of distereoselectivity with regard to the relative
conguration of the two sp3 stereocenters. In each case, the
corresponding allenyl pyrroldine was obtained as a diastereomerically pure product with regard to the two adjacent
C(sp3) chiral centers. Interestingly, although the introduction
of chirality within the propargylic acetate motif did not aect
the diastereoselectivity of the two C(sp3) chiral centers, the
allene moiety was formed as a 1:1 mixture of stereoisomers.
Subsequently, we analyzed the inuence of the heteroatom
within the linker unit. Whereas dierent sulfonylamides have
comparable reactivities, a signicant decrease in reactivity was
observed upon using an ether linker. Finally, we prepared
starting materials possessing dierent substitutions at the
olenic moiety and were happy to see that even trisubstituted
olens are reactive. However, the higher degree of substitution
goes to the expense of reactivity. Furthermore, Z-congured
olens resulted in a sluggish reaction with only minor amounts
of the product being formed.
Subsequent test experiments were performed to shed some
light on the probable mechanism of this cycloisomerization.
Enynes 34 and 35-d3 were subjected in a cross-experiment to
All reactions were performed on a 0.2 mmol scale using 10 mol %
catalyst in dry dichloromethane at 50 C under a nitrogen atmosphere
for 22 h. bIsolated yield.
the use of catalytic amounts of HBF4 that might be formed
from complex 6 by reaction with water led to a fast degradation
of 9 to a mixture of dierent products (entry 5, Table 1).
A catalytic impact of the BF4 anion was excluded by
employing [Bu4N][BF4] in catalytic amounts in the absence of
the Fe complex to the test reaction under otherwise identical
conditions (entry 6, Table 1). As for the reaction in the absence
of any catalyst (entry 1, Table 1), no conversion was observed.
Importantly, 18-electron complexes 7 and 8 did not show any
activity under the given reaction conditions (entries 3 and 4,
respectively, Table 1).
With these results in hand, a screening of the scope and
limitation of this transformation was performed (Scheme 3).
A broad set of dierent densely functionalized tri- and
tetrasubstituted allenyl pyrrolidines were prepared. The cycloisomerization shows good functional group tolerance. Halides,
esters, ethers, and amides are tolerated. Additional olenic moieties
7149
DOI: 10.1021/acscatal.6b02260
ACS Catal. 2016, 6, 71487151
Research Article
ACS Catalysis
corresponding Fe-catalyzed transformation of enyne 9, they
could be potential intermediates from which allenyl pyrrolidine 10
might be formed. However, neither 36 nor 37 gave the corresponding allene 10 when either was subjected to the Fe catalysis
conditions (eqs 2 and 3, Scheme 4).
Importantly, treating the isoprenyl-substituted amide 38 with
either Fe complex 6 or Echavarrens Au catalyst 39 under
otherwise identical conditions (eqs 4 and 5, Scheme 4) gave
product 30 in 87 or 69% yield, respectively.26 Finally, enantiomerically pure enyne acetate 40 was tested using Fe catalyst
6 (eq 6, Scheme 4) and Au complex 39 (eq 7, Scheme 4).
In both cases, no transfer of chirality was observed. A racemic
mixture of allenes was observed in the presence of Fe catalyst 6,
and the two C(sp3) chiral centers were formed with exclusive
diastereoselectivity. Treatment of enantiopure enyne acetate 40
with Au catalyst 39 led to the formation of the corresponding vinylcyclopropane 41 as a single diastereomer as a racemic
mixture.
On the basis of these results, we propose a mechanism that
involves -activation of the alkyne moiety to III that sets the
stage for the 1,2-attack of the carboxyl group at the activated
alkyne moiety to give the corresponding vinylFe species IV.
This species is in equilibrium with Fecarbene species V.
At this point, any stereochemical information within the starting
material is lost.24 The subsequent [2+2]-cycloaddition yields
the corresponding metallacyclobutane VI, which in contrast to
Au catalysis does not undergo a fast reductive elimination to
vinylcyclopropane VII but rather an acetyl migration to give
nal product VIII (Figure 1).
Scheme 4. Control Experiments
analyze the level of deuterium scrambling (eq 1, Scheme 4).
Approximately 20% scrambling was observed; however, control
experiments indicate the scrambling to be a Fe-catalyzed
follow-up reaction between the two products, 13 and 29-d3.
This result indicates an intramolecular acyl group transfer to be
part of the mechanism. Propargylic acetates are a frequently
used structural motif in Pt as well as Au catalysis.23,24 Dierent
mechanisms for the mode of activation have been discussed,
i.e., the formal [3,3]-acyl rearrangement to allenyl acetates as
well as a [1,2]-acyl shift to vinylAu complexes or Aucarbene
complexes and the subsequent cyclopropanation of adjacent
double bonds.24 To obtain some indication of a probable
mechanistic scenario, we screened dierent noble metal-based
catalysts in the isomerization using enyne 9. Whereas Pt(II)
complexes catalyze a rearrangement of enyne acetate 9 to the
corresponding allene 36, catalytic amounts of either AuCl3 or
Echavarrens Au(I) complex [(o-Ph)C6H4Pt-Bu2]Au(CH3CN)PF6 3925 result in the formation of cyclopropane 37. Despite
the fact that both 36 and 37 were not observed in the
Figure 1. Mechanistic proposal.
CONCLUSION
Herein, we report that cationic FeNO complex 6 is reactive
in the redox-neutral cycloisomerization of 1,6-enyne acetates.
A variety of substrates underwent reaction to the corresponding
allenyl pyrrolidines27 with exclusive diastereoselectivities regarding the two new C(sp3) chiral centers. This novel reactivity
opens a new eld in Fe catalysis and adds an interesting feature
to the steadily growing area of base metal catalysis.
ASSOCIATED CONTENT
S Supporting Information
*
The Supporting Information is available free of charge on the
ACS Publications website at DOI: 10.1021/acscatal.6b02260.
7150
DOI: 10.1021/acscatal.6b02260
ACS Catal. 2016, 6, 71487151
Research Article
ACS Catalysis
(19) (a) Plietker, B.; Dieskau, A.; Mows, K.; Jatsch, A. Angew. Chem.,
Int. Ed. 2008, 47, 198201. (b) Holzwarth, M.; Dieskau, A.;
Tabassam, M.; Plietker, B. Angew. Chem., Int. Ed. 2009, 48, 7251
7255. (c) Dieskau, A. P.; Holzwarth, M. S.; Plietker, B. J. Am. Chem.
Soc. 2012, 134, 50485051. (d) Lin, C.-H.; Pursley, D.; Klein, J. E. M.
N.; Teske, J.; Allen, J. A.; Rami, F.; Kohn, A.; Plietker, B. Chem. Sci.
2015, 6, 70347043.
(20) (a) Belger, C.; Plietker, B. Chem. Commun. 2012, 48, 5419
5421. (b) Rommel, S.; Hettmanczyk, L.; Klein, J. E. M.N.; Plietker, B.
Chem. - Asian J. 2014, 9, 21402147.
(21) (a) Roustan, J. L. A.; Forgues, A. J. Organomet. Chem. 1980, 184,
C13C16. (b) Roustan, J. L. A.; Merour, J. Y.; Forgues, A. J.
Organomet. Chem. 1980, 186, C23C26.
(22) (a) Performing the same reaction on a 2.0 mmol scale gave the
desired product in 59% isolated yield. (b) For further optimization of
catalyst loading, see the Supporting Information. (c) Cycloisomerizations using related substrates were reported to be catalyzed
by AgSbF6 ( Ji, K.-G.; Shu, X.-Z.; Zhao, S.-C.; Zhu, H.-T.; Niu, Y.-N.;
Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2009, 11, 32063209. ) or mediated
by TiCl4 ( Zhang, Z.; Shi, M. Eur. J. Org. Chem. 2011, 2011, 2610
2614 ). However, different products were obtained.
(23) Zhang, L.; Sun, J.; Kozmin, S. A. Adv. Synth. Catal. 2006, 348,
22712296.
(24) Fensterbank, L.; Malacria, M. Acc. Chem. Res. 2014, 47, 953
965.
(25) Nieto-Oberhuber, C.; Perez-Galan, P.; Herrero-Gomez, E.;
Lauterbach, T.; Rodrguez, C.; Lopez, S.; Bour, C.; Rosellon, A.;
Cardenas, D. J.; Echavarren, A. M. J. Am. Chem. Soc. 2008, 130, 269
279.
(26) Harrak, Y.; Simonneau, A.; Malacria, M.; Gandon, V.;
Fensterbank, L. Chem. Commun. 2010, 46, 865867.
(27) -Allenols are versatile building blocks in organic synthesis. For
example, for recent applications of -allenols, see: (a) Brel, V. K.;
Belsky, V. K.; Stash, A. I.; Zavodnik, V. E.; Stang, P. J. Eur. J. Org.
Chem. 2005, 2005, 512521. (b) Oh, C. H.; Gupta, A. K.; Park, D. I.;
Kim, N. Chem. Commun. 2005, 45, 56705672. (c) Gockel, B.;
Krause, N. Org. Lett. 2006, 8, 44854488. (d) Zriba, R.; Gandon, V.;
Aubert, C.; Fensterbank, L.; Malacria, M. Chem. - Eur. J. 2008, 14,
14821491. (e) Cheng, X.; Jiang, X.; Yu, Y.; Ma, S. J. Org. Chem.
2008, 73, 89608965. (f) Kim, S.-H.; Oh, S.-J.; Ho, P.-S.; Kang, S.-C.;
O, K.-J.; Yu, C.-M. Org. Lett. 2008, 10, 265268. (g) Bates, R. W.;
Nemeth, J. A.; Snell, R. H. Synthesis 2008, 2008, 10331038.
(h) Kong, W.; Cui, J.; Yu, Y.; Chen, G.; Fu, C.; Ma, S. Org. Lett. 2009,
11, 12131216. (i) Alcaide, B.; Almendros, P.; Carrascosa, R.;
Martinez del Campo, T. Chem. - Eur. J. 2009, 15, 24962499.
(j) Arbour, J. L.; Rzepa, H. S.; White, A. J. P.; Hii, K. K. Chem.
Commun. 2009, 71257127. (k) Cheng, J.; Tang, X.; Ma, S. ACS
Catal. 2013, 3, 663666.
Additional observations and data (PDF)
Crystallographic information (CIF)
Crystallographic information (CIF)
Crystallographic information (CIF)
AUTHOR INFORMATION
Corresponding Author
*E-mail: bernd.plietker@oc.uni-stuttgart.de.
Notes
The authors declare no competing nancial interest.
ACKNOWLEDGMENTS
The authors are grateful to the Deutsche Forschungsgemeinschaft for nancial support and to Dr. Wolfgang Frey for X-ray
analysis.
DEDICATION
Dedicated to Prof. Dr. Dieter Hoppe on the occasion of his
75th birthday.
REFERENCES
(1) Lautens, M.; Klute, W.; Tam, W. Chem. Rev. 1996, 96, 4962.
(2) Dorel, R.; Echavarren, A. M. Chem. Rev. 2015, 115, 90289072.
(3) Espinosa-Jalapa, N. A .; Ke, D.; Nebra, N.; Le Goanvic, L.; MalletLadeira, S.; Monot, J.; Martin-Vaca, B.; Bourissou, D. ACS Catal. 2014,
4, 36053611.
(4) Masutomi, K.; Noguchi, K.; Tanaka, K. J. Am. Chem. Soc. 2014,
136, 76277630.
(5) Dieckmann, M.; Jang, Y.-S.; Cramer, N. Angew. Chem., Int. Ed.
2015, 54, 1214912152.
(6) Trost, B. M.; Ryan, M. C.; Rao, M.; Markovic, T. Z. J. Am. Chem.
Soc. 2014, 136, 1742217425.
(7) Aubert, C.; Buisine, O.; Malacria, M. Chem. Rev. 2002, 102, 813
834.
(8) Jimenez-Nunez, E.; Echavarren, A. M. Chem. Rev. 2008, 108,
33263350.
(9) Garcia, P.; Harrak, Y.; Diab, L.; Cordier, P.; Ollivier, C.; Gandon,
V.; Malacria, M.; Fensterbank, L.; Aubert, C. Org. Lett. 2011, 13,
29522955.
(10) Koo, B. S.; McDonald, F. E. Org. Lett. 2007, 9, 17371740.
(11) Rauniyar, V.; Wang, Z. J.; Burks, H. E.; Toste, F. D. J. Am. Chem.
Soc. 2011, 133, 84868489.
(12) Hamlin, A. M.; de Jesus Cortez, F.; Lapointe, D.; Sarpong, R.
Angew. Chem., Int. Ed. 2013, 52, 48544857.
(13) Tsuji, H.; Yamagata, K.; Itoh, Y.; Endo, K.; Nakamura, M.;
Nakamura, E. Angew. Chem., Int. Ed. 2007, 46, 80608062.
(14) (a) Holzwarth, M. S.; Plietker, B. ChemCatChem 2013, 5,
16501679. (b) Accounts of Chemical Research; Special Issue Earth
Abundant Metals in Homogeneous Catalysis; American Chemical
Society: Washington, DC, 2015.
(15) (a) Bauer, I.; Knolker, H.-J. Chem. Rev. 2015, 115, 31703387.
(b) Plietker, B. Iron-catalysis in Organic Synthesis; Wiley-VCH:
Weinheim, Germany, 2008.
(16) (a) Sylvester, K. T.; Chirik, P. J. J. Am. Chem. Soc. 2009, 131,
87728774. (b) Lin, A.; Zhang, Z.-W.; Yang, J. Org. Lett. 2014, 16,
386389.
(17) (a) Furstner, A.; Martin, R.; Majima, K. J. Am. Chem. Soc. 2005,
127, 1223612237. (b) Furstner, A.; Majima, K.; Martin, R.; Krause,
H.; Kattnig, E.; Goddard, R.; Lehmann, C. W. J. Am. Chem. Soc. 2008,
130, 19922004.
(18) (a) Klein, J. E. M. N.; Miehlich, B.; Holzwarth, M. S.; Bauer, M.;
Milek, M.; Khusniyarov, M. M.; Knizia, G.; Werner, H.-J.; Plietker, B.
Angew. Chem., Int. Ed. 2014, 53, 17901794. (b) Klein, J. E. M. N.;
Knizia, G.; Miehlich, B.; Kastner, J.; Plietker, B. Chem. - Eur. J. 2014,
20, 72547257.
7151
DOI: 10.1021/acscatal.6b02260
ACS Catal. 2016, 6, 71487151
S-ar putea să vă placă și
- Stereoselective Synthesis of Tetralins Using Cationic CyclisationsDocument4 paginiStereoselective Synthesis of Tetralins Using Cationic CyclisationsRyan JosephÎncă nu există evaluări
- Organic SynthesisDocument35 paginiOrganic SynthesisAshok PareekÎncă nu există evaluări
- Poly EneDocument3 paginiPoly EneMohammed TarekÎncă nu există evaluări
- Ocando Mavarez1998 PDFDocument7 paginiOcando Mavarez1998 PDFMateus PinheiroÎncă nu există evaluări
- Chapter 5: Solutions 51 - 100: A Radical Cascade From A Ketene DithioacetalDocument148 paginiChapter 5: Solutions 51 - 100: A Radical Cascade From A Ketene DithioacetalLuis Angel Perez GonzalezÎncă nu există evaluări
- The Metathesis Reactions From A Historical Perspective ToDocument15 paginiThe Metathesis Reactions From A Historical Perspective TonetsomÎncă nu există evaluări
- qt65w527wn NosplashDocument4 paginiqt65w527wn Nosplashlssoy655Încă nu există evaluări
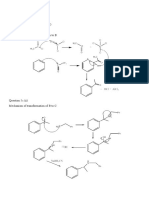
- Question 5C (I) Reagents - Lewis Acid (Alcl3) - Acetyl Chloride (CH Cocl) Mechanism of Transformation of A To BDocument9 paginiQuestion 5C (I) Reagents - Lewis Acid (Alcl3) - Acetyl Chloride (CH Cocl) Mechanism of Transformation of A To BKIBET SHADRACKÎncă nu există evaluări
- Alkyne Zipper ReactionDocument28 paginiAlkyne Zipper ReactionRajeswari RajiÎncă nu există evaluări
- O-Acylation of Ketone Enolates by Allyl 1H-Imidazole-1-Carboxylate Mediated With Boron Trifluoride EtherateDocument8 paginiO-Acylation of Ketone Enolates by Allyl 1H-Imidazole-1-Carboxylate Mediated With Boron Trifluoride EtheratedoubleffectÎncă nu există evaluări
- 815 824 PDFDocument10 pagini815 824 PDFDiogo DiasÎncă nu există evaluări
- Jacs AsapDocument2 paginiJacs AsapMohamadMostafaviÎncă nu există evaluări
- Tetrahedron Letters: Fumihiko Yoshimura, Masaki Takahashi, Keiji Tanino, Masaaki MiyashitaDocument4 paginiTetrahedron Letters: Fumihiko Yoshimura, Masaki Takahashi, Keiji Tanino, Masaaki MiyashitaLuis MoralesÎncă nu există evaluări
- Chapter 13: Olefinations: O R R LG R R LG PR, P (O) R, So R, Sir ADocument12 paginiChapter 13: Olefinations: O R R LG R R LG PR, P (O) R, So R, Sir AVictor BlackÎncă nu există evaluări
- Tetrahedron 2010 66 10 01902 - 01910 CiclicoDocument9 paginiTetrahedron 2010 66 10 01902 - 01910 Ciclicoteodoro11Încă nu există evaluări
- A Simple and Straightforward Approach To Quinoxalines by Iron/Sulfur-Catalyzed Redox Condensation of o Nitroanilines and PhenethylaminesDocument4 paginiA Simple and Straightforward Approach To Quinoxalines by Iron/Sulfur-Catalyzed Redox Condensation of o Nitroanilines and PhenethylaminesClaudia BocanegraÎncă nu există evaluări
- Dai Cheng, Shirong Zhu, Zhifang Yu, and Theodore CohenDocument5 paginiDai Cheng, Shirong Zhu, Zhifang Yu, and Theodore CohenSveti JeronimÎncă nu există evaluări
- Interrupted Carbonyl-Olefin Metathesis Via Oxygen Atom TransferDocument8 paginiInterrupted Carbonyl-Olefin Metathesis Via Oxygen Atom TransferFeniletilaminÎncă nu există evaluări
- J. Am. Chem. Soc. 2011, 133, 5791-5793Document3 paginiJ. Am. Chem. Soc. 2011, 133, 5791-5793NoimurÎncă nu există evaluări
- One Electron Oxidation of 12-Tungstocobalt (III) Ate Microsomal Cytochrome P450Document4 paginiOne Electron Oxidation of 12-Tungstocobalt (III) Ate Microsomal Cytochrome P450Narsinh M. DodiaÎncă nu există evaluări
- Catalytic Oxidation of Olefins 3pDocument3 paginiCatalytic Oxidation of Olefins 3pLi HojunÎncă nu există evaluări
- O Nucleophilic Amino Alcohol Acyl Transfer Catalysts The Effect of Acidity of The Hydroxyl Group On The Activity of The CatalystDocument4 paginiO Nucleophilic Amino Alcohol Acyl Transfer Catalysts The Effect of Acidity of The Hydroxyl Group On The Activity of The Catalystbruenor304amancaboihÎncă nu există evaluări
- Organic Reactions Volume 57Document250 paginiOrganic Reactions Volume 57vigogiff1594Încă nu există evaluări
- Zhao 2012Document9 paginiZhao 2012AndreaÎncă nu există evaluări
- Coupling of Enamides With Alkynes or Arynes For Synthesis of Substituted Pyridines and Isoquinolines Via Amide ActivationwDocument3 paginiCoupling of Enamides With Alkynes or Arynes For Synthesis of Substituted Pyridines and Isoquinolines Via Amide ActivationwBalaji ChandrasekharÎncă nu există evaluări
- Enantioselective DADocument4 paginiEnantioselective DASatyaki MajumdarÎncă nu există evaluări
- B 3Document2 paginiB 3bijuarÎncă nu există evaluări
- Electrochemical MethodDocument9 paginiElectrochemical MethodSumit VakhariaÎncă nu există evaluări
- IzomerizareDocument22 paginiIzomerizareTogan CristianÎncă nu există evaluări
- Synthesis of Novel Functional Polyolefin Containing Carboxylic Acid Via Friedelecrafts Acylation ReactionDocument7 paginiSynthesis of Novel Functional Polyolefin Containing Carboxylic Acid Via Friedelecrafts Acylation ReactionhusseinhshÎncă nu există evaluări
- Acc Chem Res 2008 Hiyama-Denmark Coupling ReviewDocument14 paginiAcc Chem Res 2008 Hiyama-Denmark Coupling Reviewsleekboy25Încă nu există evaluări
- Sem-4 - Active Methylene CompoundsDocument16 paginiSem-4 - Active Methylene CompoundshazemÎncă nu există evaluări
- Ate Complexes of Secondary Boronic Esters As Chiral Organometallic-Type Nucleophiles For Asymmetric SynthesisDocument4 paginiAte Complexes of Secondary Boronic Esters As Chiral Organometallic-Type Nucleophiles For Asymmetric SynthesisludoÎncă nu există evaluări
- Chem. Eur. J. 2015, 21, 7379-7383Document5 paginiChem. Eur. J. 2015, 21, 7379-7383NoimurÎncă nu există evaluări
- Formation Pathways of Magnetite Nanoparticles by CoprecipitationDocument8 paginiFormation Pathways of Magnetite Nanoparticles by CoprecipitationShweta KaurÎncă nu există evaluări
- A Nucleophilic Aromatic Displacement Reactions of Aryl HalidesDocument5 paginiA Nucleophilic Aromatic Displacement Reactions of Aryl HalidesSAGNIK PALÎncă nu există evaluări
- Microreview: Oleg KulinkovichDocument13 paginiMicroreview: Oleg KulinkovichSangvenkatÎncă nu există evaluări
- Modular Terpene Synthesis Enabled by Mild Electrochemical CouplingsDocument18 paginiModular Terpene Synthesis Enabled by Mild Electrochemical Couplingsgabriel martinezÎncă nu există evaluări
- J. Am. Chem. Soc. 2011, 133, 752-755Document4 paginiJ. Am. Chem. Soc. 2011, 133, 752-755Sidney Ramos SantanaÎncă nu există evaluări
- Olefin Polymerization With Ziegler-Natta Catalyst - Chemistry LibreTextsDocument3 paginiOlefin Polymerization With Ziegler-Natta Catalyst - Chemistry LibreTextsasad100% (1)
- 2011 OLAu MigracionDocument4 pagini2011 OLAu MigracionLoloÎncă nu există evaluări
- AngewandteDocument5 paginiAngewandteMunesh MeenaÎncă nu există evaluări
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesDe la EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesÎncă nu există evaluări
- Organo Cat Alys IsDocument15 paginiOrgano Cat Alys IsrajendickÎncă nu există evaluări
- Com 97 8051Document5 paginiCom 97 8051bruna_0410Încă nu există evaluări
- Bioinorganic Reaction Mechanisms: From High-Valent Iron To Bioorganometallic ChemistryDocument4 paginiBioinorganic Reaction Mechanisms: From High-Valent Iron To Bioorganometallic ChemistryBeh IranpoorÎncă nu există evaluări
- Organic Reactions Volume 58 ZincDocument246 paginiOrganic Reactions Volume 58 ZincKybernetikumÎncă nu există evaluări
- Organometallic Chemistry at The Nanoscale. Dendrimers For Redox Processes and CatalysisDocument21 paginiOrganometallic Chemistry at The Nanoscale. Dendrimers For Redox Processes and CatalysisVeselina GeorgievaÎncă nu există evaluări
- Aldehydes and Ketones IIDocument46 paginiAldehydes and Ketones IImidohemaÎncă nu există evaluări
- Problem 2Document4 paginiProblem 2王將方Încă nu există evaluări
- Anchimeric AssistanceDocument7 paginiAnchimeric AssistanceBen Duncan Málaga EspichánÎncă nu există evaluări
- Kimia Organik Strategi 6Document7 paginiKimia Organik Strategi 6Elfin LopeÎncă nu există evaluări
- Stereochemical Aspects of Organic SynthesisDocument9 paginiStereochemical Aspects of Organic SynthesisNoor NÎncă nu există evaluări
- Base Catalyzed RearrangementsDocument7 paginiBase Catalyzed RearrangementsAlvaroEnriqueQuinterosÎncă nu există evaluări
- CHEM 43.1 Exercise 5Document5 paginiCHEM 43.1 Exercise 5paradoxcomplexÎncă nu există evaluări
- Strategies Toward The Design of Energetic Ionic Liquids: Nitro-And Nitrile-Substituted N, N - Dialkylimidazolium SaltswDocument10 paginiStrategies Toward The Design of Energetic Ionic Liquids: Nitro-And Nitrile-Substituted N, N - Dialkylimidazolium SaltswZhan FangÎncă nu există evaluări
- Organic Electrochemistry As A Tool For SynthesisDocument7 paginiOrganic Electrochemistry As A Tool For SynthesismagicianchemistÎncă nu există evaluări
- Organic 2022Document134 paginiOrganic 2022xapodi8776Încă nu există evaluări
- Silicon in Organic Synthesis: Butterworths Monographs in Chemistry and Chemical EngineeringDe la EverandSilicon in Organic Synthesis: Butterworths Monographs in Chemistry and Chemical EngineeringÎncă nu există evaluări
- Chemica ChemicalDocument3 paginiChemica ChemicalAnonymous btFsuBkbwÎncă nu există evaluări
- Chemical Profile - MIBK PDFDocument3 paginiChemical Profile - MIBK PDFAnonymous btFsuBkbwÎncă nu există evaluări
- Self-Terminated Cascade Reactions That Produce ACS INTERESSANTEDocument5 paginiSelf-Terminated Cascade Reactions That Produce ACS INTERESSANTEAnonymous btFsuBkbwÎncă nu există evaluări
- Celc 201402017 PDFDocument8 paginiCelc 201402017 PDFAnonymous btFsuBkbwÎncă nu există evaluări
- Synthesis of Highly Ordered Hydrothermally Stable Mesoporous DUMESICDocument12 paginiSynthesis of Highly Ordered Hydrothermally Stable Mesoporous DUMESICAnonymous btFsuBkbwÎncă nu există evaluări
- Handouts 6 Residual PropertiesDocument17 paginiHandouts 6 Residual PropertiesAnonymous btFsuBkbwÎncă nu există evaluări
- Review of Proton Conductors For Hydrogen SeparationDocument13 paginiReview of Proton Conductors For Hydrogen SeparationAnonymous btFsuBkbwÎncă nu există evaluări
- Site-Selective Ethanol Conversion Over Supported Copper CatalystsDocument5 paginiSite-Selective Ethanol Conversion Over Supported Copper CatalystsAnonymous btFsuBkbwÎncă nu există evaluări
- Proton and Oxygen Ionic Conductivity of Doped CeriaDocument16 paginiProton and Oxygen Ionic Conductivity of Doped CeriaAnonymous btFsuBkbwÎncă nu există evaluări
- Re RefiningDocument68 paginiRe RefiningBibhu PratapÎncă nu există evaluări
- Chemical Tests PDFDocument2 paginiChemical Tests PDFSyafiqah ArinaÎncă nu există evaluări
- Materials: A Study On Tannery Sludge As A Raw Material For Cement MortarDocument14 paginiMaterials: A Study On Tannery Sludge As A Raw Material For Cement MortarKumar PallavÎncă nu există evaluări
- Solid StatesDocument4 paginiSolid StatesAbhi RamÎncă nu există evaluări
- Calabano Clinical Bacteriology Activity 1 (Lab)Document6 paginiCalabano Clinical Bacteriology Activity 1 (Lab)MarkJasperCalabanoÎncă nu există evaluări
- Zlib - Pub Toxicity of Nanomaterials Environmental and Healthcare ApplicationsDocument259 paginiZlib - Pub Toxicity of Nanomaterials Environmental and Healthcare ApplicationsEdwin Juan Mercado LenguaÎncă nu există evaluări
- Cefpodoxime Tablets: Ml. ML ML ML MLDocument2 paginiCefpodoxime Tablets: Ml. ML ML ML MLTống Ái Linh NguyễnÎncă nu există evaluări
- Fenvik, მარტი 2012Document50 paginiFenvik, მარტი 2012emediageÎncă nu există evaluări
- Determination of Partition Coefficient of Iodine in Water and Carbon Tetra ChlorideDocument15 paginiDetermination of Partition Coefficient of Iodine in Water and Carbon Tetra ChlorideNanda SatishÎncă nu există evaluări
- Organic Chemistry Principles and Mechanisms 2nd Edition Joel KartyDocument41 paginiOrganic Chemistry Principles and Mechanisms 2nd Edition Joel KartyjennieÎncă nu există evaluări
- Qa QC of Semisolid SDocument16 paginiQa QC of Semisolid SNita YesitaÎncă nu există evaluări
- 10th PS EM-02 TQADocument5 pagini10th PS EM-02 TQAksvvslan raju kÎncă nu există evaluări
- CHEMISTRY PROJECT - MergedDocument14 paginiCHEMISTRY PROJECT - Mergedarpitarathore024Încă nu există evaluări
- CHEM 101 - Principles of ChemistryDocument4 paginiCHEM 101 - Principles of ChemistrySaad Abdul AleemÎncă nu există evaluări
- Biocatalysis and Agricultural BiotechnologyDocument12 paginiBiocatalysis and Agricultural BiotechnologyMufidatun Nafi'ahÎncă nu există evaluări
- TB Unit3progresscheckfrq 5dc331c19f6919Document3 paginiTB Unit3progresscheckfrq 5dc331c19f6919api-486324042Încă nu există evaluări
- Intermolecular Forces of AttractionDocument26 paginiIntermolecular Forces of AttractionEsaïe GreñaÎncă nu există evaluări
- Pre Foundation ChemistryDocument68 paginiPre Foundation ChemistryAARYAN SURESH V. X DÎncă nu există evaluări
- List of Polyatomic IonsDocument1 paginăList of Polyatomic IonsSk. Salahuddin Ahammad100% (1)
- Ferrioxalate SystemDocument6 paginiFerrioxalate SystemRohit ChauhanÎncă nu există evaluări
- Glycine Leaching Kinetics of Chalcocite-92939546Document10 paginiGlycine Leaching Kinetics of Chalcocite-92939546Chad IrungÎncă nu există evaluări
- 8.ionic EquilibriumDocument64 pagini8.ionic EquilibriumhosifaÎncă nu există evaluări
- Determination of The Empirical Formula of MgODocument7 paginiDetermination of The Empirical Formula of MgOCicy IrnaÎncă nu există evaluări
- Carbide Burrs: High Performance Cutting ToolsDocument30 paginiCarbide Burrs: High Performance Cutting ToolsRam SinghÎncă nu există evaluări
- Sci 7 1FDocument9 paginiSci 7 1FLyra LlantadaÎncă nu există evaluări
- Assessment Schedule - 2011 Chemistry: Demonstrate Understanding of Aspects of Carbon Chemistry (90932)Document4 paginiAssessment Schedule - 2011 Chemistry: Demonstrate Understanding of Aspects of Carbon Chemistry (90932)Suv ViseÎncă nu există evaluări
- General Chemistry I: Senior High SchoolDocument14 paginiGeneral Chemistry I: Senior High SchoolJerry De Leon TaayÎncă nu există evaluări
- Chemistry 2Document38 paginiChemistry 2James Loyed TabasanÎncă nu există evaluări
- Replacement of Gas Phase With Liquid HexamineDocument6 paginiReplacement of Gas Phase With Liquid HexaminePradhita Ramdani HÎncă nu există evaluări
- Isomerism ReviewDocument7 paginiIsomerism Reviewayesha sheikhÎncă nu există evaluări