Documente Academic
Documente Profesional
Documente Cultură
Efectos en El Metabolismo Del Hypo e Hipertiroidismo
Încărcat de
Luis Ricardo Hernandez Garcia0 evaluări0% au considerat acest document util (0 voturi)
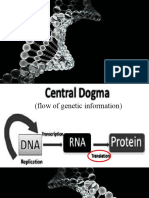
42 vizualizări16 pagini1) The thyroid hormones act directly on mitochondria to control the conversion of energy from oxidation into a usable form for cells.
2) Through their direct action on mitochondria, thyroid hormones also indirectly control the rate of protein synthesis and the amount of oxidative apparatus in cells.
3) In hypothyroidism, slow fuel consumption leads to low energy output. In hyperthyroidism, rapid fuel consumption leads to high energy output initially but efficiency decreases so usable energy production decreases.
Descriere originală:
Metabolismo en el hipo e hipertiroidismo
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest document1) The thyroid hormones act directly on mitochondria to control the conversion of energy from oxidation into a usable form for cells.
2) Through their direct action on mitochondria, thyroid hormones also indirectly control the rate of protein synthesis and the amount of oxidative apparatus in cells.
3) In hypothyroidism, slow fuel consumption leads to low energy output. In hyperthyroidism, rapid fuel consumption leads to high energy output initially but efficiency decreases so usable energy production decreases.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
42 vizualizări16 paginiEfectos en El Metabolismo Del Hypo e Hipertiroidismo
Încărcat de
Luis Ricardo Hernandez Garcia1) The thyroid hormones act directly on mitochondria to control the conversion of energy from oxidation into a usable form for cells.
2) Through their direct action on mitochondria, thyroid hormones also indirectly control the rate of protein synthesis and the amount of oxidative apparatus in cells.
3) In hypothyroidism, slow fuel consumption leads to low energy output. In hyperthyroidism, rapid fuel consumption leads to high energy output initially but efficiency decreases so usable energy production decreases.
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 16
Postgrad. med. J. (May 1968) 44, 347-362.
Biochemistry of hyperthyroidism and hypothyroidism*
FREDERIC L. HOCH
B.S., M.S., M.D.
Biophysics Research Division, Institute of Science and Technology,
The University of Michigan, Ann Arbor, Michigan 48104
Summary effect. The preponderance of evidence at present
The thyroid hormones act directly on mito- supports the first hypothesis. It seems feasible
chondria, and thereby control the transformation therefore to attempt to reduce the complex
of the energy derived from oxidations into a form pathologic pictures to subcellular phenomena
utilizable by the cell. Through their direct actions and their consequences.
on mitochondria, the hormones also control in- Recent advances in the understanding of where
directly the rate of protein synthesis and thereby and how the thyroid hormones act in the cell
the amount of oxidative apparatus in the cell. A support a simplification of thyrotoxicosis and
rationale for the effects of thyroid hormone excess hypothyroidism, although our understanding is
or deficiency is based upon studies of the not as yet so far advanced as to permit a final
mechanism of thyroid hormone action. In hypo- 'explanation' of the diseases in molecular terms.
thyroidism, slow fuel consumption leads to a low A brief history of the evolution of studies on
output of utilizable energy. In hyperthyroidism, the mechanism of thyroid hormone action serves
rapid fuel consumption leads to a high energy to outline the present state of knowledge, the
output, but as efficiency decreases, the utilizable areas in which future advances may be made,
energy produced decreases. Many of the chemical and a basis for a rationale of hyperthyroidism
and physical features of these diseases can be and hypothyroidism.
reduced to changes in available energy.
Actions and effects of thyroid hormones
Introduction Ever since Magnus-Levy (1895) showed that
Excess or deficiency in the amount of thyroid the thyroid gland controlled the rate of oxygen
hormones in humans produces clinical and consumption in mammals, attention has been
chemical manifestations that involve a number fixed on oxidative processes as a target of the
of organ and metabolic systems. Variations of hormone. Kendall (1929) showed the structure of
thyroid hormone concentrations in vivo change thyroxine and suggested the hormone might be
oxygen consumption, temperature regulation, a component or coenzyme of an oxidative
growth and development, the response to other enzyme, undergoing a redox cycle between the
hormones, nerve function, and the metabolism of phenol and semiquinone forms. No evidence
proteins, fats, carbohydrates, nucleic acids, vita- has as yet been found to support Kendall's hypo-
mins, and inorganic anions and cations. On the thesis conclusively. In the years 1940-50 it be-
other hand, thyroxine and triiodothyronine are came clear that 90% or more of the cell's
relatively simple molecules, and their small size oxygen was consumed via processes occurring
and limited number of reactive groups suggest in mitochondria, and experiments were done
either that the variety of the effects they pro- with thyroid hormones in vivo and in vitro to
duce are due to a few types of primary inter- determine their effects on mitochondria.
actions at the molecular level, or that the hor- One should differentiate, in considering these
mones are changed in the body to analogues studies, between actions of the hormones and
each having a different and specific physiologic effects of the hormones. Actions may be defined
as those functional or structural changes that
*Abbreviations: L-T,, L-thyroxine; L-T,, L-triiodo- are primary and depend upon the presence of
thyronine; Triac, triiodothyroacetic acid; ATP, ADP and the hormone at a site where it interacts with a
AMP, adenosine tri-, di- and mono-phosphate; P1, inorganic molecule in the cellular apparatus. Because hor-
phosphate; NADH and NADPH, reduced nicotinamide- mones are effective in small amounts we may
adenine dinucleotide and dinucleotide phosphate; DNP,
2,4-dinitrophenol; BMR, basal metabolic rate (0O con- assume that their primary molecular interactions
sumption). are reversible, so that the hormones are not
348 Frederic L. Hoch
used up. Effects may be defined as those func- 1966), then increases in ribosomal RNA-content
tional, structural, or compositional changes that and aggregation (about 40 hr) (Tata, 1967). How-
are secondary and do not depend upon the pres- ever, although all these phenomena showed an
ence of the hormone; they should not be important relationship between the thyroid hor-
reversed if the hormone is removed after acting. mones and the processes supplying information
The differentiation between actions and effects to and controlling the rate of protein synthesis,
makes no judgement on their relative importance they did not show the primary locus of hormone
in the cell. The thyroid hormones are peculiarly action. When L-T3 was added to isolated nuclei,
suitable for the resolution of primary actions RNA-metabolism was not stimulated (Widnell &
from secondary effects, because their iodine Tata, 1963; Tata & Widnell, 1966; Sokoloff,
moieties can be used experimentally as a tracer Francis & Campbell, 1964). Tata's conclusions
for quantitative analysis. Methods for detecting are diagrammed in Fig. 1.
other hormones not possessing this useful pro-
perty are less specific or more tedious. Protein Mitochondrion
As will be detailed below, the thyroid hor- T- -/- Nucleus -:> Ribosome = >
mones were shown to affect mitochondria as synthesis
2,4-dinitrophenol did: both agents increased 3-16 40 48 70-90
mitochondrial respiration, and the energy lib-
erated was transformed into heat rather than Time(hr) /i vivo
into the normal utilizable form, the high-energy
phosphate bond. This toxic, catabolic, energy- FIG. 1. Sequence of events after injecting hypothyroid
wasting effect served as a rationale for thyro- rats with thyroid hormone (T L-T,), according to
Tata and co-workers.
=
toxicosis (Hoch, 1962a), but not for the anabolic
energy-conserving effects that the smaller doses The studies of Sokoloff (Sokoloff & Kaufman,
of thyroid hormones exerted in euthyroid or
hypothyroid subjects (Hoch, 1962b). Nor was 1959, 1961; Sokoloff et al., 1963, 1964) have
hypothyroidism made more understandable by recently drawn attention back to the mitochon-
the 'uncoupling' hypothesis. Accordingly, atten- drion as a site of action of the hormone (Fig. 2).
tion was directed away from the mitochrondrion
in the search for the mechanism. o2
In the early 1960s the groups of Tata and of ASH.2 t-RNA-AA
Sokoloff showed that thyroid hormones affected T Mitochondrion =>xn >
ATP Ribosomez= Protein synthesis
protein synthesis. It had been demonstrated GTP
earlier by Dutoit (1952) that protein was syn- 5min Time 2hr:
thesized abnormally slowly in the livers of hypo- in vitro in vivo
thyroid rats. L-T3 given in vivo accelerated FIG. 2. Sequence of events after injecting hypothyroid
the synthesis of proteins by ribosomes after rats with thyroid hormone, or after addition of thyroid
about 48 hr after injection; the doses necessary hormone to mitochondria (T - L-T,), according to
were small and physiologic, smaller than those Sokoloff and co-workers.
producing uncoupling in mitochondria, and the
effect of the hormone was obviously anabolic Adding L-T3 to a homogenate in vitro stimu-
(see Tata, 1967). Puromycin and actinomycin D, lated ribosomal synthesis of proteins. The pro-
agents that block protein synthesis by acting on cesses whereby t-RNA-amino-acyl complexes
nucleic acids, blocked the calorigenic action of interacted with the ribosomes were the locus of
thyroid hormones (Tata, 1963; Weiss & Sokoloff, the stimulation. Mitochondria oxidizing a sub-
1963). No changes were observed in mitochon- strate were necessary, and they apparently pro-
drial respiratory control after hormone injec- duced a substance that accelerated the ribosomal
tion (Tata et al., 1963). Respiratory acceleration translation; adding ATP, GTP or glutathione did
could be demonstrated in mitochondria 70-90 not replace the effect of hormone-treated mito-
hr after hormone injections, but it was due to chondria. What it is that mitochondria produce
increases in the number of depleted respira- to control ribosomal protein synthesis is not yet
tory assemblies in the mitochondria of hypo- clear; studies by Bronk (1963) have suggested
thyroid rats (Tata et al., 1963; Roodyn, Freeman that mitochondrial non-phosphorylated, high-
& Tata, 1965), and so represented the specific energy intermediates may support protein syn-
results of earlier protein synthesis. Increases in thesis.
nuclear RNA-metabolism were shown early (3- Our recent studies have shown that L-T4 in-
16 hr) after hormone injection (Tata & Widnell, jected in vivo can act rapidly and directly on
Biochemistry of hyperthyroidism and hypothyroidism 349
mitochondria (Hoch, 1968a). Bronk (1966) has not yet clear, the rate of translation of t-RNA-
also shown a very short latent period for L-T8. amino-acyl complexes by ribosomes to synthesize
In hypothyroid rats, a subcalorigenic dose of proteins. Among the proteins synthesized are
L-T4 (at least fifty times less than those used the enzymatic components of the mitochondrial
to stimulate protein synthesis) partly corrected respiratory chain. The nucleus is also involved
the excessive respiratory control in liver mito- early, but the relation between the rises in nuclear
chondria 3 hr after injection (Hoch, 1966). A RNA-metabolism and the earlier changes in
larger dose did the same when the rats were mitochondrial function, as well as the later
killed 2 min after injection (Hoch, 1967, 1968b). changes in ribosomal metabolism and mitochon-
The hormone content of mitochondria, as mea- drial composition, are also not yet finally de-
sured by the total iodine or the butanol- fined. Thus, mitochondria show changes in func-
extractable iodine, was 20% of normal in un- tion dependent upon the hormone's presence or
treated hypothyroid rats, and rose progressively absence, and changes in enzyme content secon-
with the functional changes up to 3 hr after dary to the alterations in protein synthesis that
injection (Hoch, 1967; Dillon & Hoch, 1968); the depend ultimately upon the functional changes.
amount of hormone was one to five molecules It now appears profitable to consider mito-
per respiratory assembly in the treated rats, and chondrial respiration and energy-transformation
about 50 /uM in the mitochondrion (a concen- as the loci at which the thyroid hormone nor-
tration effective in vitro in Sokoloff's experi- mally acts, and at which excess or deficiency in
ments). This early or instantaneous action of the hormone content exerts its primary action.
hormone was completely reversed when bovine
serum albumin was added to the mitochondria, Early functional changes
and the greater part of the hormone was thereby Mitochondria may be regarded as energy
removed (Hoch & Motta, 1968). The features transforming or transducing machines perform-
of the reversibility of the functional changes ing oxidative phosphorylation: liberating energy
demonstrated the following: (a) that the hor- by oxidizing a substrate, and transforming this
mone acted directly on mitochondria, but per- energy into a chemically utilizable form for
haps through an intermediate or synergistically endergonic reactions, the high-energy phosphate
with endogenous mitochondrial components; (b) bonds of adenosine-triphosphate (ATP). The
that synthesis of a mitochondrial protein was molecular events of this process are the subject
not involved; and (c) that observations (Tata et of intensive investigation (see Lehninger, 1964;
al., 1963) that the hormone produced new mito- Racker, 1965) but are as yet incompletely under-
chondrial enzymes demonstrated an effect and stood. Oxidative phosphorylation is measured by
not an action of the hormone, because those the rate of oxygen consumption (energy input
workers had routinely added bovine serum al- per unit of time) and the efficiency of energy
bumin to their assay mixtures and so had ob- transfer, the P:O ratio (utilizable energy output
served only the late irreversible effects of L-T3. per energy input). The amount of work the
At the present time, the actions and the effects machine can do per unit of time (the utilizable
of the thyroid hormones appear to be related energy output per unit of time) is in physical
as in Fig. 3. Low doses of the hormone act units, power. The useful output of the mito-
chondrion, its oxidative power, consists of high-
energy phosphate bonds.
t-RNA-AA Physiologically, the most important feature of
T = Mitochondrion
? ? ?
=>
Ex
- -. Ribosome => Protein synthesis
oxidative phosphorylation is that it is self-regulat-
Nucleus- -
_
ing. The rate of output of high-energy phos-
2 min phate bonds controls the rate of input, i.e. the
/n v/vo
Time consumption of oxygen and the oxidation of
substrates. ADP controls the rate of oxidation;
FIG. 3. Sequence of events after injecting hypothyroid ADP accepts the high-energy phosphate groups
rats with thyroid hormone (T L-T4), according to
= from a hypothetical mitochondrial intermediate
Hoch and co-workers. to form ATP. In the absence of added ADP,
or in the absence of any agency removing the
rapidly or instantaneously, and reversibly, on terminal phosphate groups of ATP to form ADP,
mitochondria (only liver mitochondria have so mitochondria oxidize substrates very slowly
far shown unmistakable results of treating hypo- (State 4) as in Fig. 4. Addition of ADP, or the
thyroid rats with the hormone). The functional presence of an enzyme system hydrolysing ATP
changes in mitochondria accelerate, by a process to ADP, increases the rate of oxidation markedly
350 Frederic L. Hoch
(State 3) as in Fig. 5. The respiratory control mitochondria subsequently isolated from the
ratio is the ratio of the rates of respiration in treated animal phosphorylate with decreased effi-
State 3: State 4. The mitochondria in resting ciency.
living cells function as if little ADP were avail- The efficiency of oxidative phosphorylation
able; that is, cells respire in State 4, in a con- also controls the oxidative rate. Uncoupling
trolled condition in which large demands for raises the respiratory rate markedly (State 3u),
utilizable energy (production of ADP) can be and depresses the respiratory control ratio (Fig.
met with bursts of high oxidative activity (see 6); addition of ADP does not then accelerate
Hoch, 1968b). the already rapid respiration. Low concentrations
of uncoupling agents, insufficient to decrease the
efficiency of phosphorylation measurably, also
H2 X P
increase oxygen consumption and lower respira-
tsA ) tory control; this is termed 'loose coupling' (Fig.
7). The mechanism that ordinarily limits the rate
of respiration when ADP is absent (i.e. when
-X,-.P remains undischarged) now permits both
rapid respiration and transfer of energy.
SH2
Oxidation Phosphorylation
H 0 ;
FIG. 4. Normal mitochondrial oxidative phosphorylation
in State 4, with no P-acceptor. The - X P group
exerts a braking effect on the oxidation cycle. 2 / j
ADP 02
H2O
( SH2
FIG. 6. Uncoupled oxidative phosphorylation in the
')ATP absence of ADP (State 3u). The -X group exerts no
braking effect on the oxidation cycle. Adding ADP will
not accelerate oxidation. Phosphorylation is abolished
(efficiency = 0).
Pi
SH2
FIG. 5. Normal oxidative phosphorylation in State 3, in
the presence of ADP. The free - X group exerts no
braking effect on the oxidation cycle, and oxidation is
accelerated.
H20 Pf
The efficiency of phosphorylation, the P:O
ratio, is the number of moles of high-energy 02p
phosphate produced per gram atom of oxygen SH2
consumed. This ratio is 3 for most compounds
oxidized via diphosphopyridine-nucleotide-depen- FIG. 7. Loose-coupled oxidative phosphorylation in the
absence of ADP (State 4). The - X P group now
dent dehydrogenases. The translation of the exerts no braking effect on the oxidation cycle. Adding
energy liberated by oxidation into phosphoryl- ADP will not accelerate oxidation. Phosphorylation is
bond energy has been termed 'coupling' by at almost normal efficiency.
mechanical analogy (Loomis & Lipmann, 1948).
Phosphorylation can be decreased or abolished L-T4 and L-T3 can act like uncoupling agents
selectively without diminishing mitochondrial oxi- in many respects. Large doses in vivo and high
dations. Physical agencies (e.g. heat or hypotoni- concentrations in vitro uncouple oxidative phos-
city) or a variety of chemical agents (the classical phorylation (see Hoch, 1962b; Hoch & Lipmann,
one is 2,4-dinitrophenol) 'uncouple' oxidative 1954; Maley & Lardy, 1953; Martius & Hess,
phosphorylation, and decrease the phosphoryla- 1952). By either route, the hormone accelerates
tion quotient by decreasing its numerator. Many the State 4 oxidation of most substrates and de-
of the chemical agents also uncouple in vivo presses the respiratory control. Much smaller
when administered to normal animals, i.e. the doses or concentrations also lower respiratory
Biochemistry of hyperthyroidism and hypothyroidism 351
control by raising State 4 oxidation, but they Qo2) and the efficiency of the machine (the
do not depress the P: ratio (see Fig. 7), nor
interfere with the inhibitory action of oligomycin
,PP:O ratio) are plotted as a function of the
concentration of an agent that can uncouple,
(Hoch, 1968b), an agent specific for phosphory- e.g. DNP or thyroid hormone. It will be seen
lating respiration. that low concentrations of DNP or thyroid hor-
Mitochondria from hypothyroid animals, that mone stimulate energy consumption before effi-
contain only 20% of the normal amount of ciency is depressed; the result is increased power.
thyroid hormone, are 'over-coupled', respiring Higher concentrations uncouple and depress
too slowly in State 4 with excessive respiratory efficiency. Power therefore is decreased, and it
control (Maley & Lardy, 1955), as in Fig. 8. It is this catabolic effect that is seen in thyrotoxi-
cosis. In hypothyroid subjects, mitochondrial
energy consumption is depressed below normal,
H2 0 = but efficiency is normal. Power is therefore de-
creased. Treatment with L-T4 raises energy con-
sumption, but does not affect efficiency: power
is restored to normal, an anabolic action. DNP
-
has been shown to possess such an anabolic
02 %Pp
action as well, in accelerating protein synthesis
SH2 in vitro (Sokoloff & Kaufman, 1961).
However, the differences between the thyroid
FIG. 8. 'Over-coupled' oxidative phosphorylation in hormones and the uncoupling agents should be
State 4. The - X P group now exerts a greater than
-
stressed at this point: certain effects of the thy-
normal braking effect on the oxidation cycle. Adding
ADP will accelerate oxidation to (almost) normal roid hormones are biologically specific. Agents
levels. Phosphorylation is normal. like DNP do not relieve all the defects seen in
hypothyroid subjects, nor do they stimulate
seems that the optimal amount of L-T4 is neces- growth and development, nor do they induce
sary to poise mitochondrial energy-transfer be- metamorphosis in Anura. The specificity must
tween inertia and inefficiency. reside in the differences between their actions at
Consideration of oxidative power as an index the mitochondrial level. The mechanisms of
of the performance of the mitochondrial mech- action of thyroxine and DNP are known to
anism offers a basis for rationalizing many of differ: thyroxine makes mitochondria swell and
the diverse effects of the thyroid hormones. In DNP does not; and thyroxine and DNP act
Fig. 9, oxidative power is viewed as a resultant synergistically on mitochondrial respiration, not
of changes in the rate of oxidation and/or the additively.
efficiency of energy transfer (Hoch, 1962a, At least two factors may govern the effect
1968a). The rate of energy consumption (the of thyroid hormones: the thyroid state of the
organism (presumably a function of the concen-
% of tration of thyroid hormone in contact with the
normal Energy target sites) and the additional amount of the
/ consumed administered hormone reaching the target. Thus,
/
/ (Q02) thyroid hormone given to a hypothyroid subject
raises oxidative metabolic power to normal levels;
-P/ 10
Efficiency similar dosage in a euthyroid subject increases
9Q02
_o _ /
/ (NP/0) power above normal; higher dosage decreases
power.
Late compositional changes
DNP: O ==Cr- The functional changes in mitochondrial res-
piration discussed in the previous section involve
Thyroid -Hypo -Eu Hyper --
specific respiratory activity, in the sense of the
state rate of respiration per amount of respiring mass.
Power
(-P/t)
j-- I- The thyroid hormones also affect the total res-
piratory mass in the mitochondrion. They con-
FIG. 9. Fuel consumption (Qo,), efficiency (- P:O), trol, through protein synthesis, the amount of
and power (- P:t) of the mitochondrion as a function respiratory enzymes per gram of total protein
of the concentration of DNP or thyroid hormone (from in the mitochondrion. In hypothyroidism, the
Hoch, 1968a). rate of protein synthesis is lower than normal.
352 Frederic L. Hoch
Mitochondria from hypothyroid rats contain The increased rates of oxidation are accom-
half as much cytochrome b, c, and a+a3 as nor- panied by increased production of heat per unit
mal mitochondria (Maley, 1957; Kadenbach, of time even when efficiency is normal, and rela-
1966) and perhaps less flavoprotein catalysts (Riv- tively more heat than utilizable energy will be
lin & Langdon, 1966). But, paradoxically, there evolved as the efficiency of energy transfer de-
is twice the normal amount of pyridine nucleo- creases. In the most extreme forms of thyro-
tide coenzymes. Conversely, in mild hyperthyroid- toxicosis, where more complete uncoupling might
ism, with its raised rate of protein synthesis, occur, heat production leads to hyperpyrexia (as
the content of cytochromes and flavoproteins is in thyroid crisis; see below). Lesser degrees of
high, and of pyridine nucleotides, low (Kaden- heat production are compensated by sweating,
bach, 1966; Maley & Lardy, 1955). vascular changes, dilation, flushing, and resultant
At first glance, these compositional differences tachycardia, increased cardiac stroke volume and
seem to reflect or account for the abnormal pulse pressure. Such changes may in part be
BMRs in thyroid disease. However, the situation mediated through the action of the hormones
is not so clear. The flavoproteins and cyto- of the adrenal medulla (see pp. 357-8). Compen-
chromes have turnover numbers, and are pre- sation may be precarious, and the excessive de-
sent in such amounts in mitochondria, that they mands imposed by relatively slight increases of
do not control or limit oxidation in the respira- external temperature, the rise of heat produc-
tory chain. The 'bottle-neck' in respiration (actu- tion during muscular exercise, or administration
ally in electron-transport) appears to be at or of agents with uncoupling properties may have
near the pyridine nucleotide end of the chain drastic consequences. Increased tissue heat em-
(Chance, 1965), and there is evidence that the phasizes the increased metabolic demands and
substrate-dehydrogenase interactions are involved further accelerates oxidative rate. Weight loss
(Klingenberg, 1963). The pyridine nucleotides, and wasting of both fat and lean body mass
however, change in amount in a direction oppo- (Wayne, 1960) occur without any losses in
site to the changes in BMR, under thyroid hor- appetite.
mone influence. Thus, while the hormone seems Conversely, the hypothyroid subject consumes
to control the maximal capacity for oxidation in oxygen more slowly than normally because his
mitochondria, it also controls the specific acti- mitochondria respire slowly in State 4. Both be-
vity of respiration in the chain, and the latter cause of thyroid hormone deficiency and res-
accounts for the rate of oxygen consumption in piratory enzyme depletion, 'hypometabolism' is
the resting subject (Ernster & Luft, 1964). The here not a misnomer, phosphorylative metabolism
importance of capacity for oxidation in pro- proceeding at a low rate because of the
cesses involving large demands for energy, how- diminished liberation of oxidative energy. De-
ever, should not be minimized. creased rates of oxidation produce less heat, and
The resting hyperthyroid subject thus con- extremes result in hypothermia (see myxoedema
sumes oxygen faster because the mitochondria coma, below). Demands for increased heat pro-
in his tissues consume oxygen faster in State 4. duction, as in acclimatization to cold environ-
The term 'hypermetabolism' in this connection is ments, are met poorly by hypothyroid subjects.
a misnomer, for while oxidation is increased, the Responses to the hormones of the adrenal
metabolic processes of phosphorylative energy medulla are subnormal. It is of some interest
transfer are normal or decreased. This kind of that clinical investigators (Selenkow & Marcus,
hyperoxidation arises from the presence of ex- 1960) have commented upon the 'apathetic'
cess amounts of thyroid hormones in the mito- hyperthyroidism seen in older patients. It has
chondria. Not all organs consume more oxygen features similar to those of hypothyroidism; on
in thyrotoxic subjects; the brain, spleen and our basis, both are reflections of the decreased
testes do not. The mitochondria of brain, spleen capacities for the production of utilizable energy.
and testes do not swell in the presence of thyroid
hormones, in contrast to those from other tissues Alterations in metabolism
(Tapley & Cooper, 1956), but it is not known Certain features of clinical and experimental
if their iodine contents are high or normal in hyperthyroidism and hypothyroidism may be
thyrotoxic subjects. Another kind of hyperoxi- considered as manifestations of changes in the
dation may arise from the late adaptive increases transformation of energy. More information is
in mitochondrial enzyme content, but it is pre- available on the correlation of manifestations
sumably fully efficient and should persist for and basic changes in hyperthyroidism than in
some time even when the amount of thyroid hypothyroidism. The following are those areas
hormone in the mitochondria becomes normal. of energy utilization best studied at present.
Biochemistry of hyperthyroidism and hypothyroidism 353
Protein metabolism contents of the livers of thyroxine-treated rats
Thyroid hormones control protein synthesis (Handler, 1948), and the fat content of human
and breakdown. The effect of administered thy- bodies (Wayne, 1960) were below normal in
roid hormones depends upon the thyroid status hyperthyroidism. Thyroidectomy also decreased
of the subject. Low doses of thyroxine stimulate the rate of cholesterol synthesis (Boyd, 1959);
protein synthesis, high doses depress it. L-Ts the low rate of synthesis of cholesterol and fatty
decreased protein synthesis in euthyroid humans, acids in myxoedematous subjects was raised to
but the same dose raised the low rate of protein normal by thyroid hormones (Lipsky et al., 1955)
synthesis to normal levels in myxoedematous However, the control of lipid synthesis by thy-
patients (Crispell, Parson & Hollifield, 1956). In roid hormones has also been observed in tissue
hypothyroid rats, 5-10 ,ug of T4 daily increased preparations that were supposed to be free of
protein synthesis (Karp & Stetten, 1949; Rupp, mitochondria (Fletcher & Myant, 1960).
Paschkis & Cantarow, 1949), but in either hypo- The defects and increases in lipid synthesis
thyroid or normal animals, 50-100 j/g decreased might be ascribed to changes in the availability
or abolished synthesis (Rupp et al., 1949; Soko- of ATP at various steps in the process, even if
loff & Kaufman, 1959). Hyperthyroid humans mitochondria were not present in the lipid-
have subnormal amounts of parenchymal pro- synthesizing preparations. Thus, the effects of
tein in liver biopsies (Nikkila & Pitkanen, 1959). thyroid hormones have been laid to alterations
Consistent with the decreased synthesis of pep- in the supply of acetyl-coenzyme A, or further
tide linkages, the concentrations of free amino along the synthetic path, in the conversion of
acids in blood, liver and muscle are elevated in acetate to cholesterol, fatty acids and CO2 (Day-
thyrotoxic rats (Crispell et al., 1956; Friedberg ton et al., 1960); both require ATP. Coenzyme A
& Greenberg, 1947). Rat muscle (Ferrini, Per- concentrations do vary in the tissues of thyro-
roni & Bestagno, 1959) and human cells growing toxic animals, being low in hyperthyroid rat
in culture (Leslie & Sinclair, 1959) incorporate livers (Fraenkel-Conrat & Greenberg, 1946) and
amino acids into proteins more slowly in the in hyperthyroid humans (Gershberg & Kuhl,
presence of added thyroxine. Sokoloff & Kauf- 1950), and rising above normal when thyroidec-
man's (1961) studies have demonstrated in vitro tomized rats are treated with thyroxine, appar-
that the apparently conflicting effects of T4 upon ently via increased availability of ATP (Tabach-
protein synthesis are in reality biphasic hor- nick & Bonnycastle, 1954).
monal effects. The fatty acid oxidases are in mitochondria.
Thyroid hormones can act directly on lipid oxid-
Lipid metabolism ations by controlling ATP production, since fatty
Thyroid hormones control the rates of lipid acid activation requires ATP prior to oxidation.
synthesis, oxidation and mobilization. Biphasic Thyroxine treatment accelerated fatty acid oxid-
effects of thyroid hormone have been shown on ation in rat heart homogenates (Deitrich & Smith,
lipid synthesis (Fletcher & Myant, 1960); 20 /ug 1960), earlier than it raised the basal metabolic
of thyroxine increased the synthesis of choles- rate (Abelin & Kiirsteiner, 1928). Bacterial oxid-
terol from acetate by the cell-free fractions of ation of cholesterol was increased by added
rat livers, while 30-50 /,g decreased the syn- thyroid hormones (Wainfan & Marx, 1955).
thesis. Fatty acid synthesis was decreased at all Hypothyroidism decreased fatty acid oxidation
these levels of dosage. and ATP production from fatty acids in the
Thyroid-treated rats and humans synthesized hearts of dogs.
cholesterol more rapidly than normal (Kritch- Thyroid hormones also control fatty acid con-
evsky, 1960), and the hypocholesterolaemia of centrations in tissues through the rate of the
thyrotoxicosis, anomalous in the face of increased mobilization of fatty acids from adipose tissue,
synthesis, was ascribed to the hormonal stimu- in conjunction with the action of other hor-
lation of cholesterol excretion (Rosenman, Byers mones. T3 and Triac raised serum concentra-
& Friedman, 1952). Increased rates of choles- tions of unesterified fatty acids within 6 hr in
terol and fatty acid synthesis have been ob- humans (Rich, Bierman & Schwartz, 1959) and
served in hormone-treated rats and in their tissue enhanced their release from adipose tissue and
slices (Karp & Stetten, 1949; Dayton et al, 1960). their removal from serum in dogs. These
On the other hand, decreased rates of choles- thyroid effects are facilitations of other stimuli,
terol synthesis were observed in liver homo- particularly epinephrine (Jeanrenaud, 1961),
genates (Scaife & Migicovsky, 1957) and cell- which ordinarily liberate fatty acids (Schwartz
free preparations (Fletcher & Myant, 1962) from & Debons, 1959). Epinephrine-induced mobili-
thyrotoxic rats. The cholesterol and neutral fat zation of fatty acids in vivo requires optimal
354 Frederic L. Hoch
thyroid function, while thyroid hormone alone contents of liver and muscle were markedly
does not release fatty acids from adipose tissue diminished in hyperthyroid subjects, especially
in vitro (White & Engel, 1958). Hypothyroidism the metabolically active forms of glycogen (Chil-
prevents epinephrine-induced mobilization. In son & Sacks, 1959), but this, of course, may also
hypopituitary monkeys epinephrine injection depend on increased breakdown. Consistent with
liberated no fatty acids; thyroid-stimulating hor- a decrease in synthesis is the fact that both liver
mone partly restored, and small doses of T3 and muscle (where the major portion of gly-
fully restored, the normal fat-mobilizing res- cogen synthesis proceeds) showed decreased con-
ponse to epinephrine (Goodman & Knobil, tents of ATP after thyroid hormones were ad-
1959). Hyperthyroidism exaggerates the epine- ministered (Chatagner & Gautheron, 1960; Berg,
phrine effect on fat pads in vitro (Debons & 1937). Increases in synthetic rates have also been
Schwartz, 1961) and hypothyroidism abolishes it. observed, however, after single doses (Stern-
The fat-mobilization effects of insulin are simi- heimer, 1939) or more prolonged hormone treat-
larly affected by the thyroid state, insulin re- ment followed by liver perfusion with large
leasing six times more free fatty acids from the amounts of glucose (Burton, Robbins & Byers,
adipose tissue of T4-injected rats than normally 1958).
(Hagen, 1960). These interdependences of hor- The hyperglycaemic effect of epinephrine,
mone effects may reflect the facts that fat mobi- mediated through the increased formation of
lization depends upon the production of cyclic- cyclic-3',5'-AMP and the subsequent activation
3',5'-AMP, and that cyclic-3',5'-AMP is formed of phosphorylase, depends upon the thyroid
only from ATP; the thyroid hormones control state, and administered thyroid hormone pro-
ATP production, and it may be speculated-in duces biphasic effects. A small dose of T4 raised
the absence of definite information to date- the hyperglycaemic effect of injected epinephrine
that the ATP supply, as well as the hormone- whereas in rabbits fed 225 g of desiccated thy-
controlled activity of adenyl cyclase, controls roid, epinephrine caused little or no hypergly-
cyclic-3',5'-AMP production. caemia (Burn & Marks, 1925; Abbot & Van
Buskirk, 1931). The amount of liver glycogen
Carbohydrate metabolism also affects the hyperglycaemic response to epine-
Thyroid hormones control the rates of gly- phrine; prolonged thyrotoxicosis depletes rabbit
cogen synthesis and breakdown, and of hexose liver glycogen and then no hyperglycaemia fol-
oxidation. lows epinephrine administration. In hypothyro-
Thyroxine has a biphasic effect on glycogen dism, epinephrine produced a response smaller
synthesis. Low doses of thyroxine increased gly- than normal.
cogen synthesis in rat diaphragms either in vivo Thyroid hormones affect hexose oxidation and
or in vitro, and higher doses reversed this effect hexose phosphorylation, directly and by modifica-
(Wertheimer & Bentor, 1953). In vivo injections tions of the actions of other hormones. Oxidation
of thyroid-stimulating hormones (in normal but of hexoses was accelerated by administered thyroid
not in thyroidectomized animals), or of 20-30 /ug hormone, either through equal increases in both
of L-thyroxine, increased glycogen synthesis, the phosphogluconate and the glycolytic path-
whereas 100-200 /ug of L-thyroxine decreased ways, or mainly through increased glycolysis; the
synthesis below normal rates. In vitro, incuba- route chosen may depend upon the degree of
tion of 2 /ig of L-thyroxine with normal hyperthyroidism, low doses of hormone acceler-
rat diaphragms increased glycogen synthesis ating glycolysis mainly (Glock, McLean & White
while higher amounts either did not stimulate, head, 1956) and depressing the phosphogluconate
or irregularly depressed, synthesis. Both the in path (Dow & Allen, 1961). Hypothyroidism de-
vitro and the in vivo effects depended upon in- pressed glucose exidation via both paths (Dow
cubation of the diaphragms in homologous rat & Allen, 1961). The mechanisms of the thyroid
serum, which may have involved thyroxine-bind- hormonal effects on glycolysis may be via one
ing or lipid-binding. The lack of a measurable or more routes. An effect of thyroxine on the
rise in oxygen consumption, however, indicates cytoplasmic acylphosphatase of rat liver and
caution in accepting this system as one depend- muscle has been demonstrated: administered
ing simply on ATP-supply. hormone increases acylphosphatase activity, thy-
Most of the available evidence indicates de- roidectomy decreases it, and then low doses of
creased synthesis of glycogen in thyrotoxicosis. T4 restore it (Harary, 1958). This enzyme hydro-
Glycogen synthesis was decreased in hyperthy- lyses 1,4-diphosphoglycerate to Pi and 3-phos-
roid humans and rabbits (Coggeshall & Greene, phoglycerate; it acts as a rate-limiting ATPase,
1933; Mirsky & Broh-Kahn, 1936). The glycogen uncoupling glycolysis from phosphorylation, and
Biochemistry of hyperthyroidism and hypothyroidism 355
accelerates both glycolysis (by supplying Pi) and ism, skeletal muscles are larger and firmer than
mitochondrial oxidation (by supplying ADP). normal and contract slowly because of an ab-
And the activity of two enzymes of the glyco- normality in the contraction mechanism (Milli-
lytic pathway, enolase and lactic dehydrogenase, kan & Haines, 1957). The clinical sign of the
were increased in the livers of thyrotoxic rats 'hung-up' reflex, with its slow relaxation, reflects
(Bargoni et al., 1961), but they are probably not this defect. In hyperthyroidism, muscle contracts
rate-controlling steps; the activities of a number at the normal rate, but performs work ineffi-
of the glycolytic enzymes were decreased in hypo- ciently (Plummer & Boothby, 1923). Clinically,
thyroidism (Bargoni et al., 1964). Lastly, the 'thyrotoxic myopathy' (Thorn & Eder, 1946; Hed,
hormonal control of the generation of NADH Kirstein & Lundmark, 1958) reflects this defect.
and NADPH by mitochondria might also affect Thyroxine has a biphasic effect on muscular
both glycolysis and the phosphogluconate path- work in adrenalectomized rats, a low dose im-
way (Dow & Allen, 1961). Increased glucose proving the work done per contraction, and a
uptake or oxidation, or both, have been ob- four-times-higher dose decreasing it (Ganju &
served in the muscles and in the livers of hyper- Lockett, 1958).
thyroid animals, and also in cultures of animal The relation between muscle contraction and
cells, sperm, Saccharomyces cerevisiae, or Ace- thyroid state thus seems clearer than in the case
tobacter aerogenes treated with thyroid hormones. of some of the synthetic processes, probably be-
The rates of glucose utilization may also be cause muscle contraction and relaxation depend
affected through a hormonal control of hexose more directly upon ATP supplied by mitochon-
phosphorylation. Thyrotoxicosis raised the acti- drial oxidative phosphorylation. The other source
vity of intestinal phosphokinases (Nishikawara & of muscle ATP, the r-P of phosphocreatine, can
Gabrielson, 1961), and the observed delays in support only a few contractions and must itself
the tolerance curves for glucose and galactose be replenished from mitochondrial energy trans-
in this condition have been ascribed to rapid formations. The skeletal muscles of thyrotoxic
phosphorylation and intestinal absorption (Alth- rats showed uncoupling (Johnson et al., 1958),
ausen, 1940). However, the importance of the and those of thyrotoxic humans showed loose
phosphokinases in absorption has been ques- coupling (Ernster, Ikkos & Luft, 1959). In our
tioned (Nishikawara & Gabrielson, 1961). The terms, these mitochondria showed the action of
utilization rate of glucose, measured under con- the excessive amounts of thyroid hormones pre-
stant intravenous load, is reported to be normal sent. Other studies on apparently similar patients
in thyrotoxicosis (Macho, 1958) and normal in have shown normal muscle mitochondrial res-
hypothyroidism (Macho, 1961); in hypothyroid- piratory control, and either high controlled (State
ism, administration of thyroxine or DNP rapidly 4) and maximal (State 3) respiration (Stocker,
(4 hr) accelerated the utilization rate, suggesting Samaha & De Groot, 1966) or normal levels of
that these two agents act similarly. respiration (Dow, 1967; Peter & Lee, 1967); be-
Intravenous glucose-tolerance tests gave high cause bovine serum albumin was used in the
disappearance rates in hyperthyroid patients and preparation and assay of the mitochondria, these
low rates in hypothyroids; tolbutamide decreased results may show only the underlying enzymatic
blood glucose faster in hyperthyroids and slower composition of the muscle mitochondria as con-
in hypothyroids; and glucagon induced a lower trasted with the action of the excessive amounts
glucose response in hyperthyroids (Lamberg, of hormone present in situ.
1965). Orally administered D-xylose was norm- Heart muscle mitochondria are particularly
ally absorbed by patients with thyrotoxicosis or susceptible to thyroid hormones (Bing, 1961).
myxoedema, but oral or intravenous D-xylose Clinically, this seems to be reflected in the high
was excreted in the urine more rapidly in thyro- incidence of myocardial failure in thyrotoxicosis
toxicosis, and less rapidly in myxoedema (Broit- (the increased work load and decreased effi-
man et al., 1964). Thyroid hormones potentiate ciency of contraction are an unfortunate com-
insulin action; insulin-induced hypoglycaemia was bination), and in the lack of response of this
increased in human thyrotoxicosis (Elrick, Hlad form of failure to digitalis (which is more effec-
& Arai, 1961), and the uptake of glucose by rat tive against mechanically induced defective con-
adipose tissue was more sensitive to insulin after tractile mechanisms).
thyroxine was injected (Hagen, 1960). Alterations in creatine metabolism usually in-
volve muscle. A rationale for the observations
Muscle contraction and creatine metabolism is shown in Fig. 10. Clinically and experimentally,
Thyroid hormones control muscle contraction hyperthyroidism is accompanied by increased
and creatine metabolism. In human hypothyroid- creatine excretion, and hypothyroidism by de-
356 Frederic L. Hoch
creased creatine excretion. In hyperthyroidism, al., 1960). Pyridoxal-5-phosphate content was low
creatine synthesis was normal (Wilkins & Fleisch- because of defective phosphorylation; conversely,
mann, 1946). Injection of thyroxine rapidly de- it was high after thyroidectomy (Labouesse,
pleted muscle phosphocreatine content, well be- Chatagner & Jolles-Bergeret, 1960). Vitamin
fore creatine excretion rose (Wang, 1946), sug- B12 content was low in the tissues of thyro-
gesting a hormonal effect of phosphorylation. toxic rats and hypothyroid female rats (Gershoff
Thyrotoxic humans excreted creatine admini- et al., 1958; Kasbekar et al., 1959); hormone
stered to them, or endogenously synthesized, in administration raised renal B12 to normal in
contrast to normal subjects, because of an in- hypothyroid rats, and above normal in euthy-
ability to 'fix' creatine in their muscles, i.e. to roid rats (Okuda & Chow, 1961). Ascorbic acid
resynthesize phosphocreatine from creatine and content was low in the blood and tissues of
ATP (Shorr, Richardson & Wolff, 1933; Thorn, thyrotoxic subjects. Pantothenic acid and CoA
1936). The defective creatine load-test is so char- metabolism have been discussed under 'Lipid
acteristic that it has been used diagnostically. metabolism', and follow the same general pat-
tern as the other water-soluble vitamins.
The thyroid hormones also control the syn-
thesis of a fat-soluble vitamin. Vitamin A syn-
ADP thesis requires thyroid hormone. Both hypo- and
C-* AT
ATPP,I Muscle hyperthyroid patients had poor dark adaptation
C * contraction (Wohl & Feldman, 1939). In hypothyroidism
serum vitamin A was decreased because carotene
was not converted to the vitamin; hormone treat-
Pi ---Pi +Cr ment restored synthesis (Drill & Truant, 1947;
^ 1[
E xcretion
Johnson & Baumann, 1947). In euthyroid animals,
the hormone increased vitamin A synthesis, but
prolonged treatment produced a severe resistant
FIG. 10. Creatine and inorganic phosphate ion meta- vitamin A deficiency (Portugal'skaya, 1961), an-
bolism in relation to mitochondrial energy transfer. other example of the hormone's biphasic effect.
Creating phosphate (Cr P), usually in equilibrium
-
with the generated ATP, dissociates in hyperthyroidism Metabolism of inorganic ions
to maintain the ATP level. The extra free creatine (Cr)
and phosphate (Pi) are excreted. The Pi pool is aug- Phosphorus metabolism is strongly influenced
mented through inefficiency of oxidative re-esterification.
Similarly, administering extra Cr in a load test augments
by the thyroid state. Hyperthyroid patients are
the Cr-pool and subsequent excretion.
in negative phosphorus balance (Rawson et al.,
1955). Hypothyroid patients excreted large
amounts of phosphate soon after administration
Vitamin metabolism of the hormone, probably because of increased
The thyroid hormones control the utilization phosphocreatine hydrolysis (Beaumont, Dodds &
of the water-soluble vitamins and their synthesis Robertson, 1940; Flach et al., 1959). Phosphate
into coenzymes. The synthetic steps affected are contents were high, and ATP contents were low,
usually energy-requiring condensations and phos- in the soft tissues of thyrotoxic animals (Berg,
phorylations. Most of the information is avail- 1937; Chatagner & Gautheron, 1960; Maley,
able on thyrotoxic subjects, where conditioned 1957). The esterification of phosphate was slow
vitamin deficiences exist (Drill, 1943; Rawson, in such tissues (Johnson et al., 1958). In the
Rall & Sonenberg, 1955). Normal intake of vita- bones of hyperthyroid patients, phosphate was
mins is accompanied by deficiency symptoms turned over abnormally rapidly (Hernberg, 1960)
because of increased demands or defective utili- probably in conjunction with the changes in
zation. calcium.
Thiamine requirements are increased in hyper- Calcium metabolism also changes in thyroid
thyroid patients. The blood and liver vitamin disease. Calcium turnover in bones is accelerated
contents were subnormal, and the excretion was in hyperthyroid patients, and becomes normal
higher than normal (Williams et al., 1943). Tis- with the thyroid state upon treatment (Krane
sue cocarboxylase content was low in hyper- et al., 1956). Calcium accumulation was more
thyroid rats; it rose after thiamine was injected, striking in the livers than in the bones of thyro-
but then fell more rapidly than in euthyroid xine-treated rats; the capacity of liver mitochon-
animals, suggesting rapid destruction (Peters & dria to store Ca++ may be involved. In hypo-
Rossiter, 1939). Pyridoxine availability is limited thyroid rats, calcium incorporation into bone
in the tissues of hyperthyroid animals (Wohl et was decreased (Lengemann, Wasserman &
Biochemistry of hyperthyroidism and hypothyroidism 357
Comar, 1960) but in hypothyroid humans, hor- and to antagonize adrenomedullary hormones
mone administration did not raise calcium ex- have also been used with success.
cretion rapidly, although it raised phosphate ex- The experimental induction of the acute
cretion (Beaumont et al., 1940), perhaps because hyperthermia that is seen clinically in thyroid
the hormone acts more directly on phosphate crisis provides an insight into how the thyroid
metabolism. hormones act physiologically. Administering large
Magnesium metabolism depends upon the doses of thyroid hormone to animals usually
thyroid state, and vice versa. Mg++ and thyroid produces a loss of weight and an apathetic death,
hormones are antagonists in vivo and in vitro not a hyperthermic crisis. Much smaller doses
when mitochondrial function is measured (see of hormone, however, can produce fatal hyper-
Hoch, 1962b). Myxoedematous patients excreted thermia in conjunction with the administration
large amounts of Mg++ in their urine promptly of an agent that acts on mitochondrial oxidative
after hormone administration (Tapley, 1955). The metabolism. Among such compounds are the un-
plasma magnesium content was low in hyper- coupling agents, like dinitrophenol (Hoch, 1965a)
thyroidism and high in hypothyroidism; Mg bal- dinitro-o-cresol (Barker, 1946) and methylene
ance was positive in hyper- and negative in blue (Alwall, 1936); phosphate ions given by in-
hypothyroidism; total and cellular exchangeable fusion (Roberts et al., 1956); and antipyretic
Mg++ was strikingly low in hypothyroidism but agents, like sodium salicylate (Hoch, 1965a). A
normal in hyperthyroidism (Jones et al., 1966). dose of salicylate too small to raise the BMR
The effect of administering magnesium salts upon in a normal rat raises the BMR sharply in a
thyrotoxicosis is controversial, some finding a midly hyperthyroid rat; one-quarter of the nor-
decrease in BMR and heart rate (Hueber, 1939), mally lethal dose of salicylate rapidly induces
others finding no decrease in BMR nor change a fatal hyperthermia in such hyperthyroid rats.
in negative nitrogen and phosphate balances With dinitrophenol, this phenomenon can be
Wiswell, 1961). shown to arise from an exaggerated sensitivity
Decreased exchangeable potassium (Munro, of mitochondria to the uncoupling agent, in-
Renschler & Wilson, 1958; Wayne, 1960) and duced by thyroxine treatment (Hoch, 1968c).
hyperkalaemia and hyperkaluria (Boekelman, Whether the clinical phenomenon has a similar
1948) have been reported in thyrotoxicosis. Oc- basis remains to be seen. The association of
casionally periodic muscular paralysis is asso- thyroid crisis in thyrotoxic patients with infec-
ciated with hyperthyroidism. tions that cause fever (Means, De Groot & Stan-
bury, 1963) may be another example of an in
Temperature regulation vivo synergism.
The thyroid hormones are involved in the In hypothyroidism, heat production is
control of body temperature. About 60% of the diminished by the depressed rate of mitochon-
energy liberated by mitochondrial oxidations is drial oxidations. Again there are physiologic
normally converted to a chemically utilizable compensations to preserve body heat, and the
form, the other 40% being liberated as heat and skin is cold, circulation is slow, and cold is
thereby maintaining the body temperature of poorly tolerated. Body temperatures may be
homeotherms. In hyperthyroidism heat produc- below normal. Infections that normally elicit
tion is raised by two factors: the increased rate fever may not raise the hypothyroid patient's
of oxidation and the decreased efficiency of temperature at all, or at least not above normal.
energy conversion. Usually the excess amounts Occasionally a fatal hypothermia may supervene,
of heat can be dispelled by physiologic com- the so-called myxoedema coma, in which body
pensations such as flushing, sweating and in- temperature can no longer be maintained, and
creased circulation; many of the clinical char- has been reported as low as 74F. Experiment-
acteristics of hyperthyroid patients arise from ally the calorigenic response of hypothyroid rats
these compensations. to an administered uncoupling agent is sub-
Thyrotoxic crisis or storm can be viewed as a normal (Hoch, 1965b), because their mitochon-
failure of compensation due to increased heat dria are subnormally sensitive (Hoch, 1967,
production through a further loss of mitochon- 1968c). Administered thyroxine rapidly raises
drial efficiency. Body temperatures rise sharply mitochondrial responses, and the efficiency of
to 107F or more, muscle tone is lost, liver such treatment clinically may be evidence for a
damage (? mitochondrial) is severe and the common basis for the hypothermia.
patients may die. Body refrigeration may remove
enough heat to save the situation, but therapeutic Effects of hormones and drugs
measures to provide adrenocortical hormones The effects of a number of hormones and
358 Frederic L. Hoch
drugs depend upon the thyroid state of the sub- The adrenal cortical hormones and insulin may
ject, as has been mentioned above in connec- have synergistic effects with the thyroid hor-
tion with specific systems. In general, hyper- mones on a physiological level, but these effects
thyroidism exaggerates and hypothyroidism min- involve different rates of catabolism and produc-
mizes the changes seen after administration of tion of all three groups of hormones, as well
the agent. Physiologically, the clearest example as interactions in the tissues.
is that of the catecholamines. The relationship
is so striking that some have concluded that the Other features
apparent peripheral effects of thyroxine are There are several clinical features of hyper-
actually effects of epinephrine (Brewster et al., thyroidism and hypothyroidism that are not
1956), but there is evidence against so sweeping readily reduced to manifestations of changed cel-
a claim (see Hoch, 1962b). The biochemical lular energy transfer. These features fall into
basis of the observed interdependences may be two categories. First, there are those that arise
the inactivating effects (or actions?) of thyroid from mechanisms not due directly to the
hormones upon the enzymes that normally in- changed amounts of thyroid hormones in the
activate the catecholamines themselves. The cate- tissues, but to phenomena associated with the
chol-o-methyl-transferase (D'Iorio & Leduc, primary defect in thyroid hormone production.
1960), the amine oxidases (Zile & Lardy, 1959), Thus, exophthalmos is one of the classical signs
and a peroxidase system (Klebanoff, 1959) have in the Merseburg triad in hyperthyroidism but it
been studied, but it is still difficult to assign is not produced by hormone administration
physiologic relevance to the mechanisms. (Means et al., 1963). A pituitary factor, possibly
Another enzymatic system under scrutiny in this associated with thyrotropin, may be responsible
regard involves the formation of cyclic-3',5'- (Loeb & Friedman, 1932). The frequent persist-
AMP, the mediator of many of the catechol- ence of exophthalmos after the therapeutic res-
amine effects. Thyroid hormones have been sug- toration of euthyroidism speaks for such a
gested to control the activation of the lipase in secondary relationship.
adipose tissue via a mechanism involving cyclic- In the second category are those clinical fea-
3',5'-AMP (Fisher & Ball, 1967). Yet other pos- tures that may (and indeed seem to) arise from
sible routes are the control the thyroid hormones changed amounts of tissue thyroid hormone, but
exert over ATP availability, since ATP is the that are not reducible to cellular phenomena be-
only source of cyclic-3',5'-AMP; and the sen- cause we don't know enough yet. The hyper-
sitivity of mitochondria. Adrenochrome and irritability of the nervous system in hyperthyroi-
thyroxine act synergistically on mitochondria in dism, and its opposite in hypothyroidism, may
vitro (Park, Meriwether & Park, 1956). The be presenting symptoms clinically. The involve-
glycogenolytic and hyperglycaemic, the lipolytic, ment of ATP in nerve conduction and in re-
the inotropic, and the calorigenic effects of epine- synthesis of acetylcholine at the myoneural junc-
phrine all depend upon the thyroid state (see tion, and the involvement of K+, Ca++ and Mg++
Ellis, 1956; Brodie et al., 1966; Goodman & in neural events make it likely that hormone-
Bray, 1966). The abnormally slow pulse rate induced defects in energy transfer and ion ac-
after epinephrine administration in hypothyroid cumulation will affect the nervous system, but
patients, and the rapid rate in hyperthyroid sub- just how changes in nerve function relate to
jects, have been used diagnostically, although 'nervousness' presents complex problems not yet
caution is advised in hyperthyroids (Goetsch & conclusively approached. Similarly, the present-
Ritzmann, 1934). ing abnormality of 'myxoedema' seems to be a
The dependence of the calorigenic effect of defect in mucopolysaccharide metabolism that
epinephrine upon the thyroid state is an example leads to excessive deposition, somehow and pre-
of the generality that the thyroid state controls sumably dependent upon insufficient thyroid
the response of the body to calorigenic sub- hormone.
stances. Excessive rise in BMR is seen in hyper- The thyroid hormones obviously control
thyroidism, and little or no rise is seen in hypo- growth, development, and the striking structural
thyroidism, after the administration of glucagon and chemical changes in Anuran metamorphosis.
(Davidson, Salter & Best, 1960), nitrophenols, In general terms we may say these processes
salicylates (Hoch, 1965a, b), chloropromazine must depend upon available 'energy' but our
(unpublished data), and 'febrile toxins' (above). lack of knowledge of the details of the energy-
The only exception seems to be the enhanced dependent steps precludes a mechanistic inter-
sensitivity of hypothyroid subjects to the thyroid pretation at present. In this area, however, the
hormone itself. recent evidence that the thyroid hormones con-
Biochemistry of hyperthyroidism and hypothyroidism 359
trol mitochondrial energy metabolism directly CHANCE, B. (1965) Reaction of oxygen with the respiratory
and promptly, and thereby regulate protein syn- chain in cells and tissues. J. gen. Physiol. 49, 163.
thesis, offers promise of new and important in- CHATAGNER, F. & GAUTHERON, D. (1960) Influence des
hormones thyroidiennes sur la teneur en adenosine tri-
formation on biologic and medical problems. phosphate du foi du rat. Biochim. biophys. Acta, 41, 544.
CHILSON, O.P. & SACKS, J. (1959) Effect of hyperthyroidism
Acknowledgment on distribution of adenosine phosphates and glycogen in
This work was supported by research Grant AM-11184 liver. Proc. Soc. exp. Biol. (N. Y.), 101, 331.
from the National Institutes of Arthritis and Metabolic COGGESHALL, H.C. & GREENE, J.A. (1933) The influence of
Diseases, National Institutes of Health, Bethesda, Maryland, desiccated thyroid gland, thyroxin, and inorganic iodine,
U.S.A. upon the storage of glycogen in the liver of the albino rat
under controlled conditions. Amer, J. Physiol. 105, 103.
CRISPELL, K.R., PARSON, W. & HOLLIFIELD, G. (1956) A
References study of the rate of protein synthesis before and during
ABBOT, A.J. & VAN BUSKIRK, F.W. (1931) The blood sugar the administration of L-triiodothyronine to patients with
response to epinephrin in thyroid-fed animals. Amer. J. myxedema and healthy volunteers using N-15 glycine.
med. Sci. 182 610. J. clin. Invest. 35, 164.
ABELIN, I. & KURSTEINER, P. (1928) Ober den Einflusz der DAVIDSON, I.W.F., SALTER, J.M. & BEST, C.H. (1960) The
Schilddrusensubstanzen auf den Fettstoffwechsel. Biochem. effect of glucagon on the metabolic rate of rats. Amer. J.
Z. 198, 19. clin. Nutr. 8, 540.
ALTHAUSEN, T.L. (1940) The disturbance of carbohydrate DAYTON, S., DAYTON, J., DRIMMER, F. & KENDALL, F.E.
metabolism in thyrotoxicosis. J. Amer. med. Ass. 115, 101. (1960) Rates of acetate turnover and lipid synthesis in
ALWALL, N. (1936) Ober die Wirkung der Dinitrophenole normal, hypothyroid and hyperthyroid rats. Amer. J.
auf die Tierischen Oxydationsprozesse. Skand. Arch. Physiol. 199, 71.
Physiol. Suppl. 72, 1. DEBONS, A.F. & SCHWARTZ, I.L. (1961) Dependence of the
BARGONI, N., GRILLO, M.A., RINAUDO, M.T., FOSSA, T., lipoytic action of epinephrine in vitro upon thyroid
AYASSOT, M. & Bozzi, M.L. (1964) Glycolysis and hormone. J. Lipid Res. 2, 86.
glycogenesis in the liver of hypothyroid rats. Boll. Soc. ital. DEITRICH, R.A. & SMITH, D.L. (1960) Effect of the thyroid
Biol. sper. 40, 1888; (Chem. Abstr., 64, 2506e, 1966). on butyrate oxidation in the rat heart. Biochem. Pharmacol.
BARGONI, N., LUZZATI, A., RINAUDO, M.T., RossINI, L. & 3, 85.
STRUMIA, E. (1961) tber die Leberglykolyse von mit DILLON, R.S. & HOCH, F.L. (1968) Iodine in mitochondria
Schildruse gefutterten Ratten. Hoppe-Seylers Z. physiol. and nuclei. Biochem. Med. (In press).
Chem. 326, 65. D'IORIO, A. & LEDUC, J. (1960) The influence of thyroxine
BARKER, S.B. (1946) Effect of thyroid activity upon metabolic on the o-methylation of catechols. Arch. Biochem. Biophys.
response to dinitro-ortho-cresol. Endocrinology, 39, 234. 87, 224.
BEAUMONT, G.E., DODDS, E.C. & ROBERTSON, J.D. (1940) Dow, D.S. (1967) The isolation from thyrotoxic and diabetic
Calcium and phosphorous metabolism in thyrotoxicosis. rats of skeletal muscle mitochondria showing tight coupl-
J. Endocr. 2, 237. ing, high respiratory indices, and normal adenosine
BERG, H. (1937) Uber den Herzmuskelstoffwechselbei Hyper- triphosphatase activities. Biochemistry, 6, 3350.
thyreose und seine Beeinflussung durch Vitamin C. Arch. Dow, D.S. & ALLEN, C.E. (1961) Steady-state oxidation of
exp. Path. Pharmak. 185, 359. glucose in hyperthyroid and hypothyroid rats. Can. J.
BING, R.J. (1961) Metabolic activity of the intact heart. Biochem. Physiol. 39, 981.
Amer. J. Med. 30, 679. DRILL, V.A. (1943) Interrelations between thyroid function
BOEKELMAN, A.J. (1948) La glande thyroide. Regulatrice du and vitamin metabolism. Physiol. Rev. 23, 355.
potassium. Presse med. 56, 23. DRILL, V.A. & TRUANT, A.P. (1947) Effect of thyroidectomy
BOYD, G.S. (1959) Thyroid function, thyroxine analogs and on conversion of carotene to vitamin A. Endocrinology,
cholesterol metabolism in rats and rabbits. Hormones 40, 259.
and Atherosclerosis (Ed. by G. Pincus), p. 49. Academic DUTOIT, C.H. (1952) The effects of thyroxine on phosphate
Press, New York. metabolism. In: Phosphorus Metabolism (Ed. by W. D.
BREWSTER, W.R., JR, ISAACS, J.P., OSGOOD, P.F. & KING, McElroy and B. Glass), p. 597. The Johns Hopkins Press,
T.L. (1956) The hemodynamic and metabolic interrela- Baltimore.
tionships in the activity of epinephrine, norepinephrine ELLIS, S. (1956) The metabolic effects of epinephrine and
and the thyroid hormones. Circulation, 13, 1. related amines. Pharmacol. Rev. 8, 485.
BRODIE, B.B., DAVIES, J.I., HYNIE, S., KRISHNA, G. &
WEISS, B. (1966) Interrelations of catechol amines with ELRICK, H., HLAD, C.J. & ARM, Y. (1961) Influence of
other endocrine systems. Pharmacol. Rev. 18, 273. thyroid function on carbohydrate metabolism and a new
BROITMAN, S.A., BONDY, D.C., YACHNIN, I., HOSKINS, L.C., method for assessing response to insulin, J. clin. Endocr.
INGBAR, S. & ZAMCHEK, N. (1964) Absorption and dis- 21, 387.
position of D-xylose in thyrotoxicosis and myxedema. ERNSTER, L., IKKOS, D. & LUFT, R. (1959) Enzymic activities
New Engl. J. Med. 270, 333. of human skeletal muscle mitochondria: a tool in clinical
BRONK, J.R. (1963) The nature of the energy requirement for metabolic research. Nature (Lond.), 184, 1851.
amino acid incorporation by isolated mitochondria and ERNSTER, L. & LUFT, R. (1964) Mitochondrial respiratory
its significance for thyroid hormone action. Proe. nat. control: biochemical, physiological, and pathological
Acad. Sci. (Wash.), 50, 524. aspects. Advanc. Metab. Disorders, 1, 95.
BRONK, J.R. (1966) Thyroid hormone: effects on electron FERRINI, O., PERRONI, G.L. & BESTAGNO, M. (1959) Distri-
transport. Science, 153, 638. bution of methionine-S36 in the rat. Hormonal influence
BURN, J.H. & MARKS, H.P. (1925) The relation of the thyroid on the incorporation of amino acids into muscle protein.
gland to the action of insulin. J. Physiol. (Lond.), 60, 131. Minerva nucleare, 3, 210; (Chem. Abstr. 55, 18922G, 1961).
BURTON, S.D., ROBBINS, E.D. & BYERS, S.O. (1958) Utiliza- FISHER, J.N. & BALL, E.G. (1967) Studies on the metabolism
tion of glucose by hyperthyroid isolated rat liver. Proc. of adipose tissue. XX. The effect of thyroid-status upon
Soc. exp. Biol. (N. Y.), 92, 272. oxygen consumption and lipolysis. Biochemistry, 6, 637.
B
360 Frederic L. Hoch
FLACH, F.F., CELIAN, C.I., STOKES, P.E. & RAWSON, R.W. HOCH, F.L. (1968c) Thyroid hormone action on mitochon-
(1959) The influence of thyroid hormones on metabolism dria. IX. Effects of dinitrophenol. Arch. Biochem. Biophys.
in psychiatric disorders. I. The effect of 3:5:3'-triiodo- (In press).
thyronine on calcium and phosphorus metabolism in HOCH, F.L. & LIPMANN, F. (1954) The uncoupling of respira-
psychiatric patients. J. clin. Endocr. 19, 455. tion and phosphorylation by thyroid hormones. Proc. nat.
FLETCHER, K. & MYANT, N.B. (1960) Effects of thyroxine Acad. Sci. (Wash.), 40, 909.
on the synthesis of cholesterol and fatty acids by cell-free HOCH, F.L. & MOTTA, M.V. (1968) Reversal of early thyroid
fractions of rat liver. J. Physiol. (Lond.), 154, 145. hormone action on mitochondria by bovine serum
FLETCHER, K. & MYANT, N.B. (1962) Effect of thyroxine on albumin in vitro. Proc. nat. Acad. Sci. (Wash.), 59, 118.
the synthesis of lipids in rat liver. Endocrinology, 71, 870. HUEBER, E.F. v. (1939) 1Jber die Beeinflussung von Hyper-
FRAENKEL-CONRAT, J. & GREENBERG, D.M. (1946) Acetyla- thyreosen durch Magnesiumglutaminat. Wien. klin.
tion of sulfanilamide as influenced by the thyroid. Proc. Wschr. 52, 932.
Soc. exp. Biol. (N. Y.), 63, 537. JEANRENAUD, B. (1961) Dynamic aspects of adipose tissue
FRIEDBERG, F. & GREENBERG, D.M. (1947) Endocrine metabolism: a review. Metabolism, 10, 535.
regulation of amino acid levels in blood and tissues. JOHNSON, R.M. & BAUMANN, C.A. (1947) The effect of
J. biol. Chem. 168, 405. thyroid on the conversion of carotene into vitamin A.
GANJU, S.N. & LOCKETT, M.F. (1958) The action of thyroid J. biol. Chem. 171, 513.
hormones on the oxygen consumption and resistance to JOHNSON, P.C., PosEY, A.F., PATRICK, D.R. & CAPUTTO, R.
cold of adrenalectomized and thyroidectomized mice. (1958) Incorporation of P,, in the muscle by normal and
J. Endocr. 16, 396. thyrotoxic resting rats. Amer. J. Physiol. 192, 279.
GERSHBERG, H. & KUHL, W.J., JR. (1950) Acetylation studies JONES, J.E., DESPER, P.C., SHANE, S.R. & FLINK, E.B. (1966)
in human subjects with metabolic disorders. J. clin. Invest. Magnesium metabolism in hyperthyroidism and hypo-
29, 1625. thyroidism. J. clin. Invest. 45, 891.
GERSHOFF, S.N., VITALE, J.J., ANTONOWICZ, I., NAKAMURA, KADENBACH, B. (1966) The influence in vivo of thyroid
M. & HELLERSTEIN, E.E. (1958) Studies of interrelation- hormones on oxidative phosphorylation and enzymic
ships of thyroxine, magnesium and vitamin B1,. J. biol. activities in mitochondria. Biochem. Z. 344, 49.
Chem. 231, 849. KARP, A. & STETTEN, D., JR. (1949) The effect of thyroid
GLOCK, G.E., MCLEAN, P. & WHITEHEAD, J.J. (1956) Path- activity on certain anabolic processes studied with the aid
ways of glucose catabolism in rat liver in alloxan diabetes of deuterium. J. biol. Chem. 179, 819.
and hyperthyroidism. Biochem. J. 63, 520.
GOETSCH, E. & RITZmANN, A.J., JR (1934) Thyroid disorders. KASBEKAR, D.K., LAVATE, W.V., REGE, D.V. & SREENIVASAN,
VI. The suprarenal factor in reactions to thyroidectomy. A. (1959) A study of vitamin B,, protection in experi-
Arch. Surg. 29, 492. mental thyrotoxicosis in the rat. Biochem. 1. 72, 374.
GOODMAN, H.M. & BRAY, G.A. (1966) Role of thyroid KENDALL, E.C. (1929) Thyroxine. The Chemical Catalogue
hormones in lipolysis. Amer. J. Physiol. 210, 1053. Co., New York.
GOODMAN, H.M. & KNOBIL, E. (1959) Mobilization of fatty KLEBANOFF, S.J. (1959) An effect of thyroxine and related
acids by epinephrine in normal and hypophysectomized compounds on the oxidation of certain hydrogen donors
Rhesus monkeys. Proc. Soc. exp. Biol. (N. Y.), 100, 195. by the peroxidase system. J. biol. Chem. 234, 2437.
HAGEN, J.H. (1960) Effect of insulin on the metabolism of adi- KLINGENBERG, M. (1963) Morphological and functional
pose tissue from hyperthyroid rats. J. biol. Chem. 235,2600. aspects of pyridine nucleotide reactions in mitochondria.
HANDLER, P. (1948) The influence of thyroid activity on the In: Energy-linked Functions of Mitochondria (Ed. by
liver and plasma lipides of choline- and cystine-deficient B. Chance), p. 121. Academic Press, New York.
rats. J. biol. Chem. 173, 295. KRANE, S.M., BROWNELL, G.L., STANBURY, J.B. & CORRIGAN,
HARARY, I. (1958) The effect in vivo of thyroxine on acyl H. (1956) The effect of thyroid disease on calcium meta-
phosphatase of rat liver and muscle. Biochim. biophys. Acta, bolism in man. J. clin. Invest. 35, 874.
29, 647. KRITCHEVSKY, D. (1960) Influence of thyroid hormones and
HED, R., KIRSTEIN, L. & LUNDMARK, C. (1958) Thyrotoxic related compounds on cholesterol biosynthesis and
myopathy. J. Neurol. Neurosurg. Psychiat. 21, 270. degradation: a review. Metabolism, 9, 984.
HERNBERG, C.A. (1960) Bone phosphorous metabolism in LABOUESSE, J., CHATAGNER, F. & JOLLES-BERGERET, B.
vitro in thyrotoxicosis. Acta endocr. (Kbh.), 33, 577. (1960) Dosage du phosphate de pyridoxal dans le foie du
HOCH, F.L. (1962a) Thyrotoxicosis as a disease of mito- rat normal, du rat thyrotoxique et du rat thyroidectemis6.
chondria. New Engl. J. Med. 266, 446 and 498. Biochim. biophys. Acta, 39, 372.
HOCH, F.L. (1962b) Biochemical actions of thyroid hormones. LAMBERG, B.A. (1965) Glucose metabolism in thyroid
Physiol. Rev. 42, 605. disease. Acta med. scand. 178, 351.
HOCH, F.L. (1965a) Synergism between calorigenic effects: LEHN1NGER, A.L. (1964) The Mitochondrion, p. 263. Benjamin,
L-thyroxine and 2,4-dinitrophenol or sodium salicylate in New York.
euthyroid rats. Endocrinology, 76, 335.
HOCH, F.L. (1965b) L-Thyroxine in subcalorigenic doses: LENGEMANN, F.W., WASSERMAN, R.H. & COMAR, C.L.
rapid potentiation of dinitrophenol-induced calorigenesis (1960) The effect of growth and thyroid hormones upon
in hypothyroid rats. Endocrinology, 77, 991. the removal by lavage of calcium and strontium from the
HOCH, F.L. (1966) Rapid effects of a subcalorigenic dose of skeleton of the rat. Endocrinology, 67, 535.
L-thyroxine on mitochondria. J. biol. Chem. 241, 524. LESLIE, I. & SINCLAIR, R. (1959) The action of thyroxine
HOCH, F.L. (1967) Early action of injected L-thyroxine on and triiodothyronine on human cells growing in tissue
mitochondrial oxidative phosphorylation. Proc. nat. Acad. culture. Exp. Cell Res. 17, 272.
Sci. (Wash.), 58, 506. LIPSKY, S.R., BONDY, P.K., MAN, E.B. & McGUIRE, J.S., JR
HOCH, F.L. (1968a) Biochemical action of thyroid hormone. (1955) The effects of triiodothyronine on the biosynthesis
In: Proc. 3rd Midwest Conference on Thyroid, 1967. of plasma lipids from acetate-l-C14 in myxedematous
University of Missouri Press, Columbia. subjects. J. clin. Invest. 34, 950.
HOCH, F.L. (1968b) Thyroid hormone action on mitochon- LOEB, L. & FRIEDMAN, H. (1932) Exophthalmos produced by
dria. I. Respiration, effects of inhibitors of respiration. injections of acid extract of anterior pituitary gland of
Arch. Biochem. Biophys. (In press). cattle. Proc. Soc. exp. Biol. (N. Y.), 29, 648.
Biochemistry of hyperthyroidism and hypothyroidism 361
LooMis, W.F. & LIPMANN, F. (1948) Reversible inhibition ROBERTS, K.E., FIRMAT, G., PRUNIER, J., SCHWARTZ, M.U.
of the coupling between phosphorylation and oxidation. & RAWSON, R.W. (1956) Effect of phosphate in enhancing
J. biol. Chem. 173, 807. action of triiodothyronine. Endocrinology, 59, 565.
MACHO, L. (1958) The influence of endocrine glands on ROODYN, D.B., FREEMAN, K.B. & TATA, J.R. (1965) The
carbohydrate metabolism. II. The glucose tolerance and stimulation by treatment in vivo with triiodothyronine of
clearance of glucose in healthy subjects and in patients amino acid incorporation into protein by isolated rat-liver
with hypo- and hyperthyroidism. Acta med. scand. 160,485. mitochondria. Biochem. J. 94, 628.
MACHO, L. (1961) Effect of thyroxine and 2,4-dinitrophenol ROSENMAN, R.H., BYERS, S.O. & FRIEDMAN, M. (1952) The
on the rate of utilization of glucose. Nature (Lond.), 191, mechanism responsible for the altered blood cholesterol
604. content in deranged thyroid states. J. clin. Endocr. 12, 1287.
MAGNUs-LEVY, A. (1895) Ober den respiratorischen Gas- Rupp, J., PASCHKIS, K.G. & CANTAROW, A. (1949) Influence
wechsel unter dem Einfluss der Thyroidea sowie unter of thyroxine on protein metabolism. Endocrinology, 44,
verschiedenen pathologischen Zustlinden. Berl. klin. 449.
Wschr. 32, 650. SCAIFE, J.F. & MIGICOVSKY, B.B. (1957) Effect of alloxan,
MALEY, G.F. (1957) Comparison of some enzyme systems insulin, and thyroxine on cholesterol and fatty acid
in normal and thyrotoxic rat livers. Amer. J. Physiol. syntheses by rat liver homogenates. Can. J. Biochem.
188, 35. 35, 15.
MALEY, G.F. & LARDY, H.A. (1953) Metabolic effects of SCHWARTZ, I.L. & DEBONS, A.F. (1959) Action of thyroid
thyroid hormones in vitro. II. Influence of thyroxine and hormone on the release of fatty acids from tissue stores.
triiodothyronine on oxidative phosphorylation. J. biol. Physiologist, 2, 104.
Chem. 204, 435. SELENKOW, H.A. & MARCUS, F.I. (1960) Masked hyper-
MALEY, G.F. & LARDY, H.A. (1955) Efficiency of phosphor- thyroidism and heart disease. Med. Clin. N. Amer. 44, 1305.
ylation in selected oxidations by mitochondria from normal SHORR, E., RICHARDSON, H.B. & WOLFF, H.G. (1933) Endo-
and thyrotoxic rat livers. J. biol. Chem. 215, 377. genous glycine formation in myopathies and Graves'
MARTIUS, C. & HEss, B. (1952) Ober den Wirkingsmechanis- disease. Proc. Soc. exp. Biol. (N. Y.), 31, 207.
mus der Schilddrusenhormons. Arch. exp. Path. Pharmak. SOKOLOFF, L., FRANCIS, C.M. & CAMPBELL, P.L. (1964)
216, 45. Thyroxine stimulation of amino acid incorporation into
MEANS, J.H., DEGROOT, L.J. & STANBURY, J.B. (1963) The protein indenendent of any action on messenger RNA
Thyroid and its Diseases, 3rd edn. McGraw-Hill, New York. synthesis. Proc. nat. Acad. Sci. (Wash.), 52, 728.
MILLIKAN, C.H. & HAINES, S.F. (1957) The thyroid gland in
relation to neuromuscular disease. Arch. intern. Med. 92, 5. SOKOLOFF, L. & KAUFMAN, S. (1959) Effects of thyroxine on
MIRSKY, I.A. & BROH-KAHN, R.H. (1936) The effect of amino acid incorporation into protein. Science, 129, 569.
experimental hyperthyroidism on carbohydrate meta- SOKOLOFF, L. & KAUFMAN, S. (1961) Thyroxine stimulation
bolism. Amer. J. Physiol. 117, 6. of amino acid incorporation into protein. J. biol. Chem.
MUNRO, D.S., RENSCHLER, H. & WILSON, G.M. (1958) 236, 795.
Exchangeable potassium and sodium in hyperthroidism SOKOLOFF, L., KAUFMAN, S., CAMPBELL, P.L., FRANCIS, C.M.
and hypothyroidism. Metabolism, 7, 124. & GELBOIN, H.V. (1963) Thyroxine stimulation of amino
NIKKILA, E.A. & PITKANEN, E. (1959) Liver enzyme pattern acid incorporation into protein. Localization of stimulated
in thyrotoxicosis. Acta endocr. (Kbh.), 31, 573. step. J. biol. Chem. 238, 1432.
NISHIKAWARA, M.T. & GABRIELSON, E. (1961) Hexokinase STERNHEIMER, R. (1939) The effect of a single injection of
and phosphatase activities of the intestinal mucosa in thyroxine on carbohydrates, protein and growth in the
hypophysectomized and thyroid-treated hypophysecto- rat liver. Endocrinology, 25, 899.
mized rats. Endocrinology, 68, 855. STOCKER, W.W., SAMAHA, F.J. & DEGROOT, L.J. (1966)
OKUDA, K. & CHOW, B.F. (1961) The thyroid and absorption Coupled oxidative phosphorylation in muscle of thyro-
of vitamin B,, in rats. Endocrinology, 68, 607. toxic patients. Program of the 23rd Annual Meeting,
PARK, J.H., MERIWETHER, B.P. & PARK, C.R. (1956) Effects Amer. Fed. Clin. Res., May 1966. J. clin. Invest. 45, viii.
of adrenochrome on oxidative phosphorylation in liver TABACHNICK, I.I.A. & BONNYCASTLE, D.D. (1954) The effect
mitochondria. Fed. Proc. 15, 141. of thyroxine on the coenzyme A content of some tissues.
PETER, J.B. & LEE, L.D. (1967) Characteristics of skeletal J. biol. Chem. 207, 757.
muscle mitochondria isolated by a new improved technique. TAPLEY, D.F. (1955) Magnesium balance in myxedematous
Biochem. biophys. Res. Commun. 29, 430. patients treated with triiodothyronine: preliminary note.
PETERS, R.A. & ROSSITER, R.J. (1939) Thyroid and vitamin Bull. Johns Hopk. Hosp. 96, 274.
B,. Biochem. J. 33, 1140. TAPLEY, D.F. & COOPER, C. (1956) Effect of thyroxine on
PLUMMER, H.S. & BOOTHBY, W.M. (1923) The cost of work the swelling of mitochondria isolated from various
in exophthalmic goiter. Amer. J. Physiol. 63, 406. tissues of the rat. Nature (Lond.), 178, 1119.
PORTUGAL'SKAYA, E.A. (1961) Effect of the thyroid hormone TATA, J.R. (1963) Inhibition of the biological action of
on conversion of carotene to vitamin A in animal body. thyroid hormones by actinomycin D and puromycin.
Vitaminy, Akad. Nauk. UKr. S.S.R, 4, 78 (Chem Abstr. Nature (Lond.), 197, 1167.
55, 737g, 1961). TATA, J.R. (1967) The formation and distribution of ribo-
RACKER, E. (1965) Mechanisms in Bioenergetics. Academic somes during hormone-induced growth and development.
Press, New York. Biochem. J. 104, 1.
RAWSON, R.W., RALL, J.E. & SONENBERG, M. (1955) The TATA, J.R., ERNSTER, L., LINDBERG, E., ARRHENIUS, E.,
chemistry and physiology of the thyroid. The Hormones, PEDERSEN, S. & HEDMAN, R. (1963) Action of thyroid
(Ed. by G. Pincus and K. V. Thimann), vol. 3, p. 433. hormones at the cell level. Biochem. J. 86, 408.
Academic Press, New York. TATA, J.R. & WIDNELL, C.C. (1966) Ribonucleic acid
RICH, C., BIERMAN, E.L. & SCHWARTZ, I.L. (1959) Plasma synthesis during the early action of thyroid hormones.
nonesterified fatty acids in hyperthyroid states. J. clin. Biochem. J. 98, 604.
Invest. 38, 275. THORN, G.W. (1936) Creatine studies in thyroid disorders.
RIVLIN, R.S. & LANGDON, R.G. (1966) Regulation of hepatic Endocrinology, 20, 628.
flavine adenine dinucleotide levels by thyroid hormone. THORN, G.W. & EDER, H.A. (1946) Studies on chronic
Advance Enzyme Regulation, 4, 45. thyrotoxic myopathy. Amer. J. Med. 1, 583.
362 Frederic L. Hoch
WAINFAN, E. & MARX, W. (1955) Effects of thyroxine and WILKINs, L. & FLEISCHMANN, W. (1946) Effects of thyroid on
some related compounds on bacterial oxidations. J. biol. creatine metabolism with a discussion of the mechanism
Chem. 214, 441. of storage and excretion of creatine bodies. J. clint. Invest.
WANG, E. (1946) Creatine metabolism and endocrine regula- 25, 360.
tion. Acta med. scand. Suppl. 169, 1. WILLIAMS, R.H., EGANA, E., ROBINSON, P., AsPER, S.P. &
WAYNE, E.J. (1960) Clinical and metabolic studies in thyroid DuToIT, C. (1943) Alterations in biologic oxidation in
disease. Brit. med. J. i, 1; 78. thyrotoxicosis. I. Thiamine metabolism. Arch intern. Med.
WEISS, W.P. & SOKOLOFF, L. (1963) Reversal of thyroxine- 72, 353.
induced hypermetabolism by puromycin. Science, 140,1324. WISWELL, J.G. (1961) Some effects of magnesium loading in
WERTHEIMER, E. & BENTOR, V. (1953) Metabolic changes patients with thyroid disorders. J. clin. Endocr. 21, 31.
in the rat diaphragm during heat regulation as a thyroxine WOHL, M.G. & FELDMAN, J.B. (1939) Vitamin A deficiency
effect. Metabolism, 2, 536. in disease of the thyroid gland: Its detection by dark
WHITE, J.E. & ENGEL, F.L. (1958) Lipolytic action of adaptation. Endocrinology, 24, 389.
corticotropin on rat adipose tissue in vitro. J. clin. Invest. WOHL, M.G., LEVY, H.A., SZUTrKA, A. & MALDIA, G.
37, 1556. (1960) Pyridoxine deficiency in hyperthyroidism. Proc.
WIDNELL, C.C. & TATA, J.R. (1963) Stimulation of nuclear Soc. exp. Biol. (N. Y.), 105, 523.
RNA polymerase during the latent period of action of ZILE, M. & LARDY, H.A. (1959) Monoamine oxidase activity
thyroid hormones. Biochim. biophys. Acta, 72, 506. in liver of thyroid-fed rats. Arch. Biochem. Biophys. 82, 411.
S-ar putea să vă placă și
- Tura KuDocument104 paginiTura KuFitzgerald BillyÎncă nu există evaluări
- A Quantitative Model of The Human Thyroid Development and ObservationsDocument9 paginiA Quantitative Model of The Human Thyroid Development and ObservationsSandu AlexandraÎncă nu există evaluări
- Neurochemical Aspects of Hypothalamic FunctionDe la EverandNeurochemical Aspects of Hypothalamic FunctionL MartiniÎncă nu există evaluări
- Rhythms in The Endocrine System of Fish: A Review: ArticleDocument34 paginiRhythms in The Endocrine System of Fish: A Review: ArticleBagas Lantip PrakasaÎncă nu există evaluări
- Accorroni Et AlDocument17 paginiAccorroni Et Alp.sepulveda.fiÎncă nu există evaluări
- Overview of Endo Physiology - HandoutDocument19 paginiOverview of Endo Physiology - HandoutVDiesel99Încă nu există evaluări
- Omcl0204 - 0181 - Synthesis of MescalineDocument10 paginiOmcl0204 - 0181 - Synthesis of MescalineOleksandr StorcheusÎncă nu există evaluări
- Role of Thyroid Hormone in Regulation of PDFDocument8 paginiRole of Thyroid Hormone in Regulation of PDFJose Leonel Fajardo RapaloÎncă nu există evaluări
- Review Article: Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in HumansDocument14 paginiReview Article: Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in HumansPreeti YadavÎncă nu există evaluări
- Pharmacology of AutacoidsDocument13 paginiPharmacology of AutacoidsInocenteÎncă nu există evaluări
- Recent Progress in Hormone Research: Proceedings of the 1984 Laurentian Hormone ConferenceDe la EverandRecent Progress in Hormone Research: Proceedings of the 1984 Laurentian Hormone ConferenceEvaluare: 5 din 5 stele5/5 (1)
- The Thyroid and Parathyroid GlandsDocument47 paginiThe Thyroid and Parathyroid GlandsRujha Haniena Ahmad RidzuanÎncă nu există evaluări
- Artículo de InvestigaciónDocument18 paginiArtículo de InvestigacióngabrielaÎncă nu există evaluări
- Biochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967De la EverandBiochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967D. RichterÎncă nu există evaluări
- Tryptophan Metabolism and Gut-Brain HomeostasisDocument23 paginiTryptophan Metabolism and Gut-Brain HomeostasisLuísa OliveiraÎncă nu există evaluări
- The Thyroid Gland: Physiology and Pathophysiology: Endocrine Series #6Document16 paginiThe Thyroid Gland: Physiology and Pathophysiology: Endocrine Series #6نذير الشرعبيÎncă nu există evaluări
- Subjects-Authors' Response High Fluid Intake Increases Urine Free Cortisol Excretion in NormalDocument5 paginiSubjects-Authors' Response High Fluid Intake Increases Urine Free Cortisol Excretion in Normallala_bojaÎncă nu există evaluări
- Nihms 165350Document15 paginiNihms 165350Il InÎncă nu există evaluări
- Gonadotropic HormonesDe la EverandGonadotropic HormonesChoh Hao LiÎncă nu există evaluări
- Enr Saqs 2Document5 paginiEnr Saqs 2Mahnoor KhanÎncă nu există evaluări
- J Physiol-1999-Duchen-1-17Document17 paginiJ Physiol-1999-Duchen-1-17nicolasÎncă nu există evaluări
- Emerging Issues in Receptor Protein Tyrosine Phosphatase Function: Lifting Fog or Simply Shifting?Document11 paginiEmerging Issues in Receptor Protein Tyrosine Phosphatase Function: Lifting Fog or Simply Shifting?LUCAS GONÇALVES DE OLIVEIRAÎncă nu există evaluări
- Editorial: Hormonal and Neuroendocrine Regulation of Energy BalanceDocument2 paginiEditorial: Hormonal and Neuroendocrine Regulation of Energy BalanceO KiÎncă nu există evaluări
- Endocrinology SupplementDocument85 paginiEndocrinology Supplementfreecouch100% (1)
- DocumentDocument45 paginiDocumentPrajwal PatilÎncă nu există evaluări
- Thyroid Gland and Its Rule in Human BodyDocument9 paginiThyroid Gland and Its Rule in Human BodyDrFarah Emad AliÎncă nu există evaluări
- Reseptor Hormon Tiroid: Mekanisme Molekuler Tiroid Dan ReseptornyaDocument8 paginiReseptor Hormon Tiroid: Mekanisme Molekuler Tiroid Dan ReseptornyaDian RahmawatiÎncă nu există evaluări
- Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in MammalsDocument40 paginiGenomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in MammalsMarco GiammancoÎncă nu există evaluări
- Chapter 29 - Thyroid Function and Thyroid DrugsDocument21 paginiChapter 29 - Thyroid Function and Thyroid DrugsCapuÎncă nu există evaluări
- Pharmacology of The Endocrine System PDFDocument74 paginiPharmacology of The Endocrine System PDFGlenn Perez71% (7)
- Bs Acc 2019 08 005Document54 paginiBs Acc 2019 08 005Angieda SoepartoÎncă nu există evaluări
- Hormones Syllabus1Document3 paginiHormones Syllabus1Ashmita KumarÎncă nu există evaluări
- Contributions of Mitochondria To Animal PhysiologyDocument17 paginiContributions of Mitochondria To Animal Physiologynorociel8132Încă nu există evaluări
- 1 LipidperoxidationDocument8 pagini1 LipidperoxidationAgharid Ali HusseinÎncă nu există evaluări
- Pharmacology Chapter 34Document5 paginiPharmacology Chapter 34languha NgatiÎncă nu există evaluări
- Eje 257Document15 paginiEje 257Sam SoeteÎncă nu există evaluări
- ERD Biochemistry Lecture 1Document15 paginiERD Biochemistry Lecture 1محمد عليÎncă nu există evaluări
- Pharmacology of The Endocrine SystemDocument74 paginiPharmacology of The Endocrine SystemarpanabiswassshetyeÎncă nu există evaluări
- Oxidative Stress TheoryDocument3 paginiOxidative Stress TheoryKayla GaigherÎncă nu există evaluări
- 10 Diversity of Endocrine System IIDocument23 pagini10 Diversity of Endocrine System IIcutethoybaÎncă nu există evaluări
- Lab 6td5tbyyvtc1920 RevDocument15 paginiLab 6td5tbyyvtc1920 RevrinidinanÎncă nu există evaluări
- Nikhil BTP Mid Term ReportDocument17 paginiNikhil BTP Mid Term ReportNikhil SaiyamÎncă nu există evaluări
- Ijerph 18 09434Document23 paginiIjerph 18 09434YHOISS SMIHT MUNOZ CERONÎncă nu există evaluări
- Regulation of The Mitochondrial Tricarboxylic Acid CycleDocument9 paginiRegulation of The Mitochondrial Tricarboxylic Acid CycleMaximiliano Tapia VazquezÎncă nu există evaluări
- Zinc and ThymulinDocument2 paginiZinc and ThymulinScottÎncă nu există evaluări
- Thyroid and Anti Thyroid DrugDocument5 paginiThyroid and Anti Thyroid Drugtamberahul1256Încă nu există evaluări
- bk978 0 7503 1302 5ch1Document30 paginibk978 0 7503 1302 5ch1Khushi BhushanÎncă nu există evaluări
- tmp9271 TMPDocument8 paginitmp9271 TMPFrontiersÎncă nu există evaluări
- Experimental Dermatology - 2008 - Schallreuter - Regulation of Melanogenesis Controversies and New ConceptsDocument10 paginiExperimental Dermatology - 2008 - Schallreuter - Regulation of Melanogenesis Controversies and New ConceptsАнударь ИчинхорлооÎncă nu există evaluări
- 1 s2.0 S0021925818824115 MainDocument11 pagini1 s2.0 S0021925818824115 Mainshravan trialÎncă nu există evaluări
- CH 45 Guided ReadingDocument7 paginiCH 45 Guided ReadingmharirajÎncă nu există evaluări
- Scholar 152Document12 paginiScholar 152Ram MaheshÎncă nu există evaluări
- Inhalation AnnnnnDocument54 paginiInhalation AnnnnnBenvenuto AxelÎncă nu există evaluări
- 1980 Marden InvolvementDocument8 pagini1980 Marden Involvementspanners 12Încă nu există evaluări
- Pyrethroids: Mammalian Metabolism and Toxicity: Hideo KanekoDocument6 paginiPyrethroids: Mammalian Metabolism and Toxicity: Hideo KanekoNacho BressánÎncă nu există evaluări
- The Hypothalamus and Pituitary GlandDocument44 paginiThe Hypothalamus and Pituitary GlandRujha Haniena Ahmad RidzuanÎncă nu există evaluări
- Neuro Nutrition 1983 v12n01 p038Document6 paginiNeuro Nutrition 1983 v12n01 p038LieMinkÎncă nu există evaluări
- Melatonin and Its Metabolites: New Findings Regarding Their Production and Their Radical Scavenging ActionsDocument9 paginiMelatonin and Its Metabolites: New Findings Regarding Their Production and Their Radical Scavenging ActionsPandi PerumalÎncă nu există evaluări
- Regulação Da MelanogeneseDocument10 paginiRegulação Da MelanogeneseMilena CristinaÎncă nu există evaluări
- Nucleus - Morphology and FunctionsDocument25 paginiNucleus - Morphology and FunctionsVytheeshwaran Vedagiri96% (24)
- Aberrant Upregulation of The Glycolytic Enzyme PFKFB3 in CLN7 Neuronal Ceroid LipofuscinosisDocument14 paginiAberrant Upregulation of The Glycolytic Enzyme PFKFB3 in CLN7 Neuronal Ceroid Lipofuscinosisezequiel.grondona.cbaÎncă nu există evaluări
- Official Bank-MusclesDocument27 paginiOfficial Bank-MusclesEbtihal AlharthiÎncă nu există evaluări
- 2.3 Living Processes in Multicellular OrganismsDocument84 pagini2.3 Living Processes in Multicellular Organismswickedbiology101Încă nu există evaluări
- POGIL Cell Size-KEYDocument5 paginiPOGIL Cell Size-KEYKali WarnkeÎncă nu există evaluări
- Birla Institute of Technology and Science Pilani K.K. Birla Goa CampusDocument3 paginiBirla Institute of Technology and Science Pilani K.K. Birla Goa CampusHarshit GargÎncă nu există evaluări
- Cell Division: Mitosis and MeiosisDocument38 paginiCell Division: Mitosis and MeiosisMaika Ysabelle RavaloÎncă nu există evaluări
- Apoptosis-Oncogenes-Tumor Suppressors & Cancer - ICS PresentationDocument30 paginiApoptosis-Oncogenes-Tumor Suppressors & Cancer - ICS PresentationRayÎncă nu există evaluări
- Risk of Opportunistic Infections Associated With Long Term GlucocorticoidDocument20 paginiRisk of Opportunistic Infections Associated With Long Term GlucocorticoidRafael CamachoÎncă nu există evaluări
- Chapter Review Clinical Immunology and SerologyDocument68 paginiChapter Review Clinical Immunology and Serologyterah debuyanÎncă nu există evaluări
- TranslationDocument19 paginiTranslationSei KoÎncă nu există evaluări
- Human Chromosomes and GenesDocument2 paginiHuman Chromosomes and GeneshomamunfatÎncă nu există evaluări
- Anti-Inflammatory Effects of Millet Bran Derived-Bound Polyphenols in LPS-induced HT-29 Cell Signaling PathwayDocument13 paginiAnti-Inflammatory Effects of Millet Bran Derived-Bound Polyphenols in LPS-induced HT-29 Cell Signaling PathwayKito TongHuiÎncă nu există evaluări
- Cell Organelles WorksheetDocument8 paginiCell Organelles WorksheetJohn OsborneÎncă nu există evaluări
- Amino Acids and ProteinDocument32 paginiAmino Acids and ProteinArchishmaan UdgataÎncă nu există evaluări
- The BRAF Inhibitor Vemurafenib Activates MitochondDocument27 paginiThe BRAF Inhibitor Vemurafenib Activates MitochondEstefania VelascoÎncă nu există evaluări
- "Central Giant Cell Granuloma" - An Update: Invited ReviewDocument3 pagini"Central Giant Cell Granuloma" - An Update: Invited ReviewPrapu RamÎncă nu există evaluări
- Cellular ReproductionDocument10 paginiCellular ReproductionCharles JÎncă nu există evaluări
- Mitosis in Onion Root Tip: Biology AssessmentDocument5 paginiMitosis in Onion Root Tip: Biology AssessmentRaashed RamzanÎncă nu există evaluări
- Dna Replication and Protein Synthesis Lesson PlanDocument10 paginiDna Replication and Protein Synthesis Lesson Planapi-356227663100% (2)
- Melaka BoosterDocument12 paginiMelaka BoosterHaifa amirahÎncă nu există evaluări
- Botany Practice Test I - Answer KeyDocument4 paginiBotany Practice Test I - Answer KeyIrene Miranda FloresÎncă nu există evaluări
- Chymosin ReninDocument1 paginăChymosin ReninArfanizam Bin ArisÎncă nu există evaluări
- Biology AssignmentDocument12 paginiBiology Assignmentsanyamjuyal4Încă nu există evaluări
- MSC Biotechnology Syllabus FINALDocument53 paginiMSC Biotechnology Syllabus FINALdeepakÎncă nu există evaluări
- Cell Disruption Extracting Target Product: Location of Biological ProductsDocument88 paginiCell Disruption Extracting Target Product: Location of Biological ProductsRoshan jaiswalÎncă nu există evaluări
- Lab 5Document13 paginiLab 5Chikaodinaka Okoro0% (4)
- Bio Practice TestDocument27 paginiBio Practice TestMichelleÎncă nu există evaluări
- Artepillin C As An Outstanding Phenolic Compound of Brazilian Green Propolis For Disease Treatment: A Review On Pharmacological AspectsDocument13 paginiArtepillin C As An Outstanding Phenolic Compound of Brazilian Green Propolis For Disease Treatment: A Review On Pharmacological AspectslyviaÎncă nu există evaluări
- Proteoglycans and Glycoproteins: DR - Nuruddin Mohammed NurDocument39 paginiProteoglycans and Glycoproteins: DR - Nuruddin Mohammed NurIffah IrdinaÎncă nu există evaluări