Documente Academic
Documente Profesional
Documente Cultură
Diffusion
Încărcat de
Robert willaimsDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Diffusion
Încărcat de
Robert willaimsDrepturi de autor:
Formate disponibile
What Is the Difference Between Osmosis and Diffusion?
Search the site
Search
GO
Science, Tech, Math Science
What Is the Difference Between Osmosis and Diffusion?
Share
You can use gummy candy to demonstrate how osmosis works.
You can use gummy candy to demonstrate how osmosis works. Water travels from an
area of high water density through the gelatin to an area of low water density,
swelling the candy. Martin Leigh, Getty Images
by Anne Marie Helmenstine, Ph.D.
Updated March 06, 2017
Question: What is the difference between osmosis and diffusion? Compare and
contrast the two forms of transport.
Answer: Both diffusion and osmosis are passive transport processes, which means
they do not require any input of extra energy to occur. In both diffusion and
osmosis, particles move from an area of higher concentration to one of lower
concentration.
Diffusion can occur in any mixture, including one which includes a semipermeable
membrane, while osmosis always occurs across a semipermeable membrane.
One big difference between osmosis and diffusion is that both solvent and solute
particles are free to move in diffusion, but when we talk about osmosis, only the
solvent molecules (water molecules) cross the membrane. This can be confusing to
understand because while the solvent particles are moving from higher to lower
solvent concentration across the membrane, they are moving from lower to higher
solute concentration (from a more dilute solution to a region of more concentrated
solution). This occurs naturally because the system seeks balance or equilibrium.
If the solute particles can't cross a barrier, the only way to equalize
concentration on both sides of the membrane is for the solvent particles to move
in. You can consider osmosis to be a special case of diffusion in which diffusion
occurs across a semipermeable membrane and only the water or other solvent moves.
When people discuss osmosis in biology, it always refers to the movement of water.
In chemistry, it's possible for other solvents to be involved.
Examples of Diffusion: perfume filling a whole room, a drop of food coloring
spreading out to uniformly color a cup of water, movement of small molecules across
a cell membrane
Examples of Osmosis: red blood cells swelling up when exposed to fresh water, plant
root hairs uptake water via osmosis
DIFFUSION VERSUS OSMOSIS
Any type of substance moves from area of highest energy or concentration to region
of lowest energy or concentration.Only water or another solvent moves from a region
of high energy or concentration to a region of lower energy or
concentration.Diffusion can occur in any medium, whether it is liquid, solid, or
gas.Osmosis only occurs in a liquid medium.Diffusion does not require a
semipermeable membrane.Osmosis requires a semipermeable membrane.Concentration of
the diffusion substance equalizes to fill the available space.Concentration of
solvent does not become equal on both sides of the membrane.Hydrostatic pressure
and turgor pressure to not normally apply to diffusion.Hydrostatic pressure and
turgor pressure oppose osmosis.Does not depend on solute potential, pressure
potential, or water potential.Depends on solute potential.Diffusion mainly depends
on the presence of other particles.Osmosis mainly depends on the number of solute
particles dissolved in the solvent.Diffusion is a passive process.Osmosis is also a
passive process.The movement in diffusion is to equalize concentration (energy)
throughout the system.The movement in osmosis seeks to equalize solvent
concentration (although it does not achieve this).
See Examples of Diffusion
Reverse osmosis can be used to purify water.
What Reverse Osmosis Is and How It Works
You can use gummy bears to demonstrate how osmosis works.
Use Candy To Demonstrate Osmosis
Diffusion
What Is Diffusion?
Diffusion is a natural mass transport mechanism that moves matter from area of high
concentration to those of low concentration.
Understand What Diffusion Is (and Isn't) in Chemistry
Digital illustration showing water travelling from less concentrated solution
through semi-permeable membrane to more concentrated solution during osmosis
Compare and Contrast Active and Passive Transport
This diagram shows effusion (left) and diffusion (right).
Understand the Difference Between Diffusion and Effusion
Here is how osmosis affects red blood cells in hypertonic, isotonic, and hypotonic
solutions.
Understand Osmotic Pressure and Tonicity
Tree Leaf Vein
Cellular process of turgidity essential for a tree's life.
Molarity and molality are two common ways to express concentration of chemical
solutions.
What is the Difference Between Molarity and Molality?
The cell membrane is an example of a selectively permeable membrane.
What Selectively Permeable Means (With Examples)
475158099.jpg
What Are Prokaryotes and Eukaryotes?
Passive Diffusion
Diffusion: Passive Transport and Facilitated Diffusion
Osmoregulation is the mechanism of controlling osmotic pressure in an organism.
Water crosses a semipermeable membrane to change the concentration of solute
molecules.
Understand How Osmoregulation Works
Illustrated world map of people enjoying having fun
How Does Culture Spread Around the World?
This is the diffusion of dye on a liquid. Dye moves from highest concentration to
lowest concentration until it is evenly distributed throughout the liquid.
Here Are Examples of Diffusion
The SEM photo shows healthy and crenated red blood cells. Crenation occurs when the
cell is exposed to a hypertonic solution.
What Is Does Hypertonic Mean?
Learn Something New Every Day
Email Address
Enter Your Email
SIGN UP
Follow Us
Science, Tech, Math
Humanities
Arts, Music, Recreation
Resources
About Us
Advertise
Privacy Policy
Careers
Contact
Terms of Use
S-ar putea să vă placă și
- MadeDocument3 paginiMadeRobert willaimsÎncă nu există evaluări
- CadeDocument2 paginiCadeRobert willaimsÎncă nu există evaluări
- HouseDocument3 paginiHouseRobert willaimsÎncă nu există evaluări
- KeepDocument3 paginiKeepRobert willaimsÎncă nu există evaluări
- AfricaDocument2 paginiAfricaRobert willaimsÎncă nu există evaluări
- GostomDocument7 paginiGostomRobert willaimsÎncă nu există evaluări
- TrackDocument3 paginiTrackRobert willaimsÎncă nu există evaluări
- FoamDocument2 paginiFoamRobert willaimsÎncă nu există evaluări
- MoreDocument3 paginiMoreRobert willaimsÎncă nu există evaluări
- GotDocument2 paginiGotRobert willaimsÎncă nu există evaluări
- NoteDocument2 paginiNoteRobert willaimsÎncă nu există evaluări
- MotionDocument6 paginiMotionRobert willaimsÎncă nu există evaluări
- Tom TomDocument2 paginiTom TomRobert willaimsÎncă nu există evaluări
- FightDocument2 paginiFightRobert willaimsÎncă nu există evaluări
- ToolDocument2 paginiToolRobert willaimsÎncă nu există evaluări
- PSD officials accused of misusing public resources in bond campaignDocument145 paginiPSD officials accused of misusing public resources in bond campaignRobert willaimsÎncă nu există evaluări
- GgtueooodDocument3 paginiGgtueooodRobert willaimsÎncă nu există evaluări
- TalkDocument22 paginiTalkRobert willaimsÎncă nu există evaluări
- Singapore Foodstuff Trade DirectoryDocument27 paginiSingapore Foodstuff Trade Directoryb4businesshelp100% (2)
- HookDocument14 paginiHookRobert willaimsÎncă nu există evaluări
- ProjectDocument81 paginiProjectRobert willaims100% (1)
- FoodDocument37 paginiFoodRobert willaimsÎncă nu există evaluări
- AttendDocument5 paginiAttendRobert willaimsÎncă nu există evaluări
- HalloweenDocument3 paginiHalloweenRobert willaimsÎncă nu există evaluări
- FundDocument7 paginiFundRobert willaimsÎncă nu există evaluări
- SpecchDocument2 paginiSpecchRobert willaimsÎncă nu există evaluări
- FakeDocument7 paginiFakeRobert willaimsÎncă nu există evaluări
- SocialDocument5 paginiSocialRobert willaimsÎncă nu există evaluări
- CouncilDocument45 paginiCouncilRobert willaimsÎncă nu există evaluări
- PricesDocument13 paginiPricesRobert willaimsÎncă nu există evaluări
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (894)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (265)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (119)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Stress BreakerDocument7 paginiStress BreakerEshan Verma33% (3)
- LearnerDocument7 paginiLearnersudhacarhrÎncă nu există evaluări
- DNA Replication Practice PDFDocument2 paginiDNA Replication Practice PDFKim100% (1)
- Biofuels Bioprod Bioref - 2022 - Souza - Bioenergy Research in Brazil A Bibliometric Evaluation of The BIOEN ResearchDocument16 paginiBiofuels Bioprod Bioref - 2022 - Souza - Bioenergy Research in Brazil A Bibliometric Evaluation of The BIOEN Researchdeborah151Încă nu există evaluări
- Psychiatric Mental Health Nursing Concepts of Care in Evidence Based Practice 8th Edition Townsend Test BankDocument36 paginiPsychiatric Mental Health Nursing Concepts of Care in Evidence Based Practice 8th Edition Townsend Test Bankquemefuloathableilljzf100% (26)
- Van Nostrands Scientific EncyclopediaDocument3.529 paginiVan Nostrands Scientific EncyclopediaMarius Popa100% (28)
- Lab Exercise 0ne: Carbohydrate Analysis Lab A.1 (Page 28)Document31 paginiLab Exercise 0ne: Carbohydrate Analysis Lab A.1 (Page 28)Goh Kae HorngÎncă nu există evaluări
- Introduction To Bioinformatics: Database Search (FASTA)Document35 paginiIntroduction To Bioinformatics: Database Search (FASTA)mahedi hasanÎncă nu există evaluări
- Module 2 NCM 114Document11 paginiModule 2 NCM 114Erven AranasÎncă nu există evaluări
- Adolescent Reproductive and Sexual HealthDocument42 paginiAdolescent Reproductive and Sexual HealthMuhammad Abbas WaliÎncă nu există evaluări
- Cell Membrane: A Selective BarrierDocument23 paginiCell Membrane: A Selective BarrierHama JamalÎncă nu există evaluări
- Short Periods of Incubation During Egg Storage - SPIDESDocument11 paginiShort Periods of Incubation During Egg Storage - SPIDESPravin SamyÎncă nu există evaluări
- Week 6. Definision and DictionaryDocument19 paginiWeek 6. Definision and DictionaryafdreliautariazizahÎncă nu există evaluări
- Effects of Different Fermentation Temperatures On Metabolites of KimchiDocument7 paginiEffects of Different Fermentation Temperatures On Metabolites of KimchiAngela ValdiviesoÎncă nu există evaluări
- General Biology 2 Budget of WorkDocument3 paginiGeneral Biology 2 Budget of WorkMaricris BalboaÎncă nu există evaluări
- AP Psychology Mnomonic DevicesDocument7 paginiAP Psychology Mnomonic DevicesBellony SandersÎncă nu există evaluări
- Correlation of Dynamic Strength in The Standing Calf Raise With Sprinting Performance in Consecutive Sections Up To 30 MetersDocument9 paginiCorrelation of Dynamic Strength in The Standing Calf Raise With Sprinting Performance in Consecutive Sections Up To 30 MetersJohn NixonÎncă nu există evaluări
- Candida Tropicalis - ACMGDocument12 paginiCandida Tropicalis - ACMGSergio Iván López LallanaÎncă nu există evaluări
- Forest Health and Biotechnology - Possibilities and Considerations (2019)Document241 paginiForest Health and Biotechnology - Possibilities and Considerations (2019)apertusÎncă nu există evaluări
- PRACTICE TEST - Gen - EdDocument93 paginiPRACTICE TEST - Gen - EddispacitoteamoÎncă nu există evaluări
- 02 Anatomy and Histology PLE 2019 RatioDocument69 pagini02 Anatomy and Histology PLE 2019 RatioPatricia VillegasÎncă nu există evaluări
- E-Portfolio Injection Study GuideDocument7 paginiE-Portfolio Injection Study Guideapi-366034042Încă nu există evaluări
- LWT - Food Science and TechnologyDocument8 paginiLWT - Food Science and TechnologyAzb 711Încă nu există evaluări
- Human Contact May Reduce Stress in Shelter DogsDocument5 paginiHuman Contact May Reduce Stress in Shelter DogsFlorina AnichitoaeÎncă nu există evaluări
- Effects of Sleep Deprivation among Students in Saint Francis of Assisi CollegeDocument50 paginiEffects of Sleep Deprivation among Students in Saint Francis of Assisi College• Cielo •Încă nu există evaluări
- Effects of Climate on Living Things"This title "TITLE "Effects of Climate on Living ThingsDocument2 paginiEffects of Climate on Living Things"This title "TITLE "Effects of Climate on Living ThingsMelannie Magalong100% (1)
- Flowering PlantsDocument43 paginiFlowering Plantskingbanakon100% (1)
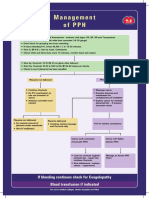
- Management of PPHDocument1 paginăManagement of PPH098 U.KARTHIK SARAVANA KANTHÎncă nu există evaluări
- The Anatomy, Physiology, and Pathophysiology of Muscular SystemDocument55 paginiThe Anatomy, Physiology, and Pathophysiology of Muscular SystemAnisaÎncă nu există evaluări
- Analisis Kualitatif Dan Kuantitatif Kandungan Kimia Dari Ekstrak Heksan, Aseton, Etanol Dan Air Dari Umbi Bawang Putih (Allium Sativum Linn.)Document11 paginiAnalisis Kualitatif Dan Kuantitatif Kandungan Kimia Dari Ekstrak Heksan, Aseton, Etanol Dan Air Dari Umbi Bawang Putih (Allium Sativum Linn.)Tari PratiwiÎncă nu există evaluări