Documente Academic
Documente Profesional
Documente Cultură
Formulas For Thermodynamics 1
Încărcat de
Stefani Ann CabalzaDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Formulas For Thermodynamics 1
Încărcat de
Stefani Ann CabalzaDrepturi de autor:
Formate disponibile
FORMULAS FOR THERMODYNAMICS 1
MEASURES OF AMOUNT OF SIZE FIRST LAW OF THERMODYNAMICS
Specific volume = Esystem + Esurroundings = 0
Esurroundings = Q W
Molar volume, Vm =
Esystem = U + PE + KE
Q W = U + PE + KE
FORCE
1 For closed systems: (PE and KE are constant)
=
E = U = Q + W
For finite changes: U = Q + W
PRESSURE For differential changes: dU = dQ + dW
= =
Enthalpy:
H = U + PV
WORK For finite changes: H = U + (PV); H = U + VP +
= U PV
For differential changes: dH = dU + d(PV)
ENERGY For constant pressure process: H = U + PV
a. Kinetic energy
1 2 Heat Capacity:
= 2 =
2 2 Constant pressure: Q = H = nCpT
b. Potential energy Constant volume: Q = U = nCvT
= =
MATERIALS & ENERGY BALANCES FOR OPEN SYSTEMS
1u1A1 = 2u2A2 (continuity equation)
OVERALL ENERGY BALANCE EQUATION
2
W + Q = Z + 2 + H
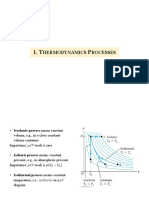
EQUATIONS FOR PROCESS CALCULATIONS: IDEAL GAS
(Mechanically Reversible Closed-System Processes)
Q U H W
Isochoric U nCvT nCpT 0
Isobaric nCvT nCpT U - Q
Isothermal -W 0 0 RT 1 = RT 2
2 1
Adiabatic 0 nCvT nCpT U
EQUATIONS OF STATE
Virial Equations
1. Application of virial equations
= = 1 + where B 2nd virial coefficient (equation 1)
= =1+ truncated in two terms (equation 2)
=
=1+ + 2
truncated in three terms (equation 3)
2. Extended virial equation: Benedict/Webb/Rubin Equation
0 0 0 / 2 2
= + 2
+ 3
+ 6 + 3 2 (1 + 2 ) /
3. Cubic equations of state
a. Van der Waals Equation of state
= 2
272 2
Where = =
64 8
b. Vapor & vapor-like roots of the generic cubic equation of state
()
= + [ ]
( )( + )
For Z: = 1 +
(+)(+)
= =
c. Vapor & vapor-like roots of the generic cubic equation of state
+
= + ( + )( + ) [ ]
For Z:
1+
= + ( + )( + ) [ ]
d. Other equations: Soave/Redlich/Kwong SRK Equation and Peng Robinson PR
Equation
Equation of
Tr Zc
state
VDW 1 0 0 1/8 27/64 3/8
RK Tr1/2 1 0 0.08664 0.42748 1/8
SRK SRK 1 0 0.08664 0.42748 1/8
PR PR 1 + 2 1 2 0.07779 0.45724 0.3074
1/2 2
= [1 + (0.480 + 1.574 0.1762 ) (1 )]
1/2 2
= [1 + (0.37464 + 1.5422 0.269922 ) (1 )]
S-ar putea să vă placă și
- Dynamics FULL Equation SheetDocument2 paginiDynamics FULL Equation SheetZachÎncă nu există evaluări
- A705F Schematic Circuit Diagram HALABTECHDocument11 paginiA705F Schematic Circuit Diagram HALABTECHbcscneg0% (1)
- MP Notes PDFDocument160 paginiMP Notes PDFElumalaiÎncă nu există evaluări
- Human Resource Development Multiple Choice Question (GuruKpo)Document4 paginiHuman Resource Development Multiple Choice Question (GuruKpo)GuruKPO90% (20)
- 2019-CH-18 ThermoDocument6 pagini2019-CH-18 ThermoMuhammad AbdullahÎncă nu există evaluări
- First Law of ThermodynamicsDocument28 paginiFirst Law of ThermodynamicsDOZPandaÎncă nu există evaluări
- Formula RioDocument3 paginiFormula RioGuillermo Prol CasteloÎncă nu există evaluări
- 02 - Derivation of Diffusivity Equation - Liquid CartesianDocument5 pagini02 - Derivation of Diffusivity Equation - Liquid CartesianHassan AmerÎncă nu există evaluări
- EB Exe. SummaryDocument12 paginiEB Exe. SummaryNajihah JaffarÎncă nu există evaluări
- EC2105 Lecture 7 E-Field4Document24 paginiEC2105 Lecture 7 E-Field4hyunyoung256Încă nu există evaluări
- Lec2 PDFDocument3 paginiLec2 PDFAbhisek RoyÎncă nu există evaluări
- Report 2Document10 paginiReport 2Karim KhalafÎncă nu există evaluări
- Lec3 - Final - Revised Stat MechDocument16 paginiLec3 - Final - Revised Stat MechnokosamÎncă nu există evaluări
- Week 01-v.2Document5 paginiWeek 01-v.2Togi Jevenson SimanjuntakÎncă nu există evaluări
- 6 Work and EnergyDocument7 pagini6 Work and EnergyHassan FulaihÎncă nu există evaluări
- Exam 3 Cheat Sheet: Heat TransferDocument2 paginiExam 3 Cheat Sheet: Heat TransferShyam PolacondaÎncă nu există evaluări
- Thermodynamic Potentials Phase Tran. Low Temp MaterialDocument26 paginiThermodynamic Potentials Phase Tran. Low Temp Materialsrinu reddyÎncă nu există evaluări
- 2022 Formula SheetDocument2 pagini2022 Formula SheetHank MoodyÎncă nu există evaluări
- Physics Class 11 DerivationDocument4 paginiPhysics Class 11 Derivation1234raoshabÎncă nu există evaluări
- Chapter 2 - Energy Balances AsdDocument17 paginiChapter 2 - Energy Balances AsdBananaliksÎncă nu există evaluări
- Chapter 23Document25 paginiChapter 23hamzaxray38Încă nu există evaluări
- Energy Method VariationalDocument23 paginiEnergy Method VariationalVaibhav ChaudhariÎncă nu există evaluări
- ICM RelatedDocument7 paginiICM Relatedprasad adsuleÎncă nu există evaluări
- Thermodynamic Revision 2021 Part 1Document70 paginiThermodynamic Revision 2021 Part 1Rawda AliÎncă nu există evaluări
- 10 A 1 EM Waves ConductorsDocument9 pagini10 A 1 EM Waves ConductorsRAJ MEENAÎncă nu există evaluări
- Hagen Poisuille EquationDocument4 paginiHagen Poisuille EquationRochakÎncă nu există evaluări
- Chapter 24-2024Document28 paginiChapter 24-2024hamzaxray38Încă nu există evaluări
- CH 6Document30 paginiCH 6tamay 95Încă nu există evaluări
- Heat Transfer Sample ProblemsDocument5 paginiHeat Transfer Sample ProblemsJc John Christoper PajoÎncă nu există evaluări
- Lecture 15Document5 paginiLecture 15JOSE CARLOS LEON GONZALEZÎncă nu există evaluări
- PHYS 102 Final Exam: 1. A Particle With Charge Q Is Moving in A Uniform Magnetic FieldDocument4 paginiPHYS 102 Final Exam: 1. A Particle With Charge Q Is Moving in A Uniform Magnetic FieldNano SuyatnoÎncă nu există evaluări
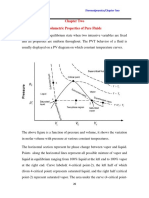
- Chapter Two Volumetric Properties of Pure FluidsDocument18 paginiChapter Two Volumetric Properties of Pure Fluidsc74zdwfbzhÎncă nu există evaluări
- ديناميك غازDocument38 paginiديناميك غازعلي عطيه منصور حسينÎncă nu există evaluări
- Hydro 1 - Open ChannelsDocument28 paginiHydro 1 - Open ChannelsJericho Alfred Rullog SapitulaÎncă nu există evaluări
- Buckling of FramesDocument48 paginiBuckling of FramesJithin KannanÎncă nu există evaluări
- Physics 1301 Equation SheetDocument2 paginiPhysics 1301 Equation Sheeth.krijestoracÎncă nu există evaluări
- All FormulasDocument32 paginiAll Formulasuzairazizsuria1Încă nu există evaluări
- Techniques To Solve: - WorkDocument9 paginiTechniques To Solve: - Workarianne denice100% (1)
- Damping: Single Degree of FreedomDocument28 paginiDamping: Single Degree of FreedomAbdulrahmanÎncă nu există evaluări
- Potential & Kinetic Energy and Flow WorkDocument10 paginiPotential & Kinetic Energy and Flow WorkCarla Francheska CalmaÎncă nu există evaluări
- Lecture String and Bar VibrationsDocument21 paginiLecture String and Bar Vibrationsanand maniÎncă nu există evaluări
- 2 Differential Equation PDFDocument17 pagini2 Differential Equation PDFyaellÎncă nu există evaluări
- Chapter Two Volumetric Properties of Pure FluidsDocument18 paginiChapter Two Volumetric Properties of Pure Fluidsson gokuÎncă nu există evaluări
- Ecuación de energía-FDT2018IDocument12 paginiEcuación de energía-FDT2018ILeon Felipe Toro NavarroÎncă nu există evaluări
- Tut 9 DriveDocument23 paginiTut 9 Drivemennaabdelrazik848Încă nu există evaluări
- Electromagnetic Formula SheetA4Document3 paginiElectromagnetic Formula SheetA4Abdullatif AlbattatÎncă nu există evaluări
- Equation Sheet APSC 131 Midterm - GrayscaleDocument2 paginiEquation Sheet APSC 131 Midterm - GrayscaleRyan WilderÎncă nu există evaluări
- Finite Element Method: - Derivation of Element PropertiesDocument7 paginiFinite Element Method: - Derivation of Element PropertiesKaran PatelÎncă nu există evaluări
- Figure 2.1 Modelling A Spring and Mass SystemDocument16 paginiFigure 2.1 Modelling A Spring and Mass Systemmehmet aliÎncă nu există evaluări
- Physics 2Document16 paginiPhysics 2Dane Mica Rint QuinonesÎncă nu există evaluări
- Single Degree of Freedom: 2.1.1 Undamped Free VibrationDocument8 paginiSingle Degree of Freedom: 2.1.1 Undamped Free VibrationMohammad Muhtasim Mashfy, 170011054Încă nu există evaluări
- EDC Unit-I RectifiersDocument12 paginiEDC Unit-I Rectifiersneha yarrapothuÎncă nu există evaluări
- Equations & ConstantsDocument5 paginiEquations & ConstantsJoserineÎncă nu există evaluări
- 8 Thermal 2Document47 pagini8 Thermal 2KingsonÎncă nu există evaluări
- DC and AC Power Fundamentals SAPDocument51 paginiDC and AC Power Fundamentals SAPPaul Danniel AquinoÎncă nu există evaluări
- Review of First and Second Law of Thermodynamics: DefinitionsDocument10 paginiReview of First and Second Law of Thermodynamics: DefinitionsSumit RijalÎncă nu există evaluări
- Table-1: Microscopic Basic Transport Equations: 1. The Continuity EquationDocument11 paginiTable-1: Microscopic Basic Transport Equations: 1. The Continuity EquationABDUL RAFEYÎncă nu există evaluări
- 2022 - SynthèseDocument9 pagini2022 - SynthèseThéo MélotteÎncă nu există evaluări
- Cheat - Sheet - Exam 1Document2 paginiCheat - Sheet - Exam 1LoganÎncă nu există evaluări
- Green's Function Estimates for Lattice Schrödinger Operators and Applications. (AM-158)De la EverandGreen's Function Estimates for Lattice Schrödinger Operators and Applications. (AM-158)Încă nu există evaluări
- Slu Online Enrollment Guide: STEP 2: Click Sign in With GoogleDocument11 paginiSlu Online Enrollment Guide: STEP 2: Click Sign in With GoogleStefani Ann CabalzaÎncă nu există evaluări
- Assignment MathDocument6 paginiAssignment MathStefani Ann CabalzaÎncă nu există evaluări
- Types of Taps, Splices and Joints of ConductorDocument10 paginiTypes of Taps, Splices and Joints of ConductorStefani Ann CabalzaÎncă nu există evaluări
- Cabigao, Abigail A. APRIL 4, 2018 Bsba - 3 Natsci 2Document3 paginiCabigao, Abigail A. APRIL 4, 2018 Bsba - 3 Natsci 2Stefani Ann CabalzaÎncă nu există evaluări
- Tabs (From Zee)Document4 paginiTabs (From Zee)Stefani Ann CabalzaÎncă nu există evaluări
- CoulsonDocument5 paginiCoulsonStefani Ann CabalzaÎncă nu există evaluări
- Nomograph: Centrifugation BiochemistryDocument1 paginăNomograph: Centrifugation BiochemistryStefani Ann CabalzaÎncă nu există evaluări
- Calc Tekniks For ProgressionDocument5 paginiCalc Tekniks For ProgressionStefani Ann CabalzaÎncă nu există evaluări
- 2 Site Development PlanDocument1 pagină2 Site Development PlanStefani Ann CabalzaÎncă nu există evaluări
- Definitions of LevelDocument10 paginiDefinitions of LevelStefani Ann CabalzaÎncă nu există evaluări
- Calc Tekniks Part 2Document4 paginiCalc Tekniks Part 2Stefani Ann CabalzaÎncă nu există evaluări
- BOD DO SAG Curve PDFDocument19 paginiBOD DO SAG Curve PDFStefani Ann CabalzaÎncă nu există evaluări
- Create A Java Program That Will Compute For The Sum of The 1st 50 NumbersDocument8 paginiCreate A Java Program That Will Compute For The Sum of The 1st 50 NumbersStefani Ann CabalzaÎncă nu există evaluări
- 19 - 22187 - Pritchard RD (1969)Document36 pagini19 - 22187 - Pritchard RD (1969)Isaias MoralesÎncă nu există evaluări
- Mechatronics Course PlanDocument3 paginiMechatronics Course PlanMohammad Faraz AkhterÎncă nu există evaluări
- KaranDocument4 paginiKarancristioronaldo90Încă nu există evaluări
- Jurong Junior College: Preliminary Examination 2009Document16 paginiJurong Junior College: Preliminary Examination 2009cjcsucksÎncă nu există evaluări
- 107 01 Covers and Side Doors A SideDocument38 pagini107 01 Covers and Side Doors A Sideben vervuurtÎncă nu există evaluări
- Maximus MHX DatasheetDocument5 paginiMaximus MHX Datasheetjulya julyaÎncă nu există evaluări
- Prestressed Concrete ProblemDocument9 paginiPrestressed Concrete ProblemPrantik Adhar SamantaÎncă nu există evaluări
- M 02 0001Document3 paginiM 02 0001Miguel ruizÎncă nu există evaluări
- Comparison of Plate Count Agar and R2A Medium For Enumeration of Heterotrophic Bacteria in Natural Mineral WaterDocument4 paginiComparison of Plate Count Agar and R2A Medium For Enumeration of Heterotrophic Bacteria in Natural Mineral WaterSurendar KesavanÎncă nu există evaluări
- Pre Post and Infix NotationsDocument12 paginiPre Post and Infix NotationsGolla GirijaÎncă nu există evaluări
- Science 8: Learning Activity SheetDocument9 paginiScience 8: Learning Activity SheetVan Amiel CovitaÎncă nu există evaluări
- 10 DLAH-Vessel Movement 28 Nov - 7 Dec 2020Document3 pagini10 DLAH-Vessel Movement 28 Nov - 7 Dec 2020herlambangÎncă nu există evaluări
- Pricing and Marketing Strategy: Rahul Mishra, Narendra Singh, Dinesh KumarDocument12 paginiPricing and Marketing Strategy: Rahul Mishra, Narendra Singh, Dinesh KumarGaurav ChauhanÎncă nu există evaluări
- Risk LogDocument1 paginăRisk LogOzu HedwigÎncă nu există evaluări
- Pism Pub Line Up - Jul-Dec - 2022Document1 paginăPism Pub Line Up - Jul-Dec - 2022Yus CeballosÎncă nu există evaluări
- Preview - ISO+8655 6 2022Document6 paginiPreview - ISO+8655 6 2022s7631040Încă nu există evaluări
- Lesson 2 - Reflection PaperDocument2 paginiLesson 2 - Reflection PaperkristhelynÎncă nu există evaluări
- Example of Presentation Planning Document 1uf6cq0Document2 paginiExample of Presentation Planning Document 1uf6cq0Wilson MorenoÎncă nu există evaluări
- Question 1: As Shown in Figure 1. A 6-Pole, Long-Shunt Lap-Wound CompoundDocument4 paginiQuestion 1: As Shown in Figure 1. A 6-Pole, Long-Shunt Lap-Wound Compoundالموعظة الحسنه chanelÎncă nu există evaluări
- Prescriptions For Closing The Seven Service Quality GapsDocument1 paginăPrescriptions For Closing The Seven Service Quality GapsReema NegiÎncă nu există evaluări
- Briefing Evaluation: Yes / No High / Low Yes / No High / Low Good / Inferior Yes / NoDocument4 paginiBriefing Evaluation: Yes / No High / Low Yes / No High / Low Good / Inferior Yes / NoAmmarah AzharÎncă nu există evaluări
- Restrictions AOP30 enDocument1 paginăRestrictions AOP30 enRicardo RamirezÎncă nu există evaluări
- Rail Inspection Vehicle Using Ir Sensor and Spot Marking SystemDocument63 paginiRail Inspection Vehicle Using Ir Sensor and Spot Marking SystemNarayananNanuÎncă nu există evaluări
- Detail Project Report: (Heidelberg Cement Group)Document42 paginiDetail Project Report: (Heidelberg Cement Group)saravananÎncă nu există evaluări
- Bachelors - Project Report 1Document43 paginiBachelors - Project Report 1divyaÎncă nu există evaluări
- Comsigua HBIDocument0 paginiComsigua HBIproxywarÎncă nu există evaluări
- Imarest 2021 Warship Development 1997Document43 paginiImarest 2021 Warship Development 1997nugrohoÎncă nu există evaluări
- Normal DistributionDocument23 paginiNormal Distributionlemuel sardualÎncă nu există evaluări