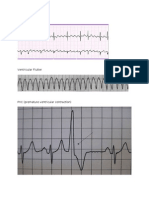
Documente Academic
Documente Profesional
Documente Cultură
Rivaroxaban Vs Warfarin Sodium in The Ultra-Early Period After Atrial Fibrillation-Related Mild Ischemic Stroke
Încărcat de
Roberto López MataTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Rivaroxaban Vs Warfarin Sodium in The Ultra-Early Period After Atrial Fibrillation-Related Mild Ischemic Stroke
Încărcat de
Roberto López MataDrepturi de autor:
Formate disponibile
Research
JAMA Neurology | Original Investigation
Rivaroxaban vs Warfarin Sodium in the Ultra-Early Period
After Atrial FibrillationRelated Mild Ischemic Stroke
A Randomized Clinical Trial
Keun-Sik Hong, MD; Sun U. Kwon, MD; Sang Hun Lee, MD; Ji Sung Lee, PhD; Yong-Jae Kim, MD; Tae-Jin Song, MD;
Young Dae Kim, MD; Man-Seok Park, MD; Eung-Gyu Kim, MD; Jae-Kwan Cha, MD; Sang Min Sung, MD;
Byung-Woo Yoon, MD; Oh Young Bang, MD; Woo-Keun Seo, MD; Yang-Ha Hwang, MD; Seong Hwan Ahn, MD;
Dong-Wha Kang, MD; Hyun Goo Kang, MD; Kyung-Ho Yu, MD; for the Phase 2 Exploratory Clinical Study to Assess
the Effects of Xarelto (Rivaroxaban) Versus Warfarin on Ischemia, Bleeding, and Hospital Stay in Acute Cerebral
Infarction Patients With Non-valvular Atrial Fibrillation (Triple AXEL) Study Group
Editorial
IMPORTANCE In atrial fibrillation (AF)related acute ischemic stroke, the optimal oral Supplemental content
anticoagulation strategy remains unclear.
OBJECTIVE To test whether rivaroxaban or warfarin sodium is safer and more effective for
preventing early recurrent stroke in patients with AF-related acute ischemic stroke.
DESIGN, SETTING, AND PARTICIPANTS A randomized, multicenter, open-label, blinded end
point evaluation, comparative phase 2 trial was conducted from April 28, 2014, to December
7, 2015, at 14 academic medical centers in South Korea among patients with mild AF-related
stroke within the previous 5 days who were deemed suitable for early anticoagulation.
Analysis was performed on a modified intent-to-treat basis.
INTERVENTIONS Participants were randomized 1:1 to receive rivaroxaban, 10 mg/d for 5 days
followed by 15 or 20 mg/d, or warfarin with a target international normalized ratio of 2.0-3.0,
for 4 weeks.
MAIN OUTCOMES AND MEASURES The primary end point was the composite of new ischemic
lesion or new intracranial hemorrhage seen on results of magnetic resonance imaging at 4
weeks. Primary analysis was performed in patients who received at least 1 dose of study
medications and completed follow-up magnetic resonance imaging. Key secondary end
points were individual components of the primary end point and hospitalization length.
RESULTS Of 195 patients randomized, 183 individuals (76 women and 107 men; mean [SD]
age, 70.4 [10.4] years) completed magnetic resonance imaging follow-up and were included
in the primary end point analysis. The rivaroxaban group (n = 95) and warfarin group (n = 88)
showed no differences in the primary end point (47 [49.5%] vs 48 [54.5%]; relative risk, 0.91;
95% CI, 0.69-1.20; P = .49) or its individual components (new ischemic lesion: 28 [29.5%] vs
31 of 87 [35.6%]; relative risk, 0.83; 95% CI, 0.54-1.26; P = .38; new intracranial hemorrhage:
30 [31.6%] vs 25 of 87 [28.7%]; relative risk, 1.10; 95% CI, 0.70-1.71; P = .68). Each group had Author Affiliations: Author
1 clinical ischemic stroke, and all new intracranial hemorrhages were asymptomatic affiliations are listed at the end of this
article.
hemorrhagic transformations. Hospitalization length was reduced with rivaroxaban
Group Information: Members of the
compared with warfarin (median, 4.0 days [interquartile range, 2.0-6.0 days] vs 6.0 days
Phase 2 Exploratory Clinical Study to
[interquartile range, 4.0-8.0]; P < .001). Assess the Effects of Xarelto
(Rivaroxaban) Versus Warfarin on
CONCLUSIONS AND RELEVANCE In mild AF-related acute ischemic stroke, rivaroxaban and Ischemia, Bleeding, and Hospital Stay
in Acute Cerebral Infarction Patients
warfarin had comparable safety and efficacy. With Non-valvular Atrial Fibrillation
(Triple AXEL) Study Group are listed
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT02042534 at the end of this article.
Corresponding Author: Sun U. Kwon,
MD, Department of Neurology,
University of Ulsan College of
Medicine, Asan Medical Center, 88,
JAMA Neurol. doi:10.1001/jamaneurol.2017.2161 Olympic-ro, Songpa-gu, Seoul 138-736,
Published online September 11, 2017. South Korea (sukwon@amc.seoul.kr).
(Reprinted) E1
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Research Original Investigation Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke
P
atients with atrial fibrillation (AF)related acute ische-
mic stroke are at high risk of recurrent ischemic stroke Key Points
and intracranial hemorrhage, including hemorrhagic
Question Is rivaroxaban safer and more effective compared with
transformation, during the early period after stroke. Paren- warfarin sodium for early anticoagulation in atrial
teral heparin is not recommended because it increases the risk fibrillationrelated acute ischemic stroke?
of symptomatic intracranial hemorrhage.1 However, in pa-
Findings In this randomized clinical trial of 195 patients with mild
tients with AF and recent ischemic stroke who are treated with
acute ischemic stroke and atrial fibrillation, new ischemic lesions
aspirin, the risk of recurrent ischemic stroke within 2 weeks or new intracranial hemorrhage on results of magnetic resonance
is 5%.2-5 The most widely used antithrombotic management imaging after 4 weeks occurred in 49.5% of patients receiving
strategy in clinical practice is to start aspirin and then to ini- rivaroxaban and 54.5% receiving warfarin, a nonsignificant
tiate oral anticoagulation after several days or 1 to 2 weeks when difference. Each group had 1 recurrence of clinical ischemic stroke,
the risk of intracranial hemorrhage is likely to have subsided. and no symptomatic intracranial hemorrhage occurred.
Nonetheless, the optimal oral anticoagulation strategy for acute Meaning Rivaroxaban and warfarin had comparable safety and
ischemic stroke, regarding when, in whom, and which drug, efficacy for early anticoagulation in mild atrial fibrillationrelated
remains unclear. acute ischemic stroke.
To our knowledge, no trial has specifically tested oral an-
ticoagulation in patients with AF-related acute ischemic stroke. of 1 cerebellar hemisphere. Key exclusion criteria included sig-
In the European Atrial Fibrillation Trial, approximately 294 pa- nificant hemorrhagic transformation, mechanical heart valve,
tients with AF were randomized to receive warfarin sodium, stroke caused by presumed small vessel occlusion, severe re-
aspirin, or placebo within 2 weeks of symptom onset, but the nal impairment (creatinine clearance <30 mL/min/1.73 m2 [to
efficacy and safety of warfarin vs aspirin or placebo in pa- convert to milliliters per second per meter squared, multiply
tients with acute cerebral ischemia was not reported.6 The piv- by 0.0167]), and high risk of intracranial or systemic bleeding
otal nonvitamin K antagonist oral anticoagulant (NOAC) trials (eTable 1 in Supplement 2). This study was designed and con-
excluded patients who had ischemic stroke within 7-30 days.7-10 ducted in accordance with the Declaration of Helsinki12 and
For early oral anticoagulation after acute ischemic stroke, Good Clinical Practice guidelines and was approved by the in-
NOACs might have several advantages compared with warfa- stitutional review boards of Asan Medical Center, University
rin: rapid onset of action, absence of transient hypercoagula- of Ulsan College of Medicine; Ilsan Paik Hospital, Inje Univer-
bility, stable and predictable anticoagulation effects, and ex- sity; Korea University Ansan Hospital, Korea University Col-
pected lower risk of intracranial hemorrhage. Accordingly, lege of Medicine; Ewha Womens University School of Medi-
this proof-of-concept trial (Acute Stroke With Xarelto to cine; Yonsei University College of Medicine; Chonnam National
Reduce Intracranial Hemorrhage, Recurrent Embolic Stroke, University Medical School; Busan Paik Hospital, Inje Univer-
and Hospital Stay [Triple AXEL]) compared the efficacy and sity; Dong-A University Hospital; Pusan National University
safety between rivaroxaban and dose-adjusted warfarin using Hospital; Seoul National University Hospital; Samsung Medi-
magnetic resonance imaging (MRI) surrogate markers and cal Center, Sungkyunkwan University School of Medicine;
hospitalization length in patients who had mild AF-related Kyungpook National University School of Medicine and Hos-
acute ischemic stroke who were considered suitable for early pital; Chosun University School of Medicine; and Hallym Uni-
anticoagulation. versity Sacred Heart Hospital as well as the Korean Ministry
of Food and Drug Administration (tracking number: 30078).
Written informed consent from eligible patients or their le-
gally authorized representatives was obtained. The trial is reg-
Methods
istered with ClinicalTrials.gov (Identifier: NCT02042534).
Study Design and Participants
Triple AXEL was a phase 2, multicenter, randomized, parallel- Randomization and Masking
group, open-label, blinded end point evaluation trial that en- We randomly allocated eligible patients in a 1:1 ratio to receive
rolled patients from 14 academic hospitals in South Korea from rivaroxaban or dose-adjusted warfarin (target international nor-
April 28, 2014, to October 30, 2015; the patients last day of the malized ratio [INR], 2-3) using an interactive web response sys-
study was December 7, 2015. Details of the study design have tem. The randomization sequence was computer generated and
been published previously.11 The trial protocol and statistical stratified by sites with a block size of 4. Patients and respon-
analysis plan are provided in Supplement 1. Briefly, patients sible physicians were aware of the assigned treatment. How-
were eligible if they had an MRI-confirmed acute ischemic ever, an independent imaging review core laboratory, blinded
stroke within 5 days due to presumed cardioembolism, had to the treatment allocation and clinical data, assessed the MRI
nonvalvular AF (including paroxysmal AF) documented by findings. Clinical events were reported by each investigator and
electrocardiogram, and were considered suitable for early an- were judged and categorized by the steering committee.
ticoagulation after taking into account the severity of the is-
chemic lesion as seen in results of diffusion-weighted imaging Procedures
(DWI): acute ischemic lesion less than one-third of the middle Prior to randomization, all patients underwent magnetic reso-
cerebral artery territory, half of the anterior cerebral artery ter- nance angiography and DWI, fluid-attenuated inversion re-
ritory, half of the posterior cerebral artery territory, and half covery, gradient-recalled echo, or susceptibility-weighted
E2 JAMA Neurology Published online September 11, 2017 (Reprinted) jamaneurology.com
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke Original Investigation Research
imaging and received aspirin after the confirmation of an acute matic intracranial hemorrhage was defined as any intracranial
ischemic lesion on MRI results. Low-dose subcutaneous hep- hemorrhage associated with clinical deterioration as judged by
arin or low-molecular-weight heparin for the prevention of the responsible investigators.
deep venous thrombosis or pulmonary embolism was used at Prespecified secondary efficacy end points were new is-
the discretion of the responsible physicians. chemic lesion; new intracranial hemorrhage; length of hospi-
After randomization, the rivaroxaban group received ri- talization; major bleeding as defined by the International So-
varoxaban, 10 mg once daily, for the first 5 days followed by ciety on Thrombosis and Hemostasis 14 ; acute coronary
20 mg once daily (patients with creatinine clearance 50 mL/ syndrome; composite of major vascular events including stroke,
min) or 15 mg once daily (patients with creatinine clearance myocardial infarction, and vascular death; composite of a ma-
of 30-49 mL/min).8 Aspirin or heparin for prophylaxis of deep jor vascular event plus major bleeding; composite of clinical is-
venous thrombosis or pulmonary embolism was stopped 24 chemic events; and 4-week modified Rankin Scale score.
hours before initiation of rivaroxaban. In the warfarin group,
aspirin or heparin for prophylaxis of deep venous thrombo- Statistical Analysis
sis or pulmonary embolism was continued until an INR of A detailed description of the analytic approach is provided in
1.7 was reached after initiation of warfarin. To reduce inter- the statistical analysis plan (Supplement 1). We designed the
investigator variation in warfarin dosing, a web-based trial to show that rivaroxaban would be superior to warfarin
Bayesian algorithm (http://www.warfarindosing.org/) was for the prevention of new ischemic lesions or new intracra-
recommended for warfarin dosing. For the anticoagulation nial hemorrhage on results of follow-up MRI. The primary and
quality in the warfarin arm, we assessed the mean INR values secondary end points were assessed in the modified ITT popu-
and the proportions of patients with an INR of 2 to 3 at day 5, lation, which included patients who were randomized, re-
2 weeks, and 4 weeks because the 4-week trial period was too ceived at least 1 dose of study drugs, and completed fol-
short to analyze the time in therapeutic range. low-up MRI. Per-protocol analysis was additionally performed
At 4 weeks, we performed MRI, including fluid-attenuated in patients who had no major protocol violation and took 80%
inversion recovery and gradient-recalled echo or susceptibility- or more of the assigned study medications. Adverse events
weighted imaging to assess for new ischemic lesions and new were assessed in the safety population, which included pa-
intracranial hemorrhage. Patients who had experienced clini- tients who were randomized and received at least 1 dose of
cal ischemic stroke or symptomatic intracranial hemorrhage be- study drugs.
fore the end of the trial underwent MRI at the time of the clini- We performed the primary end point analysis using 2 test
cal event. We allowed computed tomographic evaluation for for unadjusted analysis and Poisson regression analysis for ad-
patients who were unstable and unable to undergo MRI. How- justed analysis. In the adjusted analysis, we included vari-
ever, all patients included in the modified intent-to-treat (ITT) ables of age, sex, center, prior use of a vitamin K antagonist
population completed the follow-up MRI. (VKA), concomitant use of an antiplatelet agent, and vari-
During most of the trial period, the Korean insurance sys- ables showing difference between the 2 groups. Secondary and
tem did not cover the cost of NOACs for most patients with safety end point analyses were conducted using 2 test, Fisher
AF.13 Therefore, patients randomized to receive rivaroxaban exact test, Wilcoxon rank sum test, or analysis of covariance,
had to make the transition from rivaroxaban to warfarin with as indicated.
a 5-day overlap to ensure adequate anticoagulation during On the basis of a review of earlier studies,4,8,15 we calcu-
the transition. Therefore, clinical ischemic and major bleed- lated the sample size by assuming that the primary end point
ing events were followed up for an additional 7 days after the rate would be 25% in the warfarin group and that the abso-
end of the trial and were included in the clinical event end lute risk reduction with rivaroxaban would be 15%. Using a
points. However, patients who were willing to pay for rivar- 1-sided superiority test, 178 patients (89 in each group) assess-
oxaban at their own expense continued taking rivaroxaban. able for the primary end point would give 80% power with a
Detailed follow-up procedures are summarized in eTable 2 in significance level of P < .05. Assuming a 10% dropout rate, we
Supplement 2). planned to enroll 196 patients. An independent statistician con-
ducted the statistical analysis. At the inception, interim analy-
Outcomes sis was not planned because this was a phase 2, proof-of-
The primary end point was the composite of new ischemic le- concept, open-label trial. However, because there had been no
sion or new intracranial hemorrhage on results of follow-up MRI trial of NOACs in patients with acute ischemic stroke, the steer-
at 4 weeks. The definition of new ischemic lesion included symp- ing committee and investigators agreed to conduct an in-
tomatic or asymptomatic new ischemic lesions on results of fol- terim analysis for safety after enrolling 100 patients. No safety
low-up fluid-attenuated inversion recovery imaging. Sympto- concern was raised, and we continued to enroll the planned
matic ischemic lesions were defined as clinical ischemic stroke patients.
recurrence associated with a relevant new ischemic lesion. New Consistency of treatment effect for the primary end point
intracranial hemorrhage (hemorrhagic transformation, intra- and individual components was assessed for 8 post hoc sub-
cerebral hemorrhage, subarachnoid hemorrhage, subdural he- groups regarding age, sex, prior use of a VKA within 30 days
matoma, or epidural hematoma) included symptomatic or before randomization, prestroke CHA2DS2-VASc (congestive
asymptomatic hemorrhage on results of follow-up gradient- heart failure, hypertension, age 75 years [doubled], diabe-
recalled echo or susceptibility-weighted imaging. Sympto- tes, stroke [doubled], vascular disease, age 65-74 years, sex cat-
jamaneurology.com (Reprinted) JAMA Neurology Published online September 11, 2017 E3
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Research Original Investigation Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke
Figure. Study Flowchart
195 Patients randomized
101 Randomized to receive 94 Randomized to receive
rivaroxaban warfarin sodium
3 Withdrew consent and 4 Did not receive treatment
did not receive treatment 3 Withdrew consent
1 Withdrawn by investigators
98 Received treatment and 90 Received treatment and
were included in the safety were included in the safety
population population
3 Withdrew and did not 2 Withdrew consent and did
undergo follow-up MRI not undergo follow-up MRI
2 Withdrawn by investigators
1 Withdrew consent
95 Underwent follow-up MRI 88 Underwent follow-up MRI
and were included in the and were included in the
modified intent-to-treat modified intent-to-treat
population population
2 Protocol violations 1 Protocol violation (took
1 Did not meet the inclusion <80% of assigned treatment)
criteria
1 Took <80% of assigned
treatment
93 Included in the 87 Were included in the
per-protocol population per-protocol population
MRI indicates magnetic resonance
imaging.
egory [female]) score, prestroke HAS-BLED (hypertension, leaving 180 patients (93 in the rivaroxaban group and 87 in the
abnormal renal/liver function, stroke, bleeding history or pre- warfarin group) in the per-protocol population (Figure).
disposition, labile INR, elderly, drugs/alcohol concomitantly) Baseline demographic and clinical characteristics were well
score, baseline DWI volume, concomitant use of an antiplatelet balanced except that there were more patients in the rivar-
agent, and creatinine clearance level. oxaban group than the warfarin group with a history of type 2
diabetes (24 [25.3%] vs 10 [11.4%]), and patients in the rivar-
oxaban group had a smaller median initial ischemic lesion vol-
ume on DWI than did patients in the warfarin group (2.6 cm3
Results [interquartile range, 0.3-10.8] vs 5.5 cm3 [interquartile range,
We randomized 195 patients to 1 of 2 treatment groups: 101 to 1.1-14.5]) (Table 1). The median interval from stroke onset to ran-
rivaroxaban and 94 to warfarin. All patients had an acute is- domization was 2 days (interquartile range, 2.0-3.0) (eFigure 1
chemic lesion confirmed by MRI results before randomiza- in Supplement 2), the mean (SD) prestroke CHA2DS2-VASc score
tion. Of the 195 patients randomized, 7 did not receive study was 2.5 (1.6) (Table 1), the mean (SD) prestroke HAS-BLED score
treatments, and 5 withdrew and did not undergo follow-up was 1.3 (0.9), and the median National Institutes of Health Stroke
MRI. Therefore, 183 patients (95 in the rivaroxaban group and Scale score at the time of randomization was 2.0 (interquar-
88 in the warfarin group) were included in the modified ITT tile range, 0.0-4.0). There was a history of VKA use within 30
population for the primary end point analysis, and 188 pa- days before randomization in 75 patients (41.0%), and 57 pa-
tients (98 in the rivaroxaban group and 90 in the warfarin tients (31.1%) received concomitant antiplatelet therapy dur-
group) were included in the safety population. Comparisons ing the trial.
of patients included in the modified ITT population and those Of the modified ITT population, the proportion of pa-
excluded from the modified ITT population are provided in tients taking more than 80% of the study medications was
eTable 3 in Supplement 2. Three patients had major protocol 98.9% in the rivaroxaban group (n = 94) and 97.7% in the war-
violations or had less than 80% adherence to the study treat- farin group (n = 86) at a mean (SD) of 5 (2) days and 98.9% in
ment (2 in the rivaroxaban group and 1 in the warfarin group), the rivaroxaban group (n = 94) and 98.9% in the warfarin group
E4 JAMA Neurology Published online September 11, 2017 (Reprinted) jamaneurology.com
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke Original Investigation Research
Table 1. Baseline Characteristics of the Patients Included in the Modified Intent-to-Treat Population
Rivaroxaban Group Warfarin Sodium Group Total
Characteristic (n = 95) (n = 88) (n = 183)
Age, mean (SD), y 70.2 (10.1) 70.6 (10.9) 70.4 (10.4)
Female sex, No. (%) 40 (42.1) 36 (40.9) 76 (41.5)
Time from onset to randomization, 2.0 (2.0-3.0) 2.0 (2.0-3.0) 2.0 (2.0-3.0)
median (IQR), d
NIHSS score at randomization, 2.0 (1.0-4.0) 2.0 (0.0-4.0) 2.0 (0.0-4.0)
median (IQR)
Initial DWI volume, median (IQR),a cm3 2.6 (0.3-10.8) 5.5 (1.1-14.5) 3.5 (0.5-12.3)
Prestroke CHA2DS2-VASc scoreb
0 11 (11.6) 10 (11.4) 21 (11.5)
1 16 (16.8) 15 (17.0) 31 (16.9)
2 14 (14.7) 26 (29.5) 40 (21.9)
3 20 (21.1) 17 (19.3) 37 (20.2)
4 22 (23.2) 14 (15.9) 36 (19.7)
5 8 (8.4) 6 (6.8) 14 (7.7)
6 3 (3.2) 0 3 (1.6)
Abbreviations: CHA2DS2-VASc,
7 1 (1.1) 0 1 (0.5)
congestive heart failure,
Mean (SD) 2.7 (1.7) 2.3 (1.4) 2.5 (1.6) hypertension, age 75 years
Prestroke HAS-BLED scorec (doubled), diabetes, stroke
(doubled), vascular disease, age
0 13 (13.7) 15 (17.0) 28 (15.3)
65-74 years, sex category (female);
1 46 (48.4) 44 (50.0) 90 (49.2) CT, computed tomography;
2 25 (26.3) 25 (28.4) 50 (27.3) DWI, diffusion-weighted imaging;
FLAIR, fluid-attenuated inversion
3 9 (9.5) 4 (4.5) 13 (7.1) recovery; GRE, gradient-recalled
4 2 (2.1) 0 2 (1.1) echo; HAS-BLED, hypertension,
Mean (SD) 1.4 (0.9) 1.2 (0.8) 1.3 (0.9) abnormal renal/liver function, stroke,
bleeding history or predisposition,
Paroxysmal atrial fibrillation, No. (%) 25 (26.3) 24 (27.3) 49 (26.8) labile international normalized ratio,
Prior VKA use, No. (%) d
39 (41.1) 36 (40.9) 75 (41.0) elderly, drugs/alcohol concomitantly;
IQR, interquartile range;
Risk factor, No. (%)
MRI, magnetic resonance imaging;
Hypertension 65 (68.4) 52 (59.1) 117 (63.9) NIHSS, National Institutes of Health
Diabetes mellituse 24 (25.3) 10 (11.4) 34 (18.6) Stroke Scale; SWI, susceptibility-
weighted imaging; VKA, vitamin K
Hyperlipidemia 16 (16.8) 18 (20.5) 34 (18.6) antagonist.
Coronary artery disease 13 (13.7) 12 (13.6) 25 (13.7) SI conversion factor: To convert
Baseline neuroimaging performed, creatinine to micromoles per liter,
No. (%) multiply by 88.4.
CT 67 (70.5) 63 (71.6) 130 (71.0) a
P = .04.
b
MRI 95 (100.0) 88 (100.0) 183 (100.0) The CHA2DS2-VASc scores range
DWI 95 (100.0) 87 (98.9) 182 (99.5) from 0 to 9, with higher scores
indicating a greater risk of stroke:
FLAIR 95 (100.0) 87 (98.9) 182 (99.5) congestive heart failure,
GRE 87 (91.6) 79 (89.8) 166 (90.7) hypertension, diabetes, 65 to 74
years of age, female sex, and
SWI 9 (9.5) 9 (10.2) 18 (9.8)
vascular disease are each assigned 1
Other 18 (18.9) 20 (22.7) 38 (20.8) point, and prior stroke or transient
Blood pressure at study entry, mean (SD), ischemic attack and being 75 years
mm Hg of age or older are assigned
Systolic 131.8 (19.1) 128.0 (17.0) 130.0 (18.2) 2 points.
c
Diastolic 76.8 (11.7) 77.4 (10.9) 77.1 (11.3) The HAS-BLED scores range from 0
to 9, with higher scores indicating a
Heart rate, mean (SD), beats per minute 84.5 (22.7) 83.3 (23.3) 83.9 (22.9) greater bleeding risk: hypertension,
Serum creatinine, mean (SD), mg/dL 0.89 (0.24) 0.89 (0.25) 0.89 (0.24) abnormal renal function, abnormal
liver function, prior stroke history,
Concomitant antiplatelet agent use, 34 (35.8) 23 (26.1) 57 (31.1)
No. (%) bleeding, labile international
normalized ratios, elderly (>65 years
Single antiplatelet agent 28 (29.5) 20 (22.7) 48 (26.2)
of age), prior alcohol or drug use,
Aspirin 25 (26.3) 11 (12.5) 36 (19.7) and medication use predisposing to
Clopidogrel bisulfate 2 (2.1) 7 (8.0) 9 (4.9) bleeding are each assigned 1 point.
d
Other 1 (1.1) 2 (2.3) 3 (1.6) Use of VKA within 30 days before
randomization.
Dual antiplatelet agents 6 (6.3) 3 (3.4) 9 (4.9) e
P = .02.
jamaneurology.com (Reprinted) JAMA Neurology Published online September 11, 2017 E5
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Research Original Investigation Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke
Table 2. End Points in the Modified Intent-to-Treat Population
Rivaroxaban Warfarin Sodium Adjusted
Group, No. (%) Group, No. (%) Risk Difference Relative Risk Relative Risk
End Point (n = 95) (n = 88) (95% CI) (95% CI) P Value (95% CI)a P Value
Intracranial hemorrhage 47 (49.5) 48 (54.5) 5.07 0.91 .49 0.97 .73
or recurrent ischemic (19.52 to 9.49) (0.69 to 1.20) (0.79 to 1.18)
lesion on results of 4-wk
MRI (primary end point)
Recurrent ischemic lesion 28 (29.5) 31 (35.6) 6.16 0.83 .38 0.85 .45
on results of 4-wk MRIb (20.48 to 8.45) (0.54 to 1.26) (0.56 to 1.30)
Intracranial hemorrhage 30 (31.6) 25 (28.7) 2.84 1.10 .68 1.17 .50
on results of 4-wk MRIb (11.68 to 17.29) (0.70 to 1.71) (0.74 to 1.85)
Clinical recurrent 1 (1.1) 1 (1.1) 0.08 0.93 >.99 NA NA
ischemic stroke (14.54 to 14.42) (0.06 to 14.59)
Symptomatic hemorrhagic 0 0 NA NA >.99 NA NA
conversion or hemorrhagic
stroke
Major bleeding 1 (1.1) 0 1.05 NA >.99 NA NA
(13.44 to 15.53)
Systemic embolism 0 0 NA NA >.99 NA NA
Acute coronary syndrome 0 0 NA NA >.99 NA NA
Composite of stroke, MI, 1 (1.1) 1 (1.1) 0.08 0.93 >.99 NA NA
or vascular death (14.54 to 14.42) (0.06 to 14.59)
Composite of stroke, MI, 2 (2.1) 1 (1.1) 0.97 1.85 >.99 NA NA
vascular death, or major (13.50 to 15.46) (0.17 to 20.08)
bleeding
Composite of clinical 1 (1.1) 1 (1.1) 0.08 0.93 >.99 NA NA
ischemic events (14.54 to 14.42) (0.06 to 14.59)
Duration of hospitalization, 4.0 (2.0-6.0) 6.0 (4.0-8.0) NA NA <.001 NA .002
median (IQR), d
mRS score 01 at 4 wkc 79 (84.0) 64 (74.4) 9.62 1.13 .11 1.04 .73
(5.06 to 23.95) (0.97 to 1.31) (0.83 to 1.29)
Abbreviations: IQR, interquartile range; MI, myocardial infarction; lesion on results of 4-week MRI was not evaluated in 1 patient, and new
MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NA, not intracranial hemorrhage on 4-week MRI was not evaluated in 1 patient
applicable. (detailed description in eTable 4 in Supplement 2). Sensitivity analyses
a
Adjusted for age, sex, initial ischemic lesion volume on diffusion-weighted assuming worst-case and best-case scenarios for these patients are provided
imaging, diabetes, prior use of vitamin K antagonist, concomitant use of in eTable 4 in Supplement 2.
c
antiplatelet agent, and center. Of the intent-to-treat population, the mRS score at 4 weeks was not available
b
Of the patients in the warfarin group who were included in the intent-to-treat in 1 patient in the rivaroxaban group and in 2 patients in the warfarin group.
population and were evaluated for the primary end point, recurrent ischemic
(n = 87) at 4 weeks. After the end of the trial, 73.7% of pa- A new ischemic lesion was seen in 28 patients (29.5%) in
tients (n = 70) in the rivaroxaban group continued rivaroxa- the rivaroxaban group and 31 of 87 patients (35.6%) in the war-
ban treatment, and 26.3% (n = 25) transitioned from rivaroxa- farin group (relative risk, 0.83; 95% CI, 0.54-1.26; P = .38)
ban to warfarin. In the warfarin group, the proportion of (Table 2). Each group had 1 clinical ischemic stroke recur-
patients who achieved the target INR of 2.0-3.0 was 40.9% rence, which was presumed to be cardioembolism. The pro-
(n = 36) at 5 days, 53.4% (n = 47) at 2 weeks, and 46.6% (n = 41) portions of new ischemic lesions greater than 10 mm and mul-
at 4 weeks. The mean (SD) INR value in the warfarin group was tiple lesions did not differ between the 2 groups (eTable 6 in
2.04 (0.62) at 5 days, 2.67 (0.92) at 2 weeks, and 2.39 (0.83) at Supplement 2).
4 weeks (eFigure 2 in Supplement 2). New intracranial hemorrhage was seen in 30 patients
The primary end point occurred in 47 patients (49.5%) in (31.6%) in the rivaroxaban group and 25 of 87 patients (28.7%)
the rivaroxaban group and 48 patients (54.5%) in the warfa- in the warfarin group (relative risk, 1.10; 95% CI, 0.70-1.71;
rin group in the modified ITT population (relative risk, 0.91; P = .68) (Table 2). There was no symptomatic intracranial hem-
95% CI, 0.69-1.20, P = .49) (Table 2). After adjusting for age, orrhage in the 2 groups; all intracranial hemorrhages were
sex, initial ischemic lesion volume on DWI, diabetes, prior VKA, asymptomatic hemorrhagic transformations within or adja-
concomitant antiplatelet use, and center, the difference was cent to the qualifying ischemic lesion. Of the hemorrhagic
not significant (relative risk, 0.97; 95% CI, 0.79-1.18; P = .73). transformations, 49 of 55 (89.1%) were minimal or mild hem-
Sensitivity analyses assuming worst-case and best-case sce- orrhagic transformation without mass effect. Although there
narios for patients who received study drug but did not un- was no statistically significant difference, parenchymal
dergo follow-up MRI showed similar findings (eTable 4 in hematoma with mass effect was observed more frequently
Supplement 2). In the per-protocol population, the 2 groups in the warfarin group than in the rivaroxaban group, while
showed no difference in the primary end point rate (rivaroxa- type I hemorrhagic infarction was more frequent in the riva-
ban, 46 of 93 [49.5%] vs warfarin, 47 of 87 [54.0%]; relative risk, roxaban group than in the warfarin group (eTable 7 in
0.92; 95% CI, 0.69-1.21; P = .54) (eTable 5 in Supplement 2). Supplement 2).
E6 JAMA Neurology Published online September 11, 2017 (Reprinted) jamaneurology.com
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke Original Investigation Research
Table 3. Adverse Events in the Safety Population
No. (%)
Rivaroxaban Group Warfarin Sodium Group Risk Difference, %
Characteristic (n = 98) (n = 90) (95% CI) P Value
1 Adverse events 46 (46.9) 51 (56.7) 9.73 .18
(23.84 to 4.66)
1 Adverse drug reactions 9 (9.2) 13 (14.4) 5.26 .26
(19.41 to 9.11)
1 Serious adverse events 5 (5.1) 5 (5.6) 0.45 >.99
(14.76 to 13.82)
Withdrawal owing to 1 (1.0) 0 1.02 >.99
adverse events (13.32 to 15.32)
Death 0 0 NA >.99
Abbreviation: NA, not applicable.
The treatment effects were generally consistent across post A recent European practical guide recommends the 1-3-
hoc subgroups for the primary end point and individual com- 6-12 day rule for the initiation of NOACs after transient ische-
ponents. However, there was a significant interaction be- mic attack or acute ischemic stroke.16 However, the recom-
tween prior VKA use and treatment effect for the recurrent is- mendations are based on expert opinion and are not supported
chemic lesion end point, suggesting that rivaroxaban was by clinical trial data. An analysis of the Virtual International
associated with fewer recurrent ischemic lesions in patients Stroke Trials Archive (VISTA) database found that the early ini-
with no prior VKA use than was warfarin (eTables 8-10 in tiation of anticoagulants (2-3 days after stroke) was associ-
Supplement 2). ated with substantially fewer recurrent events during the fol-
One patient in the rivaroxaban group developed intraocu- lowing weeks without an increased risk of symptomatic
lar bleeding leading to transient visual disturbance, which was intracerebral hemorrhages.17 However, the finding is subject
categorized as major bleeding. However, it did not prolong hos- to substantial confounding by indication. In a prospective ob-
pitalization, and the patient recovered without sequelae. There servational study that enrolled 1029 consecutive patients with
was no acute coronary syndrome during the trial. The propor- acute ischemic stroke and known or newly diagnosed AF with-
tions of modified Rankin Scale scores of 0 to 1 at 4 weeks did out contraindications to anticoagulation, compared with ini-
not differ between the rivaroxaban and warfarin groups (79 of tiation of treatment before 4 days or more than 14 days after
94 [84.0%] vs 64 of 86 [74.4%]; relative risk, 1.13; 95% CI, 0.97- stroke onset, initiation of anticoagulants within 4 to 14 days
1.31; P = .11) (Table 2). However, median hospitalization length of stroke onset was associated with a significant reduction in
was significantly shorter in the rivaroxaban group than in the the composite of stroke, transient ischemic attack, sympto-
warfarin group (4.0 days [interquartile range, 2.0-6.0 days] vs matic systemic embolism, symptomatic cerebral bleeding, and
6.0 days [interquartile range, 4.0-8.0 days]; P < .001) (Table 2). major extracranial bleeding within 90 days from acute stroke.18
In the per-protocol population analysis, the secondary end In a recent small, prospective observational study of 60 pa-
point results were similar to those of the modified ITT popu- tients initiating rivaroxaban at a median time of 3 days after
lation analysis (eTable 5 in Supplement 2). mild to moderate cardioembolic stroke or transient ischemic
The rates of adverse events, adverse drug reactions, and attack, no patient developed symptomatic hemorrhagic trans-
serious adverse events were similar between the 2 groups formation, and 8 patients had asymptomatic new hemor-
(Table 3). There were no deaths during the trial. rhagic transformation or worsening of hemorrhagic transfor-
mation on results of follow-up MRI obtained 7 days after
initiation of rivaroxaban, suggesting the safety of rivaroxa-
ban treatment in the acute stage.19 Our findings support the
Discussion recommendations and are generally in accordance with the
In this first trial to compare NOAC and VKA in patients with findings of the earlier observational studies.
AF-related acute ischemic stroke, there was no difference be- Given the lower risk of intracranial hemorrhage with
tween rivaroxaban and warfarin in the combined surrogate end NOACs vs warfarin for long-term prevention therapy, pa-
point of new ischemic lesion or new intracranial hemorrhage tients with AF-related acute ischemic stroke who were treated
on results of follow-up MRI at 4 weeks. In addition, there were with rivaroxaban vs those treated with warfarin were ex-
no differences between the 2 groups in the individual compo- pected to have a lower risk of new intracranial hemorrhage,
nents of new ischemic lesion and new intracranial hemor- including hemorrhagic transformation. However, the risk was
rhage. No patient had symptomatic intracranial hemorrhage, not different between the 2 treatment groups. Although, of in-
and 1 patient (1.1%) in each group had clinical recurrence of is- tracranial hemorrhage types, minimal or mild hemorrhagic
chemic stroke. Therefore, either rivaroxaban or warfarin ini- transformation was more frequent with rivaroxaban whereas
tiated within 5 days of stroke onset was comparably safe and parenchymal hematoma was more frequent with warfarin, our
effective for preventing clinical recurrence of ischemic stroke study was not adequately powered to detect the difference in
in patients with AF and mild acute ischemic stroke. However, the types of intracranial hemorrhage.
initiation of rivaroxaban instead of warfarin reduced hospi- Although there were no statistically significant differ-
talization length by almost 2 days. ences, the rivaroxaban group had fewer recurrent ischemic
jamaneurology.com (Reprinted) JAMA Neurology Published online September 11, 2017 E7
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Research Original Investigation Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke
lesions and more new intracranial hemorrhages compared with an antithrombotic strategy in patients with AF and acute
the warfarin group. Full anticoagulation would be immedi- cerebral ischemia.
ately achieved with rivaroxaban and delayed with warfarin, We selected a low dose of 10 mg of rivaroxaban for the first
which might contribute to fewer ischemic lesions and more 5 days because no safety data on rivaroxaban in patients with
hemorrhagic lesions with rivaroxaban in the acute stage when acute ischemic stroke were available, and all patients re-
the risks of both recurrent ischemia and intracranial hemor- ceived aspirin before randomization and the residual effect of
rhage would be greatest. The post hoc analysis finding of a aspirin would remain for 5 to 7 days after rivaroxaban initia-
greater difference in the recurrent ischemic lesion end point tion. In a pharmacodynamics study in healthy Chinese indi-
in patients with no recent VKA use than in those with recent viduals, the inhibition of factor Xa activity, as measured by the
VKA use might support our speculation. However, the results median percentage change from baseline, was about 38% with
from the post hoc analysis and multiple comparisons would a single dose of rivaroxaban, 10 mg, and about 46% with a single
limit the significance of the finding. dose of rivaroxaban, 20 mg. Therefore, a single dose of rivar-
Although there were no differences in the imaging and oxaban, 10 mg, might achieve about 83% of the factor Xa ac-
clinical end points between the 2 groups, initiation of oral an- tivity inhibition as a single dose of rivaroxaban, 20 mg.25
ticoagulation with rivaroxaban vs warfarin significantly re- When NOAC is compared with warfarin, the anticoagula-
duced the length of hospitalization for acute stroke. More rapid tion quality of warfarin therapy must be adequately main-
achievement of full anticoagulation with rivaroxaban likely en- tained. Because of the short trial duration, rather than time in
ables patients to be discharged earlier. Previous studies showed therapeutic range, we assessed the mean INR and the propor-
that, for long-term therapy, use of NOACs vs warfarin was tion of INR values ranging from 2.0 to 3.0. Although the mean
cost-effective.20-24 Our trial showed that rivaroxaban would INR values in the warfarin arm were within 2.0 to 3.0 at each
reduce the cost of hospitalization for acute stroke in patients time point, the proportion of INR values from 2.0 to 3.0 ranged
who are deemed suitable for early anticoagulation, but the cost- between 40.9% (n = 36 of 88) and 53.4% (n = 47 of 88), even
saving effect might not be applicable to different health care though we followed a recommended warfarin dosing pro-
settings. gram. In the pivotal NOAC trials, the time in therapeutic range
In the current study, we found that about 50% of patients of Korean patients ranged between 48% and 56%.26-29 Our find-
had asymptomatic new ischemic lesions or intracranial hem- ings, along with those of the large trials, indicate that Korean
orrhage on results of MRI within 4 weeks after stroke, which patients have difficulty in achieving good-quality anticoagu-
was greater than expected. Despite the early use of anticoagu- lation with warfarin therapy.
lation, about 30% of the patients had a new ischemic lesion.
In a prior study, 40% of patients who had a cardioembolic Limitations
stroke within 6 hours and were treated with antiplatelet drugs This was an open-label study, which was at risk of a reporting
or anticoagulation had a recurrent ischemic lesion on MRI re- bias for clinical events. However, the primary end point was
sults within the first week.15 Our findings, along with those an imaging surrogate marker evaluated in a blinded fashion.
of the earlier study, indicate that the risk of early recurrent The quality of anticoagulation with warfarin in our study was
cerebral ischemia is high in patients with AF-related acute less than optimal. Therefore, our findings might not be appli-
ischemic stroke. However, intracranial hemorrhage was also cable to populations with high-quality anticoagulation with
observed in about 30% of patients in our study. All of the warfarin therapy. Because this study selected patients with
intracranial hemorrhages were asymptomatic and most were mild stroke severity who were considered at low risk of intra-
minor hemorrhagic transformations, which might spontane- cranial hemorrhage with early oral anticoagulation, the find-
ously develop rather than be caused by anticoagulation. ings would not be applicable to those with moderate to se-
Because we had no placebo group, we were unable to assess vere stroke.
whether rivaroxaban or warfarin increased the risk of asymp-
tomatic hemorrhagic transformation. Although the ischemic
and hemorrhagic lesions were clinically silent in most
patients, their effect on cognitive function was not explored
Conclusions
in this study and would be of interest as part of a future Both rivaroxaban and warfarin initiated within 5 days of
investigation. In addition, the high frequency of both new stroke onset in patients with mild AF-related acute ischemic
ischemic and hemorrhagic lesions emphasizes the impor- stroke were safe and effective for preventing early clinical
tance of a proper balance between the efficacy and safety of stroke recurrence.
ARTICLE INFORMATION Seoul, South Korea (Kwon, D.-W. Kang); Seoul, South Korea (Y. D. Kim); Department of
Accepted for Publication: June 12, 2017. Department of Neurology, Korea University Ansan Neurology, Chonnam National University Medical
Hospital, Korea University College of Medicine, School, Gwangju, South Korea (Park); Department
Published Online: September 11, 2017. Ansan, South Korea (S. H. Lee); Clinical Research of Neurology, Busan Paik Hospital, Inje University,
doi:10.1001/jamaneurol.2017.2161 Center, Asan Medical Center, Seoul, South Korea Busan, South Korea (E.-G. Kim); Department of
Author Affiliations: Department of Neurology, (J. S. Lee); Department of Neurology, Ewha Neurology, Dong-A University Hospital, Busan,
Ilsan Paik Hospital, Inje University, Goyang, South Womens University School of Medicine, Seoul, South Korea (Cha); Department of Neurology,
Korea (Hong); Department of Neurology, University South Korea (Y.-J. Kim, Song); Department of Pusan National University Hospital, Busan, South
of Ulsan College of Medicine, Asan Medical Center, Neurology, Yonsei University College of Medicine, Korea (Sung); Department of Neurology, Seoul
E8 JAMA Neurology Published online September 11, 2017 (Reprinted) jamaneurology.com
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke Original Investigation Research
National University Hospital, Seoul, South Korea Sankyo Korea outside the submitted work. atrial fibrillation after transient ischaemic attack or
(Yoon); Department of Neurology, Samsung Dr Hwang reported receiving grants from Bayer minor stroke. Lancet. 1993;342(8882):1255-1262.
Medical Center, Sungkyunkwan University School Korea Ltd during the conduct of the study and 7. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY
of Medicine, Seoul, South Korea (Bang, Seo); grants from Kyungpook National University outside Steering Committee and Investigators. Dabigatran
Department of Neurology and Cerebrovascular the submitted work. Dr D.-W. Kang reported versus warfarin in patients with atrial fibrillation.
Center, Kyungpook National University School of receiving grants from National Research N Engl J Med. 2009;361(12):1139-1151.
Medicine and Hospital, Daegu, South Korea Foundation of Korea funded by the Korea
(Hwang); Department of Neurology, Chosun government (NRF-2014R1A2A1A11051280) and 8. Patel MR, Mahaffey KW, Garg J, et al; ROCKET
University School of Medicine, Gwangju, South Korea Health Technology R&D Project (Ministry for AF Investigators. Rivaroxaban versus warfarin in
Korea (Ahn, H. G. Kang); Department of Neurology, Health & Welfare, Republic of Korea [HI12C1847 and nonvalvular atrial fibrillation. N Engl J Med. 2011;
Hallym University Sacred Heart Hospital, Anyang, HI14C1983]) outside the submitted work. Dr K.-H. 365(10):883-891.
South Korea (Yu). Yu reported receiving grants from Bayer Korea Ltd 9. Granger CB, Alexander JH, McMurray JJ, et al;
Author Contributions: Drs Hong and Kwon had full during the conduct of the study and personal fees ARISTOTLE Committees and Investigators.
access to all the data in the study and take from Bristol-Myers Squibb and Bayer outside the Apixaban versus warfarin in patients with atrial
responsibility for the integrity of the data and the submitted work. No other conflicts were reported. fibrillation. N Engl J Med. 2011;365(11):981-992.
accuracy of the data analysis. Drs Hong and Kwon Funding/Support: This study was supported by 10. Giugliano RP, Ruff CT, Braunwald E, et al;
contributed equally as first authors to this article. Bayer Korea Ltd and grants HI14C1061 and ENGAGE AF-TIMI 48 Investigators. Edoxaban
Study concept and design: Hong, Kwon. HI10C2020 from the Korean Health Technology versus warfarin in patients with atrial fibrillation.
Acquisition, analysis, or interpretation of data: All R&D Project, Ministry of Health & Welfare, Republic N Engl J Med. 2013;369(22):2093-2104.
authors. of Korea. 11. Hong KS, Choi YJ, Kwon SU; Triple AXEL
Drafting of the manuscript: Hong, Kwon. Role of the Funder/Sponsor: Bayer Korea Ltd Investigators. Rationale and design of Triple AXEL:
Critical revision of the manuscript for important suggested rivaroxaban dose selection. The funding trial for early anticoagulation in acute ischemic
intellectual content: All authors. sources had no other role in the design and conduct stroke patients with nonvalvular atrial fibrillation.
Statistical analysis: Hong, Kwon, J. S. Lee. of the study; collection, management, analysis, and Int J Stroke. 2015;10(1):128-133.
Obtained funding: Kwon. interpretation of the data; preparation, review, or
Administrative, technical, or material support: Hong, 12. World Medical Association. World Medical
approval of the manuscript; and decision to submit Association Declaration of Helsinki: ethical
Kwon, J. S. Lee. the manuscript for publication.
Study supervision: Hong, Kwon. principles for medical research involving human
Group Information: Members of the Phase 2 subjects. JAMA. 2013;310(20):2191-2194.
Conflict of Interest Disclosures: Dr Hong reported Exploratory Clinical Study to Assess the Effects of
receiving grants from Bayer Korea Ltd during the 13. Bang OY, Hong KS, Heo JH, et al. New oral
Xarelto (Rivaroxaban) Versus Warfarin on Ischemia, anticoagulants may be particularly useful for Asian
conduct of the study; grants from Boehringer Bleeding, and Hospital Stay in Acute Cerebral
Ingelheim, Bayer, and Ostuka Korea; grants and stroke patients. J Stroke. 2014;16(2):73-80.
Infarction Patients With Non-valvular Atrial
personal fees from Pfizer Korea, Bayer Korea, and Fibrillation (Triple AXEL) Study Group include Sun 14. Schulman S, Kearon C; Subcommittee on
Boehringer Ingelheim Korea; and personal fees U. Kwon, MD, Dong-Wha Kang, MD, Keun-Sik Hong, Control of Anticoagulation of the Scientific and
from Sanofi Korea, Chong Kun Dang Pharm, Dong MD, Sang Hun Lee, MD, Ji Sung Lee, PhD, Yong-Jae Standardization Committee of the International
Wha Pharm, United Pharm, and Daichi Sankyo Kim, MD, Tae-Jin Song, MD, Young Dae Kim, MD, Society on Thrombosis and Haemostasis. Definition
Korea outside the submitted work. Dr Kwon Man-Seok Park, MD, Eung-Gyu Kim, MD, Jae-Kwan of major bleeding in clinical investigations of
reported receiving grants from Bayer Korea Ltd and Cha, MD, Sang Min Sung, MD, Byung-Woo Yoon, antihemostatic medicinal products in non-surgical
Korean Health Technology R&D Project (Ministry of MD, Oh Young Bang, MD, Woo-Keun Seo, MD, patients. J Thromb Haemost. 2005;3(4):692-694.
Health & Welfare, Republic of Korea [HI14C1061 and Yang-Ha Hwang, MD, Seong Hwan Ahn, MD, Hyun 15. Kang DW, Latour LL, Chalela JA, Dambrosia J,
HI10C2020]) during the conduct of the study; Goo Kang, MD, and Kyung-Ho Yu, MD. Warach S. Early ischemic lesion recurrence within a
grants from Pfizer and Boehringer Ingelheim; grants week after acute ischemic stroke. Ann Neurol.
and personal fees from Otsuka Korea; and personal REFERENCES 2003;54(1):66-74.
fees from AstraZeneca Korea, Bayer Korea, Novartis
Korea, Pfizer Korea, and Daichi Sankyo Korea 1. Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy 16. Heidbuchel H, Verhamme P, Alings M, et al.
outside the submitted work. Drs J. S. Lee, Y.-J. Kim, and safety of anticoagulant treatment in acute Updated European Heart Rhythm Association
Song, Y. D. Kim, Park, E.-G. Kim, Bang, and Ahn cardioembolic stroke: a meta-analysis of practical guide on the use of nonvitamin K
reported receiving grants from Bayer Korea Ltd randomized controlled trials. Stroke. 2007;38(2): antagonist anticoagulants in patients with
during the conduct of the study. Dr Cha reported 423-430. non-valvular atrial fibrillation. Europace. 2015;17
receiving grants from Bayer Korea Ltd during the 2. Sandercock P, Collins R, Counsell C, et al; (10):1467-1507.
conduct of the study; grants from Otsuka Korea, International Stroke Trial Collaborative Group. The 17. Abdul-Rahim AH, Fulton RL, Frank B, et al;
Bayer Korea, ESAI-Korea, Daewoong International Stroke Trial (IST): a randomised trial of VISTA Collaborators. Association of improved
Pharmaceutical Co Ltd, Daichi Sankyo, Pfizer, Sanofi aspirin, subcutaneous heparin, both, or neither outcome in acute ischaemic stroke patients with
Korea, and AstraZeneca Korea; and personal fees among 19435 patients with acute ischaemic stroke. atrial fibrillation who receive early antithrombotic
from Bayer Korea, Boehringer Ingelheim Korea, Lancet. 1997;349(9065):1569-1581. therapy: analysis from VISTA. Eur J Neurol. 2015;22
Pfizer Korea, AstraZeneca Korea, Bayer Korea, 3. CAST (Chinese Acute Stroke Trial) Collaborative (7):1048-1055.
Novartis Korea, Otsuka Korea, Pfizer Korea, and Group. CAST: randomised placebo-controlled trial 18. Paciaroni M, Agnelli G, Falocci N, et al. Early
Daichi Sankyo Korea outside the submitted work. of early aspirin use in 20,000 patients with acute recurrence and cerebral bleeding in patients with
Dr Sung reported receiving grants from Bayer Korea ischaemic stroke. Lancet. 1997;349(9066):1641-1649. acute ischemic stroke and atrial fibrillation: effect of
Ltd during the conduct of the study and personal anticoagulation and its timing: the RAF Study. Stroke.
fees from Daiichi Sankyo Korea and Pfizer 4. Berge E, Abdelnoor M, Nakstad PH, Sandset PM.
Low molecular-weight heparin versus aspirin in 2015;46(8):2175-2182.
Pharmaceuticals Korea Ltd outside the submitted
work. Dr Yoon reported receiving grants from Bayer patients with acute ischaemic stroke and atrial 19. Gioia LC, Kate M, Sivakumar L, et al. Early
Korea Ltd during the conduct of the study and fibrillation: a double-blind randomised study: rivaroxaban use after cardioembolic stroke may not
grants from Bayer HealthCare outside the HAEST Study Group: Heparin in Acute Embolic result in hemorrhagic transformation: a prospective
submitted work. Dr Seo reported receiving grants Stroke Trial. Lancet. 2000;355(9211):1205-1210. magnetic resonance imaging study. Stroke. 2016;
from Bayer Korea Ltd during the conduct of the 5. Hart RG, Palacio S, Pearce LA. Atrial fibrillation, 47(7):1917-1919.
study; grants from Korea Otsuka, Daewoong stroke, and acute antithrombotic therapy: analysis 20. Lee S, Anglade MW, Pham D, Pisacane R,
Pharmaceutical Co Ltd, Myung In Pharmaceutical of randomized clinical trials. Stroke. 2002;33(11): Kluger J, Coleman CI. Cost-effectiveness of
Co Ltd, and Korea United Pharm Inc; grants and 2722-2727. rivaroxaban compared to warfarin for stroke
personal fees from Bayer Korea, Pfizer 6. EAFT (European Atrial Fibrillation Trial) Study prevention in atrial fibrillation. Am J Cardiol. 2012;
Pharmaceuticals, Sanofi Korea, and Boehringer Group. Secondary prevention in non-rheumatic 110(6):845-851.
Ingelheim Korea; and personal fees from Daiichi
jamaneurology.com (Reprinted) JAMA Neurology Published online September 11, 2017 E9
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
Research Original Investigation Rivaroxaban vs Warfarin Sodium Early After AF-Related Mild Ischemic Stroke
21. Dorian P, Kongnakorn T, Phatak H, et al. 25. Zhao X, Sun P, Zhou Y, et al. Safety, ROCKET AF clinical trial. J Am Heart Assoc. 2013;2
Cost-effectiveness of apixaban vs current standard pharmacokinetics and pharmacodynamics of (1):e000067.
of care for stroke prevention in patients with atrial single/multiple doses of the oral, direct factor Xa 28. Wallentin L, Lopes RD, Hanna M, et al;
fibrillation. Eur Heart J. 2014;35(28):1897-1906. inhibitor rivaroxaban in healthy Chinese subjects. Br Apixaban for Reduction in Stroke and Other
22. Harrington AR, Armstrong EP, Nolan PE Jr, J Clin Pharmacol. 2009;68(1):77-88. Thromboembolic Events in Atrial Fibrillation
Malone DC. Cost-effectiveness of apixaban, 26. Wallentin L, Yusuf S, Ezekowitz MD, et al; RE-LY (ARISTOTLE) Investigators. Efficacy and safety of
dabigatran, rivaroxaban, and warfarin for stroke investigators. Efficacy and safety of dabigatran apixaban compared with warfarin at different levels
prevention in atrial fibrillation. Stroke. 2013;44(6): compared with warfarin at different levels of of predicted international normalized ratio control
1676-1681. international normalised ratio control for stroke for stroke prevention in atrial fibrillation. Circulation.
23. Shah SV, Gage BF. Cost-effectiveness of prevention in atrial fibrillation: an analysis of the 2013;127(22):2166-2176.
dabigatran for stroke prophylaxis in atrial RE-LY trial. Lancet. 2010;376(9745):975-983. 29. Shimada YJ, Yamashita T, Koretsune Y, et al.
fibrillation. Circulation. 2011;123(22):2562-2570. 27. Singer DE, Hellkamp AS, Piccini JP, et al; Effects of regional differences in Asia on efficacy
24. Magnuson EA, Vilain K, Wang K, et al; ENGAGE ROCKET AF Investigators. Impact of global and safety of edoxaban compared with
AFTIMI 48 Trial Investigators. Cost-effectiveness of geographic region on time in therapeutic range on warfarininsights from the ENGAGE AF-TIMI 48
edoxaban vs warfarin in patients with atrial warfarin anticoagulant therapy: data from the Trial. Circ J. 2015;79(12):2560-2567.
fibrillation based on results of the ENGAGE AF-TIMI
48 trial. Am Heart J. 2015;170(6):1140-1150.
E10 JAMA Neurology Published online September 11, 2017 (Reprinted) jamaneurology.com
2017 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a UNIVERSITY OF ADELAIDE LIBRARY User on 09/11/2017
S-ar putea să vă placă și
- Postoperative Critical Care for Cardiac Surgical PatientsDe la EverandPostoperative Critical Care for Cardiac Surgical PatientsÎncă nu există evaluări
- Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial FibrillationDocument18 paginiEffectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial FibrillationgaryÎncă nu există evaluări
- Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial FibrillationDocument18 paginiEffectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial FibrillationJUAN SEBASTIAN CLAVIJO CASTELLANOSÎncă nu există evaluări
- Apixaban For Patients With Atrial Fibrillation On Hemodialysis: A Multicenter Randomized Controlled TrialDocument11 paginiApixaban For Patients With Atrial Fibrillation On Hemodialysis: A Multicenter Randomized Controlled TrialMedicina UPBÎncă nu există evaluări
- STR 53 1540Document10 paginiSTR 53 1540ranggaÎncă nu există evaluări
- Effectiveness and Safety of Standard-Dose Nonvitamin K Antagonist Oral Anticoagulants and Warfarin Among Patients With Atrial Fibrillation With A Single Stroke Risk FactorDocument10 paginiEffectiveness and Safety of Standard-Dose Nonvitamin K Antagonist Oral Anticoagulants and Warfarin Among Patients With Atrial Fibrillation With A Single Stroke Risk FactorRoberto López Mata100% (1)
- Stroke Research Review Issue 27 PDFDocument4 paginiStroke Research Review Issue 27 PDFGhaidaa AlarajÎncă nu există evaluări
- Effect of Rivaroxaban Vs Enoxaparin On Major Cardiac Adverse Events and Bleeding Risk in The Acute Phase of Acute Coronary Syndrome PDFDocument13 paginiEffect of Rivaroxaban Vs Enoxaparin On Major Cardiac Adverse Events and Bleeding Risk in The Acute Phase of Acute Coronary Syndrome PDFKardiologi ManadoÎncă nu există evaluări
- Matsumoto2014 PDFDocument6 paginiMatsumoto2014 PDFNur Syamsiah MÎncă nu există evaluări
- AC After StrokeDocument6 paginiAC After StrokeDileepa chathurangaÎncă nu există evaluări
- Dual Antiplatelet Therapy Vs Alteplase For Patients With Minor NondisablingDocument10 paginiDual Antiplatelet Therapy Vs Alteplase For Patients With Minor Nondisablingbetongo Bultus Ocultus XVÎncă nu există evaluări
- Ischemic Stroke and Intracranial Hemorrhage With Aspirin, Dabigatran, and WarfarinDocument9 paginiIschemic Stroke and Intracranial Hemorrhage With Aspirin, Dabigatran, and WarfarinNur Syamsiah MÎncă nu există evaluări
- 28720644: Effects of Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease A Systematic Review and Meta-AnalysisDocument12 pagini28720644: Effects of Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease A Systematic Review and Meta-AnalysisaleksÎncă nu există evaluări
- Afire Articulo OriginalDocument11 paginiAfire Articulo OriginalElvira AnitaÎncă nu există evaluări
- Vasopressin, Steroids, and Epinephrine and Neurologically Favorable Survival After In-Hospital Cardiac Arrest A Randomized Clinical TrialDocument10 paginiVasopressin, Steroids, and Epinephrine and Neurologically Favorable Survival After In-Hospital Cardiac Arrest A Randomized Clinical Trialmonica trifitrianaÎncă nu există evaluări
- Jeong DKK, 2019Document8 paginiJeong DKK, 2019sri noviyanty yusufÎncă nu există evaluări
- Rivaroxaban Versus Apixaban For Stroke Prevention in Atrial FibrillationDocument10 paginiRivaroxaban Versus Apixaban For Stroke Prevention in Atrial FibrillationRicardo BarcoÎncă nu există evaluări
- Heparin Use in The Anterior CirculationDocument22 paginiHeparin Use in The Anterior CirculationCara MagbitangÎncă nu există evaluări
- Aca 18 565Document6 paginiAca 18 565Ketan HijauÎncă nu există evaluări
- Re LyDocument13 paginiRe LyDilawar JanÎncă nu există evaluări
- Best Vasopressor For Advanced Vasodilatory Shock Should Vasopressin Be Part of The MixDocument4 paginiBest Vasopressor For Advanced Vasodilatory Shock Should Vasopressin Be Part of The MixSurachai PraimaiÎncă nu există evaluări
- WASID Trial 2005Document12 paginiWASID Trial 2005cristhian_carvaja_13Încă nu există evaluări
- Riva Rox AbanDocument11 paginiRiva Rox AbanMr. LÎncă nu există evaluări
- Etm 14 4 3563 PDFDocument6 paginiEtm 14 4 3563 PDFUlfahÎncă nu există evaluări
- Rivaroxaban Administration After Acute Ischemic Stroke: The RELAXED StudyDocument18 paginiRivaroxaban Administration After Acute Ischemic Stroke: The RELAXED StudyLoudry ElfaÎncă nu există evaluări
- Timing of Anticoagulation After Recent Ischaemic Stroke in Patients With Atrial Fibrillation PDFDocument10 paginiTiming of Anticoagulation After Recent Ischaemic Stroke in Patients With Atrial Fibrillation PDFJulian BrownÎncă nu există evaluări
- Record 1 - THRDocument11 paginiRecord 1 - THRNorhamdan Mohd YahayaÎncă nu există evaluări
- Comparison of Dabigatran Versus Warfarin TreatmentDocument7 paginiComparison of Dabigatran Versus Warfarin TreatmentDivyesh AmarsedaÎncă nu există evaluări
- Rivaroxavan en Fa ValvularDocument5 paginiRivaroxavan en Fa Valvularcarlos pardoÎncă nu există evaluări
- Selective Serotonin Reuptake Inhibitors and Bleeding Risk in Anticoagulated Patients With Atrial FibrillationDocument9 paginiSelective Serotonin Reuptake Inhibitors and Bleeding Risk in Anticoagulated Patients With Atrial FibrillationGiancarlos GuzmanÎncă nu există evaluări
- Review Article: Vasopressin in Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Randomized Animal TrialsDocument48 paginiReview Article: Vasopressin in Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Randomized Animal TrialstendriayuÎncă nu există evaluări
- Rivaroxaban in Patients With Atrial Fibrillation and A Bioprosthetic Mitral ValveDocument11 paginiRivaroxaban in Patients With Atrial Fibrillation and A Bioprosthetic Mitral ValveJuan JoseÎncă nu există evaluări
- Atrial Fibrillation, Stroke, and Acute Antithrombotic TherapyDocument6 paginiAtrial Fibrillation, Stroke, and Acute Antithrombotic Therapyarnoldo VazquezÎncă nu există evaluări
- J Annemergmed 2018 10 001Document3 paginiJ Annemergmed 2018 10 001dandinovtiardi20Încă nu există evaluări
- ¿Que Vasopresor Retiro PrimeroDocument9 pagini¿Que Vasopresor Retiro PrimeroCeleste RamirezÎncă nu există evaluări
- Adenosina Vs VerapamiloDocument11 paginiAdenosina Vs VerapamiloJohana Zamudio RojasÎncă nu există evaluări
- Systematic Review and Network Meta-Analysis of Stroke Prevention Treatments in Patients With Atrial FibrillationDocument15 paginiSystematic Review and Network Meta-Analysis of Stroke Prevention Treatments in Patients With Atrial FibrillationAlicePastranaÎncă nu există evaluări
- Journal of Critical Care: SciencedirectDocument6 paginiJournal of Critical Care: SciencedirectJulio Castro pinedoÎncă nu există evaluări
- Cardiogenic Shock Dopamine or Norepinephrine .1Document4 paginiCardiogenic Shock Dopamine or Norepinephrine .1Diego RomeroÎncă nu există evaluări
- ClairDocument9 paginiClairMuhammad Imran MirzaÎncă nu există evaluări
- Gordon 2012Document13 paginiGordon 2012Brian Giovanni Velázquez LomeliÎncă nu există evaluări
- Acute Stroke Intervention A Systematic ReviewDocument12 paginiAcute Stroke Intervention A Systematic ReviewPaula Andrea Barreto AmadorÎncă nu există evaluări
- Comparissons NeuroanesthesiaDocument8 paginiComparissons Neuroanesthesialina yohanesÎncă nu există evaluări
- Fujiwara Et Al., 2011Document6 paginiFujiwara Et Al., 2011Giulia AndreeaÎncă nu există evaluări
- Alteplase Data 4Document9 paginiAlteplase Data 4Muhammad FirmansyahÎncă nu există evaluări
- Hirano 2017Document9 paginiHirano 2017Nur Syamsiah MÎncă nu există evaluări
- TVP y CaderaDocument6 paginiTVP y CaderaAlonso CustodioÎncă nu există evaluări
- Farmacology Del Paro CardiacoDocument14 paginiFarmacology Del Paro CardiacoNicoSwtifÎncă nu există evaluări
- Anticoagulation Management of OAC Affect Prevalence of Silent Cerebral LesionsDocument8 paginiAnticoagulation Management of OAC Affect Prevalence of Silent Cerebral LesionsDavid Santacruz PachecoÎncă nu există evaluări
- Unanswered Questions During The Live EventDocument9 paginiUnanswered Questions During The Live Eventyash_acharya007Încă nu există evaluări
- Bai 2023 Oi 231164 1697661707.60995Document12 paginiBai 2023 Oi 231164 1697661707.60995Lizeth Escobar TiconaÎncă nu există evaluări
- Oasis 5Document13 paginiOasis 5Anggita Nur Widya FebrianaÎncă nu există evaluări
- Aspirin and Aspilet Compared PDFDocument18 paginiAspirin and Aspilet Compared PDFEfrianti Viorenta HutapeaÎncă nu există evaluări
- 1 s2.0 S0929664617300487 MainDocument8 pagini1 s2.0 S0929664617300487 MainSuryati HusinÎncă nu există evaluări
- Jamaneurology - Meinel - 2023 Intravenous Thrombolysis in Patients With Ischemic Stroke and Recent Ingestion of Direct Oral AnticoagulantsDocument11 paginiJamaneurology - Meinel - 2023 Intravenous Thrombolysis in Patients With Ischemic Stroke and Recent Ingestion of Direct Oral AnticoagulantsAna Margarida J. - PsicólogaÎncă nu există evaluări
- Hayashi 2015Document5 paginiHayashi 2015Nur Syamsiah MÎncă nu există evaluări
- JRMS 17 396Document6 paginiJRMS 17 396fani rudiyantiÎncă nu există evaluări
- Graham The American Journal of MedicineDocument21 paginiGraham The American Journal of MedicinemadrugagalanfÎncă nu există evaluări
- Complementary and Alternative Medical Lab Testing Part 3: CardiologyDe la EverandComplementary and Alternative Medical Lab Testing Part 3: CardiologyEvaluare: 1 din 5 stele1/5 (1)
- Hyperglycemic CrisisDocument9 paginiHyperglycemic CrisisRoberto López MataÎncă nu există evaluări
- The Use of Cephalosporins in Penicillin-Allergic Patients - A Literature ReviewDocument9 paginiThe Use of Cephalosporins in Penicillin-Allergic Patients - A Literature ReviewRoberto López MataÎncă nu există evaluări
- Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of The 2017 American College of Cardiology:American Heart Association Hypertension GuidelineDocument10 paginiPrevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of The 2017 American College of Cardiology:American Heart Association Hypertension GuidelineRoberto López MataÎncă nu există evaluări
- 2017 ACC Expert Consensus Decision Pathway For Optimization of Heart Failure TreatmentDocument30 pagini2017 ACC Expert Consensus Decision Pathway For Optimization of Heart Failure TreatmentRoberto López MataÎncă nu există evaluări
- Atrial Fibrillation and HypertensionDocument17 paginiAtrial Fibrillation and HypertensionRoberto López MataÎncă nu există evaluări
- Diagnosis and Treatment of HyperkalemiaDocument9 paginiDiagnosis and Treatment of HyperkalemiaRoberto López MataÎncă nu există evaluări
- HTN Emergencies and UrgenciesDocument14 paginiHTN Emergencies and UrgenciesRoberto López MataÎncă nu există evaluări
- Top 10 Myths Regarding The Diagnosis and Treatment of CellulitisDocument8 paginiTop 10 Myths Regarding The Diagnosis and Treatment of CellulitisRoberto López MataÎncă nu există evaluări
- ADA 2018 Diabetes CareDocument150 paginiADA 2018 Diabetes CareRoberto López Mata100% (1)
- Update On The Management of Venous ThromboembolismDocument8 paginiUpdate On The Management of Venous ThromboembolismRoberto López MataÎncă nu există evaluări
- Necrotizing Soft-Tissue InfectionsDocument13 paginiNecrotizing Soft-Tissue InfectionsRoberto López MataÎncă nu există evaluări
- Sudden Cardiac Arrest During Participation in Competitive SportsDocument11 paginiSudden Cardiac Arrest During Participation in Competitive SportsRoberto López MataÎncă nu există evaluări
- Best Clinical Practice: AnaphylaxisDocument6 paginiBest Clinical Practice: AnaphylaxisputriÎncă nu există evaluări
- Uric Acid Is A Strong Risk Marker For Developing Hypertension From PrehypertensionDocument16 paginiUric Acid Is A Strong Risk Marker For Developing Hypertension From PrehypertensionRoberto López MataÎncă nu există evaluări
- Fluid Therapy Options and Rational SelectionDocument13 paginiFluid Therapy Options and Rational SelectionRoberto López MataÎncă nu există evaluări
- Pathophysiology of Septic ShockDocument19 paginiPathophysiology of Septic ShockRoberto López MataÎncă nu există evaluări
- The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular TherapeuticsDocument18 paginiThe Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular TherapeuticsRoberto López MataÎncă nu există evaluări
- Diuretic Treatment in Heart FailureDocument12 paginiDiuretic Treatment in Heart FailureRoberto López Mata100% (1)
- A Test in Context - D-DimerDocument10 paginiA Test in Context - D-DimerRoberto López MataÎncă nu există evaluări
- 2015 ESC Guidelines For The Diagnosis and Management of Pericardial DiseasesDocument44 pagini2015 ESC Guidelines For The Diagnosis and Management of Pericardial DiseasesRoberto López MataÎncă nu există evaluări
- Hepatitis B Vaccination, Screening, and Linkage To Care - Best Practice Advice From The American College of Physicians and The Centers For Disease Control and PreventionDocument12 paginiHepatitis B Vaccination, Screening, and Linkage To Care - Best Practice Advice From The American College of Physicians and The Centers For Disease Control and PreventionRoberto López MataÎncă nu există evaluări
- The Dark Sides of Fuid Administration in The Critically Ill PatientDocument3 paginiThe Dark Sides of Fuid Administration in The Critically Ill PatientRoberto López MataÎncă nu există evaluări
- Effects of Acarbose On Cardiovascular and Diabetes Outcomes in Patients With Coronary Heart Disease and Impaired Glucose Tolerance (ACE) - A Randomised, Double-Blind, Placebo-Controlled TrialDocument10 paginiEffects of Acarbose On Cardiovascular and Diabetes Outcomes in Patients With Coronary Heart Disease and Impaired Glucose Tolerance (ACE) - A Randomised, Double-Blind, Placebo-Controlled TrialRoberto López MataÎncă nu există evaluări
- Effects on the Incidence of Cardiovascular Events of the Addition of Pioglitazone Versus Sulfonylureas in Patients With Type 2 Diabetes Inadequately Controlled With Metformin (TOSCA.it)- A Randomised, Multicentre TrialDocument11 paginiEffects on the Incidence of Cardiovascular Events of the Addition of Pioglitazone Versus Sulfonylureas in Patients With Type 2 Diabetes Inadequately Controlled With Metformin (TOSCA.it)- A Randomised, Multicentre TrialRoberto López MataÎncă nu există evaluări
- Synopsis of The 2017 U.S. Department of Veterans Affairs: Management of Type 2 Diabetes MellitusDocument10 paginiSynopsis of The 2017 U.S. Department of Veterans Affairs: Management of Type 2 Diabetes MellitusRoberto López MataÎncă nu există evaluări
- Acute Monocular Vision LossDocument9 paginiAcute Monocular Vision LossRoberto López MataÎncă nu există evaluări
- Dual Antithrombotic Therapy With Dabigatran After PCI in Atrial FibrillationDocument12 paginiDual Antithrombotic Therapy With Dabigatran After PCI in Atrial FibrillationRoberto López MataÎncă nu există evaluări
- Evaluation and Management of Lower-Extremity UlcersDocument9 paginiEvaluation and Management of Lower-Extremity UlcersRoberto López MataÎncă nu există evaluări
- TMT Simplex 2Document78 paginiTMT Simplex 2jeetÎncă nu există evaluări
- RJPDocument38 paginiRJPjoyfullÎncă nu există evaluări
- Permanent Junctional Reciprocating Tachycardia in Infants & Children 2019Document5 paginiPermanent Junctional Reciprocating Tachycardia in Infants & Children 2019Võ Từ NhấtÎncă nu există evaluări
- Atrium FlutterDocument11 paginiAtrium FlutterSiti Maryam NatadisastraÎncă nu există evaluări
- Chapter 1.2 Algorithym of Risk StratificationDocument26 paginiChapter 1.2 Algorithym of Risk StratificationMUHAMMAD DANISH AMIN BIN BORHANUDDINÎncă nu există evaluări
- Ischemic Heart Disease (IHD)Document55 paginiIschemic Heart Disease (IHD)rameshbmc100% (5)
- Family Medicine EORDocument159 paginiFamily Medicine EORAndrew BowmanÎncă nu există evaluări
- Exercise Program For Heart Failure PatientsDocument24 paginiExercise Program For Heart Failure PatientsandrianhoerulanwarÎncă nu există evaluări
- Pathophysiology of Hypertension and Heart FailureDocument1 paginăPathophysiology of Hypertension and Heart Failurejake251996Încă nu există evaluări
- Antiarrhythmic DrugsDocument42 paginiAntiarrhythmic DrugsDr Hotimah HotimahÎncă nu există evaluări
- Cardio-Vascular ExaminationDocument43 paginiCardio-Vascular ExaminationDimas FrasesaÎncă nu există evaluări
- Circulationaha 119 043780Document2 paginiCirculationaha 119 043780Robby Paguh TariganÎncă nu există evaluări
- Congenital Heart DiseasesDocument21 paginiCongenital Heart DiseasesfahmiÎncă nu există evaluări
- Ifort Sport Fitness Screening Protocol - DR U.satyanarayanaDocument8 paginiIfort Sport Fitness Screening Protocol - DR U.satyanarayanau2satyascibdÎncă nu există evaluări
- Left Bundle Branch Block LBBBDocument5 paginiLeft Bundle Branch Block LBBBFathimah afifah zahrahÎncă nu există evaluări
- Acute Coronary Syndrome Case FileDocument4 paginiAcute Coronary Syndrome Case Filehttps://medical-phd.blogspot.comÎncă nu există evaluări
- 2018.the Right-Sided ECG For The Right DiagnosisDocument3 pagini2018.the Right-Sided ECG For The Right DiagnosisWeila dos Santos VieiraÎncă nu există evaluări
- Daftar Pustaka - Aneurisma IntrakranialDocument2 paginiDaftar Pustaka - Aneurisma IntrakranialTheofilus AswadiÎncă nu există evaluări
- ECG Rhythms Guide: Normal, Abnormal & Example TracingsDocument9 paginiECG Rhythms Guide: Normal, Abnormal & Example Tracingsdiani arisandhi100% (1)
- Contoh Soal PPDS Anak (Sub Kardiologi)Document3 paginiContoh Soal PPDS Anak (Sub Kardiologi)rezkadehaÎncă nu există evaluări
- Pathophysiology of Angina PectorisDocument2 paginiPathophysiology of Angina PectorisLeroy LoyÎncă nu există evaluări
- Atrioventricular Spetal Defect Avsd Partial EditDocument2 paginiAtrioventricular Spetal Defect Avsd Partial EditFelicia Shan SugataÎncă nu există evaluări
- Medmastery Handbook ACHD 0Document127 paginiMedmastery Handbook ACHD 0Vladlena Cucoș-Caraiman100% (1)
- The EMpret EKG Book (2020)Document28 paginiThe EMpret EKG Book (2020)nt69xmwrj9Încă nu există evaluări
- Rhy THM Cheat Sheet: Initial Question Answer Additional Question Rhythm Diagnosis 1Document1 paginăRhy THM Cheat Sheet: Initial Question Answer Additional Question Rhythm Diagnosis 1Hari KCÎncă nu există evaluări
- Introduction of EcgDocument52 paginiIntroduction of Ecgmiss_studyÎncă nu există evaluări
- 2020 ESC Guidelines For The Management of Adult Congenital Heart Disease (Previously Grown-Up Congenital Heart Disease)Document84 pagini2020 ESC Guidelines For The Management of Adult Congenital Heart Disease (Previously Grown-Up Congenital Heart Disease)Liliana BrochadoÎncă nu există evaluări
- 3-CVS-Valvular Diseases-Prof. Magdy-Student-Final (Compatibility Mode)Document11 pagini3-CVS-Valvular Diseases-Prof. Magdy-Student-Final (Compatibility Mode)أميرةÎncă nu există evaluări
- Zuñega - Congenital Heart Disease Educational MaterialsDocument13 paginiZuñega - Congenital Heart Disease Educational MaterialsAprilene Angel Balaque ZunegaÎncă nu există evaluări
- Basic Ecg: Internal Medicine Cardiology Critical Care SpecialistDocument128 paginiBasic Ecg: Internal Medicine Cardiology Critical Care SpecialistEzraManzanoÎncă nu există evaluări