Documente Academic
Documente Profesional
Documente Cultură
Labelling
Încărcat de
Tinnysumardi0 evaluări0% au considerat acest document util (0 voturi)
11 vizualizări2 paginifood
Drepturi de autor
© © All Rights Reserved
Formate disponibile
DOCX, PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentfood
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca DOCX, PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
11 vizualizări2 paginiLabelling
Încărcat de
Tinnysumardifood
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca DOCX, PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 2
TITLE 21--FOOD AND DRUGS
CHAPTER I--FOOD AND DRUG ADMINISTRATION
DEPARTMENT OF HEALTH AND HUMAN SERVICES
SUBCHAPTER B--FOOD FOR HUMAN CONSUMPTION
PART 107 INFANT FORMULA
Subpart A--General Provisions
Sec. 107.1 Status and applicability of the regulations in part 107.
(a) The criteria in subpart B of this part describe the labeling
requirements applicable to infant formula under section 403 of the
Federal Food, Drug, and Cosmetic Act (21 U.S.C 343). Failure to comply
with any regulation in subpart B of this part will render an infant
formula misbranded under section 403 of the Federal Food, Drug, and
Cosmetic Act.
(b) The criteria in subpart C of this part describe the terms and
conditions for the exemption of an infant formula from the requirements
of section 412(a), (b), and (c) of the Federal Food, Drug, and Cosmetic
Act (21 U.S.C. 350a(a), (b), and (c)). Failure to comply with any
regulations in subpart C of this part will result in withdrawal of the
exemption given under section 412(h)(1) of the Federal Food, Drug, and
Cosmetic Act.
(c) Subpart D of this part contains the nutrient requirements for
infant formula under section 412(i) of the Federal Food, Drug, and
Cosmetic Act. Failure to comply with any regulation in subpart D of
this part will render an infant formula adulterated under section
412(a)(1) of the Federal Food, Drug, and Cosmetic Act.
(d) An exempt infant formula is subject to the provisions of 107.50 and
other applicable Food and Drug Administration food regulations.
[79 FR 8074, Feb. 10, 2014]
Sec. 107.3 Definitions.
The following definitions shall apply, in addition to the definitions
contained in section 201 of the Federal Food, Drug, and Cosmetic Act
(the act):
Exempt formula. An exempt infant formula is an infant formula intended
for commercial or charitable distribution that is represented and
labeled for use by infants who have inborn errors of metabolism or low
birth weight, or who otherwise have unusual medical or dietary
problems.
Manufacturer. A person who prepares, reconstitutes, or otherwise
changes the physical or chemical characteristics of an infant formula
or packages or labels the product in a container for distribution. The
term "manufacturer" does not include a person who prepares,
reconstitutes, or mixes infant formula exclusively for an infant under
his/her direct care or the direct care of the institution employing
such person.
References. References in this part to regulatory sections of the Code
of Federal Regulations are to chapter I of title 21, unless otherwise
noted.
[50 FR 48186, Nov. 22, 1985, as amended at 79 FR 8074, Feb. 10, 2014]
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- First Periodical Test Tl.e Home EconomicsDocument3 paginiFirst Periodical Test Tl.e Home EconomicsNota Belz95% (93)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- TROUBLE SHOOTING in Biscuit IndustryDocument12 paginiTROUBLE SHOOTING in Biscuit IndustryNeuro Toxin100% (6)
- Assessment Whole Foods MarketDocument20 paginiAssessment Whole Foods MarketGerry HickmanÎncă nu există evaluări
- Posters: Posters (v1.0) by Kulsoom AyyazDocument31 paginiPosters: Posters (v1.0) by Kulsoom AyyazYeeyee AungÎncă nu există evaluări
- Biodegradability of SurfactantsDocument1 paginăBiodegradability of SurfactantsTinnysumardiÎncă nu există evaluări
- Federal Register / Vol. 60, No. 183 / Thursday, September 21, 1995 / Proposed RulesDocument2 paginiFederal Register / Vol. 60, No. 183 / Thursday, September 21, 1995 / Proposed RulesTinnysumardiÎncă nu există evaluări
- Calibration, Validation and Verification of Equipment, Instruments and Other DevicesDocument2 paginiCalibration, Validation and Verification of Equipment, Instruments and Other DevicesTinnysumardiÎncă nu există evaluări
- Zoning in Processing Facilities 375003 7Document2 paginiZoning in Processing Facilities 375003 7TinnysumardiÎncă nu există evaluări
- Minireview Escherichia Coli in The Environment: Implications For Water Quality and Human HealthDocument8 paginiMinireview Escherichia Coli in The Environment: Implications For Water Quality and Human HealthTinnysumardiÎncă nu există evaluări
- Rlu PDFDocument7 paginiRlu PDFTinnysumardiÎncă nu există evaluări
- Escherichia Coli O157:H7 Issues and Ramifications: Executive SummaryDocument12 paginiEscherichia Coli O157:H7 Issues and Ramifications: Executive SummaryTinnysumardiÎncă nu există evaluări
- BookDocument155 paginiBookTinnysumardiÎncă nu există evaluări
- Escherichia Coli O157:H7 Strains Isolated From EnvironmentalDocument7 paginiEscherichia Coli O157:H7 Strains Isolated From EnvironmentalTinnysumardiÎncă nu există evaluări
- Monitoring Suhu Dan RH WH Starch 2Document4 paginiMonitoring Suhu Dan RH WH Starch 2TinnysumardiÎncă nu există evaluări
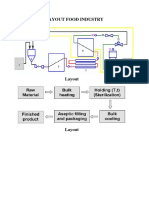
- Layout Food IndustryDocument2 paginiLayout Food IndustryTinnysumardiÎncă nu există evaluări
- Infant Formula RecallDocument4 paginiInfant Formula RecallTinnysumardiÎncă nu există evaluări
- International Formula Council: Formerly The Enteral Nutrition Council and Infant Formula CouncilDocument6 paginiInternational Formula Council: Formerly The Enteral Nutrition Council and Infant Formula CouncilTinnysumardiÎncă nu există evaluări
- Speaking DialogDocument2 paginiSpeaking DialogNovityasaÎncă nu există evaluări
- Daftar Harga PT Gloria Bisco 2Document3 paginiDaftar Harga PT Gloria Bisco 2Lalu Unggul UmbaliÎncă nu există evaluări
- Purine ListDocument9 paginiPurine Listdidodido_67Încă nu există evaluări
- FSM 5 Food Processing Module 3 AmponganDocument5 paginiFSM 5 Food Processing Module 3 AmponganShim EdwardÎncă nu există evaluări
- Grocery Products ListDocument2 paginiGrocery Products ListManoj PatelÎncă nu există evaluări
- IRDDocument2 paginiIRDasankapanawala80Încă nu există evaluări
- TLE MELCs Grade 7 PDFDocument55 paginiTLE MELCs Grade 7 PDFVincent Emmanuel Salibio40% (5)
- Nutrition Analysis ProjectDocument7 paginiNutrition Analysis Projectapi-268293649Încă nu există evaluări
- Body Composition Lab Report-CompleteDocument4 paginiBody Composition Lab Report-CompleteEmmae ThaleenÎncă nu există evaluări
- Cycling NutritionDocument29 paginiCycling NutritionPatriciaÎncă nu există evaluări
- Episode Guide - Jamie 30 Minutes MealsDocument9 paginiEpisode Guide - Jamie 30 Minutes MealsViviana MoreiraÎncă nu există evaluări
- Presenting DessertDocument46 paginiPresenting DessertAlyssa AragonÎncă nu există evaluări
- Organic ENDocument135 paginiOrganic ENAlbert ManingoÎncă nu există evaluări
- Chantypak Recipes Tcm293 71431Document24 paginiChantypak Recipes Tcm293 71431Elizabeth IbañezÎncă nu există evaluări
- Neves 2018Document6 paginiNeves 2018Maryanne CunhaÎncă nu există evaluări
- A Family Affair: Jennifer Mason Samples The Hearty Italian Fare at Enoteca Turi in PutneyDocument1 paginăA Family Affair: Jennifer Mason Samples The Hearty Italian Fare at Enoteca Turi in Putneyapi-238854528Încă nu există evaluări
- Social Forestry (Final)Document26 paginiSocial Forestry (Final)Asad ZamanÎncă nu există evaluări
- Bali Bakery Menu Hayam Wuruk 202204Document7 paginiBali Bakery Menu Hayam Wuruk 202204jayanti wulanÎncă nu există evaluări
- Super Hero Nutrition Calculator - Phase 1Document8 paginiSuper Hero Nutrition Calculator - Phase 1bhaswath2000100% (1)
- Home Bawdy: Booty On Phat + Waist On Flat 8 Week Home GuideDocument66 paginiHome Bawdy: Booty On Phat + Waist On Flat 8 Week Home GuideLeena Franklin100% (1)
- Manufacturing of Flavoured Milk: Submitted byDocument15 paginiManufacturing of Flavoured Milk: Submitted byDipu AhirÎncă nu există evaluări
- Mini Project - CashewDocument16 paginiMini Project - CashewNihar PandithÎncă nu există evaluări
- Jifood and Beverage Assessment - Test 3 (: U3 CamacDocument11 paginiJifood and Beverage Assessment - Test 3 (: U3 CamacGlobal AccountÎncă nu există evaluări
- Overview of Malnutrition in EmergenciesDocument9 paginiOverview of Malnutrition in EmergenciesAndry YonathaÎncă nu există evaluări
- En DellaNonna Menu Ver.1.1 2023Document17 paginiEn DellaNonna Menu Ver.1.1 2023JuanÎncă nu există evaluări
- Creamy Alugbati Con Quezo QuicheDocument34 paginiCreamy Alugbati Con Quezo QuicheAntonio Miguel DizonÎncă nu există evaluări