Documente Academic
Documente Profesional
Documente Cultură
Soil Nitrate SQ Chemical Indicator Sheet
Încărcat de
Sridhar KrishnamurthiTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Soil Nitrate SQ Chemical Indicator Sheet
Încărcat de
Sridhar KrishnamurthiDrepturi de autor:
Formate disponibile
Indicator Test Function
USDA Natural Resources Conservation Service C F W
Soil Quality Indicators
Soil Nitrate
issues. Climate (temperature and precipitation) affects the
rate of nitrification; the optimum temperature is between
86 and 95 degrees F. Poor soil drainage creates an ideal
Nitrate (NO3-) is a form of inorganic nitrogen (N) biochemical environment for denitrification and the release
naturally occurring in soils. Sources of soil NO3- include of gaseous NOx into the atmosphere, which is exacerbated
decomposing plant residues and animal manure/compost, in carbon-rich soils (e.g., wetlands or plugged tile drains).
chemical fertilizers, exudates from living plants, rainfall, Soil NO3- is sensitive to seasonal fluctuations and crop
and lightning. Eventually, nitrate ions immobilized by presence. Figure 1 shows that that the amount of NO3-
microorganisms (nitrate taken up by microorganisms) are increases as the soil warms in temperate regions (May and
converted into organic forms and released back to the soil June) and then, because of NO3- leaching during the rainy
in plant-available forms when dead soil organisms are season, sharply decreases in autumn.
fed upon or decompose. In well drained soils, ammonium
(NH4+) and ammonia (NH3) are converted into NO3 by
very specific populations of aerobic bacteria. This process
is known as nitrification.
Another biological N transformation is denitrification,
which is the conversion of NO3- into nitrous oxide (N2O),
nitrogen dioxide (NO2), and nitrogen gas (N2) that often
occurs in anaerobic soils, such as waterlogged soils and
wetlands. Even when nitrifying bacteria are very active
in the outer parts of aggregates in well aerated soils,
denitrification may still occur in anaerobic microsites
inside the aggregates. Nitrate is very soluble in water and Figure 1. Typical seasonal pattern of nitrate concentration
can be easily transported by runoff and other surface and in representative surface soil layers with and without
subsurface flows to rivers and lakes or moved downward growing plants for soils typical in humid temperate regions
to ground water. that have cool winters and rainfall uniformly distributed
throughout the year (based on Brady and Weil, 1996).
Factors Affecting Dynamic Nitrate is usually deficient in acid
soils because low soil pH (<5.5) reduces nitrification.
Inherent Soil texture influences NO3- mobility: the Nitrification ceases at pH <4.5, and the optimum pH is
coarser the soil texture (sandy), the faster NO3- leaches
between 6 and 8. Because organic matter is an important
through the soil profile because NO3- ions do not bind to
source of NO3-, accumulation may correlate with organic
sandy particles and water infiltration rates are typically
matter content patterns across the landscape. Moreover,
very high in sandy soils. Under heavy rain, NO3- in
nitrification depends on microorganisms, which proliferate
those soils can eventually leach and contaminate the
in the presence of organic matter. Residues with high
ground water. Less weathered types of clay minerals
C:N ratio (>24/1) slow down the release of NO3- from
(e.g., montmorillonite, illite, vermiculite) have lower
organic matter; microorganisms initially immobilize all
NO3- retention than very weathered clay minerals (e.g.,
available NO3--N from soil for their growth. This delays
kaolinite) found in tropical and subtropical soils. Soils
the decomposition of organic matter and the associated
with a high CEC do not retain NO3-. These soils, such as
nitrification.
those in the Mississippi River basin, have major leaching
Helping People Help the Land
Relationship to Soil Function Application of materials that slowly release nitrogen
Planting cover crop species that use residual NO3-
The primary function of NO3- is to serve as a source of Planning the timing and rates of irrigation according to
nitrogen for the nutrition and growth of plants and soil site water content
microorganisms.
Keeping the soil well drained
Additions of green manure with a high C/N ratio
Problems with Poor Activity
Denitrification results in nitrogen loss from soil and
produces some forms of intermediate gaseous nitrogen
(e.g., N2O) that are harmful to the environment. Problems
associated with high NO3- concentration include the
pollution of ground water and surface water and an
increased risk of eutrophication that threatens the survival
of aquatic life. Nitrification can potentially result in soil
acidification by hydrogen ions (H+) released during the
process.
Improving Management Figure 2. The effects of ammonium nitrate
and tillage (=no-till; =conventional-till;
In a study conducted at the University of Maryland 0X=no manure) on nitrate concentrations
of soil collected to a depth of 210 cm during
Research Center, soil NO3- concentrations at any depth
the spring of 1986 (after Angle et al., 1993).
(except 0-30 cm) have been found to be consistently lower
in no-till plots than in conventional-till plots (see Figure2)
and were related to the amount of N fertilizer applied.
The explanations by the authors of the study include: Measuring Nitrate
(i) the lack of a winter cover crop on the conventional
till plots affected the soil N content in the root zone and Soil NO3- is measured in the field using a test strip as
the subsequent rates of nitrate leaching; (ii) the no-till described in the Soil Quality Test Kit Guide. Measurement
plots had higher rates of denitrification compared to takes just 15 minutes. The test strip has two pads, one
the conventional-till plots (i.e., higher populations of to measure nitrite (NO2-) and the other to measure NO2-/
denitrifying organisms in no-till); (iii) crops in no-till plots NO3- combined. This is not intended to be a substitute for a
used N more efficiently (removal of more N from soil); fertilizer recommendation but is an indication of potential
and (iv) the conventional till plots had an accumulation of management remediation needs.
nitrate from the plant residues of previous years.
The following practices add nitrate: References
Angle, J.S., C.M. Gross, R.L. Hill, and M.S. McIntosh. 1993. Soil
Crop rotations with legumes nitrate concentrations under corn as affected by tillage, manure, and
Addition of organic residues, manure, and compost fertilizer applications. Journal of Environmental Quality 22:141-147.
Conservation tillage and field strips or no-till with a Brady, N.C., and R.R. Weil. 1996. The nature and properties of soils.
Prentice-Hall.
winter cover crop
Split applications of fertilizer that match crop growth Cabrera, M., and others. 2008. Modeling the nitrogen cycle. In Schepers
and Raun (eds.) Nitrogen in Agricultural Systems. American Society of
stages Agronomy Monograph 40.
The following practices prevent nitrate loss: Dick, R.P., R.A. Christ, J.D. Istok, and F. Iyamuremye. 2000. Nitrogen
fractions and transformations of vadose zone sediments under intensive
Autumn applications of ammonium-based fertilizer on agriculture in Oregon. Soil Science 165:505-515.
frozen soils
USDA is an equal opportunity provider and employer. January 2014
S-ar putea să vă placă și
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Breaking Down Adoption BarriersDocument12 paginiBreaking Down Adoption BarriersSridhar KrishnamurthiÎncă nu există evaluări
- Fldigi HelpDocument534 paginiFldigi HelpSridhar KrishnamurthiÎncă nu există evaluări
- A Tutorial On The DecibelDocument8 paginiA Tutorial On The DecibelSara HerzÎncă nu există evaluări
- Proposed Openemr Office Visit Workflow: Lab MessagingDocument1 paginăProposed Openemr Office Visit Workflow: Lab MessagingSridhar KrishnamurthiÎncă nu există evaluări
- FT8 Hinson Tips For HF DXersDocument81 paginiFT8 Hinson Tips For HF DXersSridhar KrishnamurthiÎncă nu există evaluări
- Lotw New User Guide: Get Going With Logbook of The WorldDocument37 paginiLotw New User Guide: Get Going With Logbook of The WorldSridhar KrishnamurthiÎncă nu există evaluări
- NCHER Knowledge Symposium Federal Contractor/TPS Session: in Ation Security & Risk Management ATO & FISMA Compliance 2017Document12 paginiNCHER Knowledge Symposium Federal Contractor/TPS Session: in Ation Security & Risk Management ATO & FISMA Compliance 2017Sridhar KrishnamurthiÎncă nu există evaluări
- Enisa - Metodologia para Avaliação de Gravidade de Violação de DadosDocument27 paginiEnisa - Metodologia para Avaliação de Gravidade de Violação de DadosWellington Watanabe FilhoÎncă nu există evaluări
- Getting Started With PSK31 and JT65 - HandoutDocument2 paginiGetting Started With PSK31 and JT65 - HandoutSridhar KrishnamurthiÎncă nu există evaluări
- Automate Response: Did You Know?Document10 paginiAutomate Response: Did You Know?Sridhar KrishnamurthiÎncă nu există evaluări
- Telemedicine BrochureDocument5 paginiTelemedicine BrochureSridhar KrishnamurthiÎncă nu există evaluări
- Introduction To Octave/Matlab: Deployment of Telecommunication InfrastructuresDocument13 paginiIntroduction To Octave/Matlab: Deployment of Telecommunication InfrastructuresSridhar KrishnamurthiÎncă nu există evaluări
- RfaxDocument130 paginiRfaxSridhar KrishnamurthiÎncă nu există evaluări
- Personal Data Breach & Incident Handling ProcedureDocument11 paginiPersonal Data Breach & Incident Handling ProcedureSanda NechiforÎncă nu există evaluări
- A Tutorial On The DecibelDocument8 paginiA Tutorial On The DecibelSara HerzÎncă nu există evaluări
- FT817 DIY Data Modes InterfaceDocument5 paginiFT817 DIY Data Modes InterfaceSridhar KrishnamurthiÎncă nu există evaluări
- Incident Response - Lessons From The Front LinesDocument33 paginiIncident Response - Lessons From The Front LinesSridhar KrishnamurthiÎncă nu există evaluări
- Icom Comicbookv1colorDocument24 paginiIcom Comicbookv1colorRejaÎncă nu există evaluări
- Price List NISCAIR PublicationsDocument16 paginiPrice List NISCAIR PublicationsSajal SahaÎncă nu există evaluări
- Syllabus - BME 694-794 - Drug Design and DevelopmentDocument2 paginiSyllabus - BME 694-794 - Drug Design and DevelopmentSridhar Krishnamurthi100% (1)
- Touch and See Your Solution: Finding Solutions For Your Problems in Bhagvad GitaDocument1 paginăTouch and See Your Solution: Finding Solutions For Your Problems in Bhagvad Gitaanu50% (2)
- Icom Comicbookv2colorDocument28 paginiIcom Comicbookv2colorRejaÎncă nu există evaluări
- The FOC Guide To Morse Code Proficiency: Gary Hinson, ZL2iFBDocument15 paginiThe FOC Guide To Morse Code Proficiency: Gary Hinson, ZL2iFBSridhar KrishnamurthiÎncă nu există evaluări
- A Tutorial On The DecibelDocument8 paginiA Tutorial On The DecibelSara HerzÎncă nu există evaluări
- High Frequency Voice Broadcasts: PurposeDocument5 paginiHigh Frequency Voice Broadcasts: PurposeSridhar KrishnamurthiÎncă nu există evaluări
- Lotw New User Guide: Get Going With Logbook of The WorldDocument37 paginiLotw New User Guide: Get Going With Logbook of The WorldSridhar KrishnamurthiÎncă nu există evaluări
- Granite Temple Brochure 4.0Document2 paginiGranite Temple Brochure 4.0Sridhar KrishnamurthiÎncă nu există evaluări
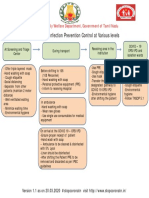
- TNSOP 5.0 Infection Prevention Control at Various Levels PDFDocument1 paginăTNSOP 5.0 Infection Prevention Control at Various Levels PDFSridhar KrishnamurthiÎncă nu există evaluări
- FT8 Hinson Tips For HF DXersDocument81 paginiFT8 Hinson Tips For HF DXersSridhar KrishnamurthiÎncă nu există evaluări
- 2019 Sohan Sensors PDFDocument9 pagini2019 Sohan Sensors PDFSridhar KrishnamurthiÎncă nu există evaluări
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (120)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- APES Erosion LabDocument3 paginiAPES Erosion Labawswenson13Încă nu există evaluări
- CVE 415 Soil ClassificationDocument17 paginiCVE 415 Soil Classificationoluwatunmise babatundeÎncă nu există evaluări
- Pennsylvania BMP Manual (Bookmarked)Document681 paginiPennsylvania BMP Manual (Bookmarked)jkg188100% (1)
- Irrigation EngineeringDocument26 paginiIrrigation EngineeringUmar NaveedÎncă nu există evaluări
- Report On Reuse of Abandoned Quarries and Mine Pits in KeralaDocument90 paginiReport On Reuse of Abandoned Quarries and Mine Pits in KeralaDrThrivikramji Kyth100% (10)
- (A) 90% Consolidation Settlement, (B) A Settlement of 100 MMDocument3 pagini(A) 90% Consolidation Settlement, (B) A Settlement of 100 MMاسامه محمدÎncă nu există evaluări
- Vermicomposting Presentation - Pamela T. HenaresDocument46 paginiVermicomposting Presentation - Pamela T. HenaresCk_psih100% (1)
- CompactionDocument28 paginiCompactionsitimarlinnachuÎncă nu există evaluări
- Navfac 7.1-2005Document391 paginiNavfac 7.1-2005Saed Asiabi100% (1)
- Role of Pore Water Pressures in Embankment Stability PDFDocument6 paginiRole of Pore Water Pressures in Embankment Stability PDFfaroeldrÎncă nu există evaluări
- Eni Et AlDocument4 paginiEni Et AlHem ShahiÎncă nu există evaluări
- Lancashire Building Stone AtlasDocument21 paginiLancashire Building Stone AtlasRichardÎncă nu există evaluări
- Geotechnical Engineering - Ii (Foundation Engineering) : Part 1 Chapter 1 Introduction To ContractsDocument37 paginiGeotechnical Engineering - Ii (Foundation Engineering) : Part 1 Chapter 1 Introduction To ContractsPascasio PascasioÎncă nu există evaluări
- Graphical Log 1: Proposed Manhole For 60 MLD Valenzuela STPDocument1 paginăGraphical Log 1: Proposed Manhole For 60 MLD Valenzuela STPBilly DÎncă nu există evaluări
- PirometalurgiaDocument12 paginiPirometalurgiaElvis Alanya TinocoÎncă nu există evaluări
- A Study On The Concentration Tests and Beneficiation of The Uludağ Tungsten OreDocument28 paginiA Study On The Concentration Tests and Beneficiation of The Uludağ Tungsten OreravibelavadiÎncă nu există evaluări
- Small FryDocument9 paginiSmall FryTimea MravikÎncă nu există evaluări
- Strength Characteristics of Fly Ash Mixed With Lime Stabilized SoilDocument4 paginiStrength Characteristics of Fly Ash Mixed With Lime Stabilized SoilCosminÎncă nu există evaluări
- Stratigraphy of IndiaDocument109 paginiStratigraphy of IndiaVaibhav TanwarÎncă nu există evaluări
- Soil Investigation Report For The Proposed Residential Building Construction PDFDocument32 paginiSoil Investigation Report For The Proposed Residential Building Construction PDFParvez KhanÎncă nu există evaluări
- EarthworkDocument20 paginiEarthworkJonathanÎncă nu există evaluări
- Các giải pháp gia cố nền đất yếu-Fico coreaDocument33 paginiCác giải pháp gia cố nền đất yếu-Fico coreaTri huỳnhÎncă nu există evaluări
- How We Used To Live in Burnley: Lime HushingDocument104 paginiHow We Used To Live in Burnley: Lime HushingDBarraclough100% (1)
- Kibali Gold Mine ProjectDocument388 paginiKibali Gold Mine ProjectCatalina Luna100% (1)
- Phosphate Rocks (Ptacek 2016)Document48 paginiPhosphate Rocks (Ptacek 2016)Laura JohnsonÎncă nu există evaluări
- Soil Forming ProcessesDocument3 paginiSoil Forming ProcessesWanambwa SilagiÎncă nu există evaluări
- SUBSURFACE WATER PPT - Hydrology ReportDocument11 paginiSUBSURFACE WATER PPT - Hydrology ReportMj AustriaÎncă nu există evaluări
- The California Bearing Ratio: Aashto T 193 / Astm D 1883Document13 paginiThe California Bearing Ratio: Aashto T 193 / Astm D 1883Arham SheikhÎncă nu există evaluări
- Lesson 2 MINERAL AGGREGATESDocument10 paginiLesson 2 MINERAL AGGREGATESDeniell Kahlil Kyro GabonÎncă nu există evaluări