Documente Academic
Documente Profesional
Documente Cultură
Znco HNO Gas Y X H O Heating Crystallisation: Cut It Short
Încărcat de
NHani IderisTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Znco HNO Gas Y X H O Heating Crystallisation: Cut It Short
Încărcat de
NHani IderisDrepturi de autor:
Formate disponibile
CUT IT SHORT
(a) Diagram 8.2 shows the reaction scheme of zinc carbonate.
ZnCO3 + HNO3 X + Gas Y + H2 O
Heating Crystallisation
Z + Gas Y Salt X
Diagram 8.2
Zinc carbonate reacts with nitric acid to produced salt X, gas Y and water. Gas Y turns lime water chalky.
(i) Based on Diagram 8.2, identify salt X, gas Y and Substance Z.
[3 marks]
(ii) Describe briefly chemical tests to verify. The cation and anion in substance X.
[4 marks]
(iii) Excess zinc carbonate is added to 100 cm3 of 1.0 mol dm-3 of nitric acid.
Write the chemical equation for the reaction and calculate the volume of gas Y
produced at room condition.
[5 marks]
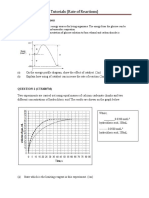
QUESTION 9
A group of students carried out three experiments to investigate the factors affecting the rate of reaction
between hydrochloric acid and zinc.
Table 8.1 shows the results of the experiments.
Time taken to collect 40
Experiment Reactants
cm3 of hydrogen gas (s)
50 cm3 of 1.0 mol dm-3 hydrochloric acid +
I zinc granule + a few drops of copper(II) 90
sulphate solution
50 cm of 1.0 mol dm-3 hydrochloric acid +
3
II zinc granule 150
50 cm3 of 0.5 mol dm-3 hydrochloric acid +
III zinc granule 270
Based on Table 8.1, compare the rate of reaction between
Experiment I and Experiment II
Experiment II and Experiment III
Explain the difference in the rate of reaction based on the Collision Theory.
[10 marks]
S-ar putea să vă placă și
- RTS Chemistry SPM Question Bank Chapter 10Document8 paginiRTS Chemistry SPM Question Bank Chapter 10Scorched ZenÎncă nu există evaluări
- RTS Chemistry SPM Question Bank Chapter 10Document8 paginiRTS Chemistry SPM Question Bank Chapter 10dobbybibiÎncă nu există evaluări
- The Meaning of The Rate of Reaction Two Factors That Affect The Rate of ReactionDocument2 paginiThe Meaning of The Rate of Reaction Two Factors That Affect The Rate of ReactionKlvin TeeÎncă nu există evaluări
- 1996 2009 Kcse Chemistry 1Document177 pagini1996 2009 Kcse Chemistry 1W GÎncă nu există evaluări
- Kcse Chemistry Marking SchemeDocument174 paginiKcse Chemistry Marking SchemeDavid Musila ToywaÎncă nu există evaluări
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDocument29 paginiChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanÎncă nu există evaluări
- Marking Scheme AssayDocument5 paginiMarking Scheme AssaySmoi TaiÎncă nu există evaluări
- Bengkel Ambang SPM 2009paper 2Document31 paginiBengkel Ambang SPM 2009paper 2Mimi MaliniÎncă nu există evaluări
- Rates of Reaction o LevelDocument19 paginiRates of Reaction o Leveljosephmadrara2Încă nu există evaluări
- As Level Chemistry Amount of Substance 1. Ammonium Sulfate Reacts With Sodium Hydroxide To Form Ammonia, Sodium Sulfate and Water As ShownDocument5 paginiAs Level Chemistry Amount of Substance 1. Ammonium Sulfate Reacts With Sodium Hydroxide To Form Ammonia, Sodium Sulfate and Water As Shownnan doeÎncă nu există evaluări
- Experiment 3 LabrepDocument10 paginiExperiment 3 LabrepDI LacsonÎncă nu există evaluări
- AP Chemistry Unit 2 Packet 3Document3 paginiAP Chemistry Unit 2 Packet 3Brandon BaxterÎncă nu există evaluări
- 5 Set Model Question - Chemistry (112) - Sci XI - UGHSSDocument10 pagini5 Set Model Question - Chemistry (112) - Sci XI - UGHSSSachin ChakradharÎncă nu există evaluări
- Chemistry SPM State Trial Papers-Form5chap1Document17 paginiChemistry SPM State Trial Papers-Form5chap1Law Jin YaoÎncă nu există evaluări
- Rate of ReactionQP2020Document5 paginiRate of ReactionQP2020Yash TandonÎncă nu există evaluări
- Test 0N Redox EquilibriaDocument5 paginiTest 0N Redox EquilibriaLittle WizardÎncă nu există evaluări
- Rate of Reaction 6 QPDocument9 paginiRate of Reaction 6 QPmalak tahaÎncă nu există evaluări
- Chemistry Form 4 PP2Document12 paginiChemistry Form 4 PP2jmwalimu81Încă nu există evaluări
- 2010 Chemistry P1 QuestionsDocument7 pagini2010 Chemistry P1 QuestionsBensonÎncă nu există evaluări
- BIO1103PE1Document6 paginiBIO1103PE1bambi leeÎncă nu există evaluări
- S.5 P525 Chemistry 2 EOT1-2Document6 paginiS.5 P525 Chemistry 2 EOT1-2Talemwa ALFRED KAKORAKIÎncă nu există evaluări
- Experiment Reactants Condition of Reaction: SMK Kepong Kimia Tingkatan 5 Cube 1Document1 paginăExperiment Reactants Condition of Reaction: SMK Kepong Kimia Tingkatan 5 Cube 1Klvin TeeÎncă nu există evaluări
- Acids, Bases - Salts 4 QPDocument8 paginiAcids, Bases - Salts 4 QPANIKA DHANIKACHALAMÎncă nu există evaluări
- S6 Chepre Reg2Document3 paginiS6 Chepre Reg2ANYWAR SIMONÎncă nu există evaluări
- Questions and Answers Chem Paper 1 2021Document13 paginiQuestions and Answers Chem Paper 1 2021PremierLeagueÎncă nu există evaluări
- 8-18.1 Revision TestDocument14 pagini8-18.1 Revision Testku haÎncă nu există evaluări
- Class Test 1 Rate of Reaction For Edexcel A2 ChemistryDocument7 paginiClass Test 1 Rate of Reaction For Edexcel A2 Chemistryjeffydaniel1972Încă nu există evaluări
- Section B: Structured Questions (65 Marks) : F.4 Chemistry Final Exam (2010-2011)Document8 paginiSection B: Structured Questions (65 Marks) : F.4 Chemistry Final Exam (2010-2011)harrynghomanÎncă nu există evaluări
- Pharm AnalysisDocument6 paginiPharm AnalysisMirumbi Kefa MomanyiÎncă nu există evaluări
- Revision STPM Term 1Document15 paginiRevision STPM Term 1Wong WengSiongÎncă nu există evaluări
- CHEMISTRY-PP2-Form-4-END TERMDocument10 paginiCHEMISTRY-PP2-Form-4-END TERMKevinÎncă nu există evaluări
- Chem pp1Document9 paginiChem pp1ewawireÎncă nu există evaluări
- Rates of ReactionDocument77 paginiRates of ReactionFatema KhatunÎncă nu există evaluări
- A Level Chemistry Paper 2 Exam 5Document5 paginiA Level Chemistry Paper 2 Exam 5Anthony AndyÎncă nu există evaluări
- 01 Kadar Tindak Balas Soalan StrukturDocument7 pagini01 Kadar Tindak Balas Soalan StrukturAzalida Md YusofÎncă nu există evaluări
- Chem PP1 MSDocument9 paginiChem PP1 MSianmutwiriÎncă nu există evaluări
- Chemistry Mocks 2016 Questions Champions ExamsDocument383 paginiChemistry Mocks 2016 Questions Champions ExamskandeabigaelÎncă nu există evaluări
- 2811 Jan 01MSDocument10 pagini2811 Jan 01MSThatchani GundasamyÎncă nu există evaluări
- Sem1 Unit1 MatterDocument9 paginiSem1 Unit1 Mattershehdilanun100% (2)
- Chapter 1 Reaction KineticsDocument8 paginiChapter 1 Reaction KineticsDinesh RamaÎncă nu există evaluări
- 3 - Chemical Cells and ElectrolysisDocument6 pagini3 - Chemical Cells and Electrolysisapi-3700944100% (1)
- Chapter 1: Moles and Equations: Homework QuestionsDocument2 paginiChapter 1: Moles and Equations: Homework QuestionsareebÎncă nu există evaluări
- Of Hydroge N Gas (CM) V 2V III II: SPM 2010/paper 2/ Section B/ Question 8: Rate of ReactionDocument1 paginăOf Hydroge N Gas (CM) V 2V III II: SPM 2010/paper 2/ Section B/ Question 8: Rate of ReactionSaya RizalÎncă nu există evaluări
- Electron Configuration and Periodicity - Paper 2Document30 paginiElectron Configuration and Periodicity - Paper 2elenaÎncă nu există evaluări
- ALEVELREVISIONQUESTIONSDocument7 paginiALEVELREVISIONQUESTIONSAnthony AndyÎncă nu există evaluări
- 4003 Chemistry Section Topic by TopicDocument32 pagini4003 Chemistry Section Topic by Topicpercymtetwa25Încă nu există evaluări
- Chemistry QuestionsDocument6 paginiChemistry Questionshotbytecyber991Încă nu există evaluări
- Acids and Bases - HL - 002: (153 Marks)Document36 paginiAcids and Bases - HL - 002: (153 Marks)VedantÎncă nu există evaluări
- VVDocument5 paginiVVLisaam De YesteÎncă nu există evaluări
- TTTTDocument1 paginăTTTTAnonymous BT988qyÎncă nu există evaluări
- NSS Chemistry Part 3 Metals - LQDocument25 paginiNSS Chemistry Part 3 Metals - LQNicole ChanÎncă nu există evaluări
- Chapter-1 & 2 W.SDocument3 paginiChapter-1 & 2 W.SAslanÎncă nu există evaluări
- Chemistry Take-Home Test-Set A, 2020. Answer All Questions. Q. 1 Solid Sodium Carbonate Reacts With Aqueous Hydrochloric Acid To Form Aqueous SodiumDocument2 paginiChemistry Take-Home Test-Set A, 2020. Answer All Questions. Q. 1 Solid Sodium Carbonate Reacts With Aqueous Hydrochloric Acid To Form Aqueous SodiumMuyatwa LiksÎncă nu există evaluări
- Tutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Document7 paginiTutorials (Rate of Reactions) : QUESTION 1 (2006 CT3 Jan)Subesh ShanmugamÎncă nu există evaluări
- IB Chemistry: Unit 4 Stoichiometry QuestionsDocument37 paginiIB Chemistry: Unit 4 Stoichiometry QuestionsmjohnmccÎncă nu există evaluări
- MRSM Chemistry Trial Paper 2 Marking SchemeDocument7 paginiMRSM Chemistry Trial Paper 2 Marking SchemeRayChinÎncă nu există evaluări
- Chemistry Mocks Set 1Document167 paginiChemistry Mocks Set 1Citron AkhalaÎncă nu există evaluări
- CHAP 1.pmd4Document3 paginiCHAP 1.pmd4Ezhil CÎncă nu există evaluări
- Chem Topic 8 PracticeDocument8 paginiChem Topic 8 Practicekakahem246Încă nu există evaluări
- Industrial Chemistry of Oxides for Emerging ApplicationsDe la EverandIndustrial Chemistry of Oxides for Emerging ApplicationsÎncă nu există evaluări
- YLMYCollection Form VODocument1 paginăYLMYCollection Form VONHani IderisÎncă nu există evaluări
- Znco HNO Gas Y X H O Heating Crystallisation: Cut It ShortDocument1 paginăZnco HNO Gas Y X H O Heating Crystallisation: Cut It ShortNHani IderisÎncă nu există evaluări
- Keyword and PenDocument6 paginiKeyword and PenNHani IderisÎncă nu există evaluări
- Keyword and PenDocument6 paginiKeyword and PenNHani IderisÎncă nu există evaluări
- Acid Base Titration ExperimentDocument2 paginiAcid Base Titration ExperimentNHani IderisÎncă nu există evaluări
- Application in Daily Life Activities HandoutDocument5 paginiApplication in Daily Life Activities HandoutNHani IderisÎncă nu există evaluări
- 1Document1 pagină1NHani IderisÎncă nu există evaluări
- 2 Heat of PrecipitationDocument21 pagini2 Heat of PrecipitationNHani Ideris100% (1)
- Isomers of PenteneDocument1 paginăIsomers of PenteneNHani IderisÎncă nu există evaluări
- Thermodynamic Equilibrium For The Esterification of Acrylic Acid With Different Alcohols Catalyzed by Ion Exchange ResinDocument2 paginiThermodynamic Equilibrium For The Esterification of Acrylic Acid With Different Alcohols Catalyzed by Ion Exchange Resin1995CLÎncă nu există evaluări
- Technical Data Sheet: For Further Information Regarding This Product Please Refer To: Tel: +49 6108 919 394Document1 paginăTechnical Data Sheet: For Further Information Regarding This Product Please Refer To: Tel: +49 6108 919 394sarhan yasmenÎncă nu există evaluări
- Synthesis, Characterization and Pharmacological of Metal Complexes From Chalcones.Document6 paginiSynthesis, Characterization and Pharmacological of Metal Complexes From Chalcones.KassimÎncă nu există evaluări
- Biomolecules PDFDocument21 paginiBiomolecules PDFVedant SalgudeÎncă nu există evaluări
- Ul 746B 2011 PDFDocument56 paginiUl 746B 2011 PDFShibu1992Încă nu există evaluări
- Agro Waste Materials - NiyalDocument12 paginiAgro Waste Materials - NiyalHyjan JacobÎncă nu există evaluări
- Pioglitazone A Review of Analytical Met - 2014 - Journal of Pharmaceutical AnalDocument8 paginiPioglitazone A Review of Analytical Met - 2014 - Journal of Pharmaceutical AnalDarian HerascuÎncă nu există evaluări
- Revised 2Document61 paginiRevised 2Stephannie SyÎncă nu există evaluări
- Chemistry Study Notes Grade 10Document10 paginiChemistry Study Notes Grade 10Jynxx1387% (15)
- Sika PDS - E - Sika Pocket Grout (BBCD)Document2 paginiSika PDS - E - Sika Pocket Grout (BBCD)lwin_oo2435Încă nu există evaluări
- Lasers in Restorative DentistryDocument283 paginiLasers in Restorative Dentistrybalajishanker147Încă nu există evaluări
- Chemical Bonds: Writing Formulas and Naming CompoundsDocument8 paginiChemical Bonds: Writing Formulas and Naming CompoundsPercen7Încă nu există evaluări
- Q2 Science 9 Module 2 1editedDocument20 paginiQ2 Science 9 Module 2 1editedSamantha BecieraÎncă nu există evaluări
- A New Class of Solvent-in-Salt Electrolyte ForDocument9 paginiA New Class of Solvent-in-Salt Electrolyte ForTong YichenÎncă nu există evaluări
- Module 1Document4 paginiModule 1welpÎncă nu există evaluări
- Usp Description and SolubilityDocument1 paginăUsp Description and SolubilityvafaashkÎncă nu există evaluări
- Read These Instructions FirstDocument7 paginiRead These Instructions FirstSalman Ul MoazzamÎncă nu există evaluări
- Acetone Peroxide:: Synthesis Written in Red Means It Is Highly Advised NOT To Make This. For Informational Use OnlyDocument5 paginiAcetone Peroxide:: Synthesis Written in Red Means It Is Highly Advised NOT To Make This. For Informational Use OnlyRUZCHEMISTRYÎncă nu există evaluări
- Biology Practical NotesDocument9 paginiBiology Practical NotesJessica KhoÎncă nu există evaluări
- Tannin Drugs - Dr.U.Srinivasa, Professor and Head, Srinivas College of Pharmacy, Mangalore - 574143, KarnatakaDocument16 paginiTannin Drugs - Dr.U.Srinivasa, Professor and Head, Srinivas College of Pharmacy, Mangalore - 574143, KarnatakaDR.U.SrinivasaÎncă nu există evaluări
- Coordination Compounds PDFDocument68 paginiCoordination Compounds PDFAsmita SinghÎncă nu există evaluări
- Aldehydes and Ketones-02 Solved ProblemsDocument13 paginiAldehydes and Ketones-02 Solved ProblemsRaju SinghÎncă nu există evaluări
- Seminar Sekolah Form 4 & Form 5 Chem 30.05.2023Document14 paginiSeminar Sekolah Form 4 & Form 5 Chem 30.05.2023Joehaimi ImanÎncă nu există evaluări
- General Chemistry 2 - Grade 11Document16 paginiGeneral Chemistry 2 - Grade 11Adoneza Gwyn AgustinÎncă nu există evaluări
- Human DNA ExtractionDocument1 paginăHuman DNA ExtractionJosaphat M. AnteÎncă nu există evaluări
- Project FileDocument18 paginiProject FileAdarsh SrivastavaÎncă nu există evaluări
- Amalie GreasesDocument2 paginiAmalie GreasesDavid PomaÎncă nu există evaluări
- Ge Ni U S: Chemical ReactionsDocument25 paginiGe Ni U S: Chemical ReactionsRouda AljÎncă nu există evaluări
- Chemistry: PAPER 5 Practical Test InstructionsDocument4 paginiChemistry: PAPER 5 Practical Test InstructionsVarun PanickerÎncă nu există evaluări
- Principles and Applications of Electrochemical Capacitors-CarlenDocument8 paginiPrinciples and Applications of Electrochemical Capacitors-CarlenwyeoÎncă nu există evaluări