Documente Academic
Documente Profesional
Documente Cultură
Ukmec For Contraceptives
Încărcat de
Shahid BobatTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Ukmec For Contraceptives
Încărcat de
Shahid BobatDrepturi de autor:
Formate disponibile
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL
AND INTRAUTERINE CONTRACEPTION
Cu-IUD = Copper-bearing intrauterine device; LNG-IUS = Levonorgestrel-releasing intrauterine system;
IMP = Progestogen-only implant; DMPA = Progestogen-only injectable: depot medroxyprogesterone acetate;
POP = Progestogen-only pill; CHC = Combined hormonal contraception
CONDITION Cu-IUD LNG-IUS IMP DMPA POP CHC
I = Initiation, C = Continuation
PERSONAL CHARACTERISTICS AND REPRODUCTIVE HISTORY
Pregnancy NA NA NA NA NA NA
Age Menarche Menarche After Menarche After Menarche
to <20=2, to <20=2, menarche to <18=2, menarche to <40=1,
20=1 20=1 =1 18-45=1, =1 40=2
>45=2
Parity
a) Nulliparous 1 1 1 1 1 1
b) Parous 1 1 1 1 1 1
Breastfeeding
a) 0 to <6 weeks postpartum 1 2 1 4
b) 6 weeks to <6 months
See below 1 1 1 2
(primarily breastfeeding)
c) 6 months postpartum 1 1 1 1
Postpartum (in non-breastfeeding women)
a) 0 to <3 weeks
(i) With other risk factors for VTE 1 2 1 4
See below
(ii) Without other risk factors 1 2 1 3
b) 3 to <6 weeks
(i) With other risk factors for VTE 1 2 1 3
(ii) Without other risk factors See below 1 1 1 2
c) 6 weeks 1 1 1 1
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
01 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Postpartum (in breastfeeding or non-
breastfeeding women, including post-
caesarean section)
a) 0 to <48 hours 1 1
b) 48 hours to <4 weeks 3 3
See above
c) 4 weeks 1 1
d) Postpartum sepsis 4 4
Post-abortion
a) First trimester 1 1 1 1 1 1
b) Second trimester 2 2 1 1 1 1
c) Post-abortion sepsis 4 4 1 1 1 1
Past ectopic pregnancy 1 1 1 1 1 1
History of pelvic surgery
1 1 1 1 1 1
Smoking
a) Age <35 years 1 1 1 1 1 2
b) Age 35 years
(i) <15 cigarettes/day 1 1 1 1 1 3
(ii) 15 cigarettes/day 1 1 1 1 1 4
(iii) Stopped smoking <1 year 1 1 1 1 1 3
(iv) Stopped smoking 1 year 1 1 1 1 1 2
Obesity
a) BMI 3034 kg/m2 1 1 1 1 1 2
b) BMI 35 kg/m2 1 1 1 1 1 3
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
02 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
History of bariatric surgery
a) With BMI <30 kg/m2 1 1 1 1 1 1
b) With BMI 3034 kg/m2 1 1 1 1 1 2
c) With BMI 35 kg/m 2
1 1 1 1 1 3
Organ transplant
a) Complicated: graft failure (acute or I C I C
chronic), rejection, cardiac allograft 2 2 2 3
vasculopathy 3 2 3 2
b) Uncomplicated 2 2 2 2 2 2
CARDIOVASCULAR DISEASE (CVD)
Multiple risk factors for CVD (such as
smoking, diabetes, hypertension, obesity 1 2 2 3 2 3
and dyslipidaemias)
Hypertension
a) Adequately controlled hypertension 1 1 1 2 1 3
b) Consistently elevated BP levels
(properly taken measurements)
(i) Systolic >140159 mmHg or
1 1 1 1 1 3
diastolic >9099 mmHg
(ii) Systolic 160 mmHg or
1 1 1 2 1 4
diastolic 100 mmHg
c) Vascular disease 1 2 2 3 2 4
History of high BP during pregnancy 1 1 1 1 1 2
Current and history of ischaemic heart 1 I C I C 3 I C
disease 4
2 3 2 3 2 3
Stroke (history of cerebrovascular 1 I C I C 3 I C
accident, including TIA) 4
2 3 2 3 2 3
Known dyslipidaemias 1 2 2 2 2 2
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
03 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Venous thromboembolism (VTE)
a) History of VTE 1 2 2 2 2 4
b) Current VTE (on anticoagulants) 1 2 2 2 2 4
c) Family history of VTE
(i) First-degree relative age <45 years 1 1 1 1 1 3
(ii) First-degree relative age 45 years 1 1 1 1 1 2
d) Major surgery
(i) With prolonged immobilisation 1 2 2 2 2 4
(ii) Without prolonged immobilisation 1 1 1 1 1 2
e) Minor surgery without immobilisation 1 1 1 1 1 1
f) Immobility (unrelated to surgery) (e.g.
1 1 1 1 1 3
wheelchair use, debilitating illness)
Superficial venous thrombosis
a) Varicose veins 1 1 1 1 1 1
b) Superficial venous thrombosis 1 1 1 1 1 2
Known thrombogenic mutations (e.g.
factor V Leiden, prothrombin mutation,
1 2 2 2 2 4
protein S, protein C and antithrombin
deficiencies)
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
04 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Valvular and congenital heart disease
a) Uncomplicated 1 1 1 1 1 2
b) Complicated (e.g. pulmonary
hypertension, history of subacute 2 2 1 1 1 4
bacterial endocarditis)
Cardiomyopathy
a) Normal cardiac function 1 1 1 1 1 2
b) Impaired cardiac function 2 2 2 2 2 4
Cardiac arrhythmias
a) Atrial fibrillation 1 2 2 2 2 4
b) Known long QT syndrome I C I C
1 2 1 2
3 1 3 1
NEUROLOGICAL CONDITIONS
Headaches
a) Non-migrainous (mild or severe) I C
1 1 1 1 1
1 2
b) Migraine without aura, at any age I C I C
1 2 2 2
1 2 2 3
c) Migraine with aura, at any age 1 2 2 2 2 4
d) History (5 years ago) of migraine
1 2 2 2 2 3
with aura, any age
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
05 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Idiopathic intracranial hypertension (IIH) 1 1 1 1 1 2
Epilepsy 1 1 1 1 1 1
Taking anti-epileptic drugs Certain anti-epileptic drugs have the potential to affect the
bioavailability of steroid hormones in hormonal contraception.
For up-to-date information on the potential drug interactions
between hormonal contraception and anti-epileptic drugs,
please refer to the online drug interaction checker available on
the Medscape website (http://reference.medscape.com/drug-
interactionchecker ).
DEPRESSIVE DISORDERS
Depressive disorders 1 1 1 1 1 1
BREAST AND REPRODUCTIVE TRACT CONDITIONS
Vaginal bleeding patterns
a) Irregular pattern without heavy bleeding 1 1 2 2 2 1
b) Heavy or prolonged bleeding (includes I C
regular and irregular patterns) 2 2 2 2 1
1 2
Unexplained vaginal bleeding (suspicious I C I C
for serious condition) before evaluation 3 3 2 2
4 2 4 2
Endometriosis 2 1 1 1 1 1
Benign ovarian tumours (including cysts) 1 1 1 1 1 1
Severe dysmenorrhoea 2 1 1 1 1 1
Gestational trophoblastic disease
(GTD)
a) Undetectable hCG levels 1 1 1 1 1 1
b) Decreasing hCG levels 3 3 1 1 1 1
c) Persistently elevated hCG levels or
4 4 1 1 1 1
malignant disease
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
06 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Cervical ectropion 1 1 1 1 1 1
Cervical intraepithelial neoplasia (CIN) 1 2 1 2 1 2
Cervical cancer
a) Awaiting treatment I C I C
2 2 1 2
4 2 4 2
b) Radical trachelectomy 3 3 2 2 1 2
Breast conditions
a) Undiagnosed mass/breast symptoms I C
1 2 2 2 2
3 2
b) Benign breast conditions 1 1 1 1 1 1
c) Family history of breast cancer 1 1 1 1 1 1
d) Carriers of known gene mutations
associated with breast cancer (e.g. 1 2 2 2 2 3
BRCA1/BRCA2)
e) Breast cancer
(i) Current breast cancer 1 4 4 4 4 4
(ii) Past breast cancer 1 3 3 3 3 3
Endometrial cancer I C I C
1 1 1 1
4 2 4 2
Ovarian cancer 1 1 1 1 1 1
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
07 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Uterine fibroids
a) Without distortion of the uterine cavity 1 1 1 1 1 1
b) With distortion of the uterine cavity 3 3 1 1 1 1
Anatomical abnormalities
a) Distorted uterine cavity 3 3
b) Other abnormalities 2 2
Pelvic inflammatory disease (PID)
a) Past PID (assuming no current risk
1 1 1 1 1 1
factor for STIs)
b) Current PID I C I C
1 1 1 1
4 2 4 2
Sexually transmitted infections (STIs)
a) Chlamydial infection (current) I C I C
(i) Symptomatic 4 2 4 2 1 1 1 1
(ii) Asymptomatic 3 2 3 2 1 1 1 1
b) Purulent cervicitis or gonorrhoea (current) 4 2 4 2 1 1 1 1
c) Other current STIs (excluding HIV & hepatitis) 2 2 1 1 1 1
d) Vaginitis (including Trichomonas vaginalis
2 2 1 1 1 1
and bacterial vaginosis) (current)
e) Increased risk for STIs 2 2 1 1 1 1
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
08 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
HIV INFECTION
HIV infection
a) High risk of HIV infection 2 2 1 2 1 1
b) HIV infected
(i) CD4 count 200 cells/mm3 2 2 1 1 1 1
(ii) CD4 count <200 cells/mm3 I C I C
1 1 1 1
3 2 3 2
c) Taking antiretroviral (ARV) drugs Certain ARV drugs have the potential to affect the bioavailability of
steroid hormones in hormonal contraception.
For up-to-date information on the potential drug interactions
between hormonal contraception and ARV drugs, please refer to
the online HIV drugs interaction checker
(www.hiv-druginteractions.org/Interactions.aspx).
OTHER INFECTIONS
Tuberculosis
a) Non-pelvic 1 1 1 1 1 1
b) Pelvic I C I C
1 1 1 1
4 3 4 3
ENDOCRINE CONDITIONS
Diabetes
a) History of gestational disease 1 1 1 1 1 1
b) Non-vascular disease
(i) Non-insulin dependent 1 2 2 2 2 2
(ii) Insulin dependent 1 2 2 2 2 2
c) Nephropathy/retinopathy/neuropathy 1 2 2 2 2 3
d) Other vascular disease 1 2 2 2 2 3
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
09 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Thyroid disorders
a) Simple goitre 1 1 1 1 1 1
b) Hyperthyroid 1 1 1 1 1 1
c) Hypothyroid 1 1 1 1 1 1
GASTROINTESTINAL CONDITIONS
Gallbladder disease
a) Symptomatic
(i) Treated by cholecystectomy 1 2 2 2 2 2
(ii) Medically treated 1 2 2 2 2 3
(iii) Current 1 2 2 2 2 3
b) Asymptomatic 1 2 2 2 2 2
History of cholestasis
a) Pregnancy related 1 1 1 1 1 2
b) Past COC related 1 2 2 2 2 3
Viral hepatitis
a) Acute or flare I C
1 1 1 1 1
3 2
b) Carrier 1 1 1 1 1 1
c) Chronic 1 1 1 1 1 1
Cirrhosis
a) Mild (compensated without
1 1 1 1 1 1
complications)
b) Severe (decompensated) 1 3 3 3 3 4
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
10 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
UKMEC SUMMARY TABLE
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
Cu-IUD LNG-IUS IMP DMPA POP CHC
CONDITION
I = Initiation, C = Continuation
Liver tumours
a) Benign
(i) Focal nodular hyperplasia 1 2 2 2 2 2
(ii) Hepatocellular adenoma 1 3 3 3 3 4
b) Malignant (hepatocellular carcinoma) 1 3 3 3 3 4
Inflammatory bowel disease (including
1 1 1 1 2 2
Crohns disease and ulcerative colitis)
ANAEMIAS
Thalassaemia 2 1 1 1 1 1
Sickle cell disease 2 1 1 1 1 2
Iron deficiency anaemia 2 1 1 1 1 1
RHEUMATIC DISEASES
Rheumatoid arthritis 1 2 2 2 2 2
Systemic lupus erythematosus (SLE)
a) No antiphospholipid antibodies 1 2 2 2 2 2
b) Positive antiphospholipid antibodies 1 2 2 2 2 4
Positive antiphospholipid antibodies 1 2 2 2 2 4
DRUG INTERACTIONS
Taking medication See section on drug interactions with hormonal contraception.
UKMEC Definition of category
Category 1 A condition for which there is no restriction for the use of the method
Category 2 A condition where the advantages of using the method generally outweigh the theoretical or proven risks
Category 3 A condition where the theoretical or proven risks usually outweigh the advantages of using the method. The provision
of a method requires expert clinical judgement and/or referral to a specialist contraceptive provider, since use of the
method is not usually recommended unless other more appropriate methods are not available or not acceptable
Category 4 A condition which represents an unacceptable health risk if the method is used
11 Copyright Faculty of Sexual and Reproductive Healthcare 2006 to 2016.
S-ar putea să vă placă și
- FSRH Ukmec Summary September 2019Document11 paginiFSRH Ukmec Summary September 2019Kiran JayaprakashÎncă nu există evaluări
- Farmako Kontrasepsi HormonalDocument43 paginiFarmako Kontrasepsi HormonalMariyatul QibthiyyahÎncă nu există evaluări
- TBH IolDocument12 paginiTBH IolCheska MalubayÎncă nu există evaluări
- Legal Summary-Chart English Final Tag508Document2 paginiLegal Summary-Chart English Final Tag508Siska PindanÎncă nu există evaluări
- Sarawak Thromboprophylaxis Risk Assessment Form: NAME: HOSPITAL: Risk Factors: Tick Score AntenatalDocument2 paginiSarawak Thromboprophylaxis Risk Assessment Form: NAME: HOSPITAL: Risk Factors: Tick Score AntenatalTan Chin AunÎncă nu există evaluări
- Revision Test Series - OBG QUEDocument7 paginiRevision Test Series - OBG QUESai TejendraÎncă nu există evaluări
- Preterm Labor by Dr. Mustafe AadanDocument14 paginiPreterm Labor by Dr. Mustafe AadanMustafa AadanÎncă nu există evaluări
- CHN-2.3, MCQs Paper Community Health Nursing.Document19 paginiCHN-2.3, MCQs Paper Community Health Nursing.Gulshad AfridiÎncă nu există evaluări
- OB-GYN Board Exam QuestionsDocument11 paginiOB-GYN Board Exam QuestionsJo Anne94% (17)
- Gauss Without AnswersDocument115 paginiGauss Without AnswersAshish R. JadhavÎncă nu există evaluări
- Gynae OsceDocument11 paginiGynae OsceRagnar LothbrokÎncă nu există evaluări
- OBDocument7 paginiOBWilmaBongotanPadawilÎncă nu există evaluări
- Preterm Labor ProtocolDocument6 paginiPreterm Labor ProtocolLana kamalÎncă nu există evaluări
- Mcqs Test Unit 8 With KeyDocument5 paginiMcqs Test Unit 8 With Keyyasodha maharajÎncă nu există evaluări
- Gynae OsceDocument11 paginiGynae OsceRagnar LothbrokÎncă nu există evaluări
- Preterm Labor Evidence Based ReviewDocument68 paginiPreterm Labor Evidence Based ReviewAmmar FardhanaÎncă nu există evaluări
- Acuity of Care Patient ClassificationDocument1 paginăAcuity of Care Patient ClassificationJan Crizza Dale R. FrancoÎncă nu există evaluări
- Ijpedi2014 204807Document5 paginiIjpedi2014 204807triaanggareniÎncă nu există evaluări
- Nursing Care During Prenatal PeriodDocument7 paginiNursing Care During Prenatal Periodsands32Încă nu există evaluări
- Pregnant Obstetrics DiagnosisDocument48 paginiPregnant Obstetrics Diagnosisdocivirus100% (1)
- Premature Rupture of Membranes (Prom)Document12 paginiPremature Rupture of Membranes (Prom)KABERA RENEÎncă nu există evaluări
- Kunci MCQ Fisiologi 10 Juli 2017-1Document19 paginiKunci MCQ Fisiologi 10 Juli 2017-1Seviana AnnisaÎncă nu există evaluări
- Pre and Post PregnancyDocument23 paginiPre and Post PregnancyJitendra ChaudharyÎncă nu există evaluări
- Induction of LaborDocument31 paginiInduction of LaborRem AlfelorÎncă nu există evaluări
- Prena TAL Care: Princess PalabricaDocument98 paginiPrena TAL Care: Princess PalabricaCess PalabricaÎncă nu există evaluări
- O&g Hsajb ProtocolDocument69 paginiO&g Hsajb Protocol23-Chong Kar KinÎncă nu există evaluări
- Induction and Augmentation of LabourDocument73 paginiInduction and Augmentation of LabourSanthosh.S.U100% (4)
- 脂肪肝篩檢與監控之原則Document22 pagini脂肪肝篩檢與監控之原則find94132Încă nu există evaluări
- T and DDocument24 paginiT and DBakana6Încă nu există evaluări
- Preterm LabourDocument7 paginiPreterm LabourPrathibha FernandopulleÎncă nu există evaluări
- Week 2 OBST 7010 Quiz 2 ReviewDocument6 paginiWeek 2 OBST 7010 Quiz 2 ReviewBridget MasonÎncă nu există evaluări
- 15 - Post Term + IOL UpdatedDocument15 pagini15 - Post Term + IOL UpdatedAli QuwarahÎncă nu există evaluări
- Antepartum SurveilDocument3 paginiAntepartum Surveilapi-3712326Încă nu există evaluări
- Protocol for PROM and P-PROM ManagementDocument1 paginăProtocol for PROM and P-PROM ManagementAzizan HakimÎncă nu există evaluări
- Harriet Lane - NeonatologyDocument27 paginiHarriet Lane - Neonatologyrichardcypher12Încă nu există evaluări
- DR - Asirifi-Induction and Augmentation of Labour (Autosaved)Document19 paginiDR - Asirifi-Induction and Augmentation of Labour (Autosaved)Max ZealÎncă nu există evaluări
- WBUHS Obstetrics and Gynaecology Papers PDFDocument245 paginiWBUHS Obstetrics and Gynaecology Papers PDFAnangsha DattaÎncă nu există evaluări
- Multiple ChoiceDocument55 paginiMultiple Choicetri ebtaÎncă nu există evaluări
- Visual Summary On Neonatal Infection Determining The Need For Antibiotic Treatment of Babies Within 72 Hours of Birth PDF 9078464413Document2 paginiVisual Summary On Neonatal Infection Determining The Need For Antibiotic Treatment of Babies Within 72 Hours of Birth PDF 9078464413walaa alsharanyÎncă nu există evaluări
- Cervical Ripening & Induction of Labour: Obstetric Guideline 1Document10 paginiCervical Ripening & Induction of Labour: Obstetric Guideline 1Maizura Syahirah MohtadaÎncă nu există evaluări
- 22 STEINAUER First Trimester BleedingDocument40 pagini22 STEINAUER First Trimester BleedingWiwing MarisyaÎncă nu există evaluări
- Preterm labour د.علية شعيبDocument58 paginiPreterm labour د.علية شعيبMohammad BelbahaithÎncă nu există evaluări
- Obs Cases BlueprintsDocument46 paginiObs Cases BlueprintsYousra AhmedÎncă nu există evaluări
- 3195 12163 1 PBDocument5 pagini3195 12163 1 PBTiAs TiAriyahÎncă nu există evaluări
- ObstetricsDocument8 paginiObstetricsrevathidadam55555100% (1)
- Introduction of Ectopic PregnancyDocument14 paginiIntroduction of Ectopic PregnancymyyoyolinÎncă nu există evaluări
- CHN FinalsDocument15 paginiCHN FinalsLuna sibilityÎncă nu există evaluări
- Maternal Retake - ProctoredDocument12 paginiMaternal Retake - ProctoredMiki Frazier100% (4)
- Case PresentationDocument36 paginiCase PresentationTara LeungÎncă nu există evaluări
- Detailed Activities of Service ProviderDocument7 paginiDetailed Activities of Service ProviderJahin KhanÎncă nu există evaluări
- IOL LaborDocument22 paginiIOL LaborSingey LhendupÎncă nu există evaluări
- PregnancyDocument27 paginiPregnancyjawad_ahmedÎncă nu există evaluări
- Normal & Abnormal LabourDocument5 paginiNormal & Abnormal Labourra100% (1)
- Obstetric Operations & Procedures2Document98 paginiObstetric Operations & Procedures2mohazemalhotraÎncă nu există evaluări
- Dysfunctional Labour by Abhishek JaguessarDocument34 paginiDysfunctional Labour by Abhishek Jaguessarreedoye21Încă nu există evaluări
- Tmbool's Notes in Obstetrics and Gynecology PDFDocument113 paginiTmbool's Notes in Obstetrics and Gynecology PDFOsman SomiÎncă nu există evaluări
- Ncma219 Course Task 3Document18 paginiNcma219 Course Task 3NikoruÎncă nu există evaluări
- CME PRETERM LABOR, PROM, PPROM FDocument39 paginiCME PRETERM LABOR, PROM, PPROM FRatna Setia WatiÎncă nu există evaluări
- NCLEX: Pharmacology for Nurses: 100 Practice Questions with Rationales to help you Pass the NCLEX!De la EverandNCLEX: Pharmacology for Nurses: 100 Practice Questions with Rationales to help you Pass the NCLEX!Evaluare: 5 din 5 stele5/5 (4)
- Labor Curve & Partograph GuideDocument50 paginiLabor Curve & Partograph GuideJhervy VillanuevaÎncă nu există evaluări
- Jurnal Plasenta PreviaDocument10 paginiJurnal Plasenta Previadiah_201192Încă nu există evaluări
- Extreme Preterm Premature Rupture of Membranes: KSM Obstetri Dan Ginekologi Rumah Sakit Umum Pusat PersahabatanDocument27 paginiExtreme Preterm Premature Rupture of Membranes: KSM Obstetri Dan Ginekologi Rumah Sakit Umum Pusat Persahabatanrilla saeliputriÎncă nu există evaluări
- Obstetric History Taking OSCE GuideDocument17 paginiObstetric History Taking OSCE GuideSYDNIKA AIRA CARBONILLAÎncă nu există evaluări
- Gender StudiesDocument25 paginiGender StudiesHarun Rasul100% (1)
- Progesterone's Limited Role in Breast DevelopmentDocument7 paginiProgesterone's Limited Role in Breast DevelopmentRoseilÎncă nu există evaluări
- Angelica New PRC Cases FormattedDocument7 paginiAngelica New PRC Cases FormattedDino CamposÎncă nu există evaluări
- The Medical Laboratories (PVT) LTD.: 34, Lawrence Road, Lahore. Tel: 6361417, Mobile: 0300-8482230Document2 paginiThe Medical Laboratories (PVT) LTD.: 34, Lawrence Road, Lahore. Tel: 6361417, Mobile: 0300-8482230Wahab Ahmad KhanÎncă nu există evaluări
- Developing SMART Objectives Case StudyDocument3 paginiDeveloping SMART Objectives Case StudyVarun AchrejaÎncă nu există evaluări
- DR Bhatia Medical Coaching Institute - Online Test Platform GynaeDocument70 paginiDR Bhatia Medical Coaching Institute - Online Test Platform Gynaeanon_287080784Încă nu există evaluări
- ZandFratkin PS2016 PreSymposium Handout MenopauseDocument22 paginiZandFratkin PS2016 PreSymposium Handout Menopausedeemoney3Încă nu există evaluări
- Umbilical Cord ProlapseDocument26 paginiUmbilical Cord Prolapsesulekhaanoob100% (2)
- Ginecologia - PerimenopausiaDocument7 paginiGinecologia - PerimenopausiaHugo GutiérrezÎncă nu există evaluări
- PPH PosterDocument1 paginăPPH PosterKhaled Gaber SabaaÎncă nu există evaluări
- School of Health and Allied Health Sciences Nursing DepartmentDocument2 paginiSchool of Health and Allied Health Sciences Nursing DepartmentRosemarie R. ReyesÎncă nu există evaluări
- Inducing Labour With Acupuncture by Debra Betts PDFDocument6 paginiInducing Labour With Acupuncture by Debra Betts PDFElanghovan Arumugam100% (2)
- Daftar PustakaDocument2 paginiDaftar PustakaIntania MairudiÎncă nu există evaluări
- Unit 9 Episiotomy and Nursing Management: 9.0 ObjectivesDocument8 paginiUnit 9 Episiotomy and Nursing Management: 9.0 ObjectivesSudip Kumar DeyÎncă nu există evaluări
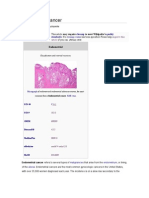
- Endometrial Cancer Diagnosis and TreatmentDocument9 paginiEndometrial Cancer Diagnosis and TreatmentAhmed Butt100% (1)
- Principles and Interpretation of CardiotocographyDocument9 paginiPrinciples and Interpretation of CardiotocographyCKÎncă nu există evaluări
- Persistent Lateral and Posterior Fetal Positions at The Onset of Labour - 3 PDFDocument7 paginiPersistent Lateral and Posterior Fetal Positions at The Onset of Labour - 3 PDFOshigitaÎncă nu există evaluări
- Postpartum hemorrhage management and treatment optionsDocument1 paginăPostpartum hemorrhage management and treatment optionsRobert Timothy YapÎncă nu există evaluări
- Oxytocin Massage Improves Breastmilk in Postpartum MothersDocument9 paginiOxytocin Massage Improves Breastmilk in Postpartum MothersNinaÎncă nu există evaluări
- Intrapartum and Newborn Care DiscussionDocument2 paginiIntrapartum and Newborn Care DiscussionAngelica Malacay RevilÎncă nu există evaluări
- European Journal of Obstetrics & Gynecology and Reproductive BiologyDocument3 paginiEuropean Journal of Obstetrics & Gynecology and Reproductive BiologyHinal VaghaniÎncă nu există evaluări
- Doctor High Yield Obgyn - Not AnnotatedDocument34 paginiDoctor High Yield Obgyn - Not AnnotatedEmanuella Gomez100% (2)
- CH2 - Male Reproductive System - 2023Document83 paginiCH2 - Male Reproductive System - 2023varyvira6677Încă nu există evaluări
- ME Sci 10 Q3 1001 PSDocument17 paginiME Sci 10 Q3 1001 PSsino56601Încă nu există evaluări
- Maternal and Child Health ServicesDocument5 paginiMaternal and Child Health ServicesElaiza RiegoÎncă nu există evaluări
- Reproductivesystem g10Document88 paginiReproductivesystem g10Mernie Grace DionesioÎncă nu există evaluări