Documente Academic
Documente Profesional
Documente Cultură
E O G D I S: Nhancement of Sseointegration by Enerating A Ynamic Mplant Urface
Încărcat de
Bagis Emre GulTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
E O G D I S: Nhancement of Sseointegration by Enerating A Ynamic Mplant Urface
Încărcat de
Bagis Emre GulDrepturi de autor:
Formate disponibile
RESEARCH
ENHANCEMENT OF OSSEOINTEGRATION BY
GENERATING A DYNAMIC IMPLANT SURFACE
Eduardo A. Anitua, DDS, MD Preparations of autologous plasma rich in growth factors (PRGF) are
used to promote healing and tissue regeneration. We seek to determine
whether covering the titanium implant surface with this preparation
KEY WORDS could enhance osseointegration. The interaction of PRGF with the
surface of titanium implants was examined by environmental scanning
Growth factors electron microscopy (ESEM). A total of 23 implants were placed in the
Titanium implants
Osseointegration tibiae and radii of 3 goats; 13 implants were inserted after covering
the surface and filling the alveolus with PRGF, and 10 more implants
were inserted following a conventional protocol and served as controls.
Histomorphometric analysis of the bone-implant interface was
performed after 8 weeks. Finally, 1391 implants were placed in 295
patients after bioactivating the surface with PRGF. Stability and implant
survival were evaluated. The implant surface adsorbed the protein-rich
material as shown by ESEM. In the animal study, osseointegration was
enhanced when the surface was covered with PRGF as shown by
histomorphometry (bone-implant contact: 51.28% 6 4.7% vs 21.89% 6
7.36%; P , .01). Finally, studies in patients showed that 99.6% of the
implants treated with PRGF were well osseointegrated. Clinical use of
this technique in oral implantology can improve the prognosis.
INTRODUCTION shown to enhance and accelerate
soft tissue repair and bone re-
n the process of tissue re-
I
generation in the preparation of
pair and restoration, the
osseointegration of dental future sites for dental implants.3,4
implants can be im- More recently, preparation with
proved and accelerated PRGF has been shown to enhance
by inducing the regener- postoperative healing of ruptured
ative capacity of surrounding Achilles tendon in professional
tissues with the appropriate stim- athletes and articular cartilage
uli. Because growth factors are repair after nontraumatic avul-
expressed during different phases sion.5,6
of tissue healing, it has been A preparation of PRGF applied
Eduardo A. Anitua, DDS, MD, is in thought that they could serve as to a titanium implant adheres to
private practice in implantology and oral the metal and might create a new
rehabilitation in Vitoria, Spain. Address therapeutic agents to promote
correspondence to Dr Anitua at c/ San tissue repair.1,2 dynamic surface that could po-
Antonio, 15-38C, 01005 Vitoria, Spain Autologous plasma rich in tentially show biological activity.7
(e-mail: eduardoanitua@eduardoanitua.com). growth factors (PRGF) has been This protein layer consists of
72 Vol. XXXII / No. Two / 2006
Eduardo A. Anitua
a fibrin net embedded with fraction located just above the red protocol (protocol B) consisting
growth factors that covers the blood cell layer was collected. of control implants without
whole implant surface and trans- Leukocytes were not included in added PRGF.
forms the initial interactions of the preparation. Fifty microliters Biopsies were taken from all
the implant surface with the of 10% w/v calcium chloride was the implants with an 8-mm tre-
surrounding tissues. It also influ- added to each tube containing phine drill after 8 weeks. Samples
ences cellular attachment, pro- 1 mL of PRGF. were numbered for blind analysis
liferation and differentiation, and and immediately fixed in 4%
bone matrix deposition. This coat- Interaction between plasma formalin. Sections were stained
ing has 2 important properties proteins and titanium implants with eosin and toluidine blue.
that may contribute to optimizing Digitalized images were obtained
Evidence that the surface of the
and accelerating the osseointegra- with a JVC TK-C1380 color video
titanium implants directly inter-
tion process: the osteoconductive camera (JVC, London, UK), and
acted with PRGF was obtained
properties attributed to fibrin8 the central section was studied
by coating implant surfaces with
and the recognized osteoinduc- with histomorphometric techni-
PRGF in the presence of Ca2
tive activities of growth factors.9 ques. Bone-implant contact (BIC),
and studying the surface by
Considering these findings, it defined as the part of the implant
ESEM (Electroscan 2020, Wil-
was important to study the ad- surface in direct contact with the
mington, NC).7
herence of PRGF to the implant bone matrix, was expressed as
surface and its permanence after a percentage of the total implant
Studies in an animal model
clot retraction. With this aim, perimeter.
environmental scanning electron Bioactive titanium dental im-
microscopy (ESEM) was utilized plants were inserted in the tibiae Patient selection
to study the interaction between and radii of 3 goats. The study
A prospective study was carried
BTI implant surfaces and PRGF. was carried out in compliance
out in all patients who underwent
The hypothesis that PRGF- with guidelines for the humane
implant rehabilitation between
coated surfaces could accelerate treatment of experimental ani-
January 2000 and June 2001. A de-
implant osseointegration was test- mals in Spain (Royal Decree
scriptive analysis was made and
ed in an animal model. In view of 2223/88). Blood was drawn from
implant success was evaluated.
the good experimental results the vena cava and collected in
The patients agreed to partic-
obtained, it was hypothesized that citrated vacutainer tubes (Veno-
ipate in this study and gave their
the clinical use of implant sur- ject, Terumo Co, Japan), and
informed consent. All patients
faces coated with PRGF would im- PRGF was prepared by centrifu-
were healthy and had no systemic
prove the outcome. To determine gation at 460 g for 8 minutes.
contraindications to the implan-
if clinical evidence supports our Animals were anesthetized
tation procedure. The areas to be
hypothesis, we prospectively with ketamine (20 mg/kg), and
treated were free from infection.
studied all implant procedures anesthesia was maintained with
performed in our office between inhaled isofluorane (2.5%). Arti-
Surgical procedures
January 2000 and June 2001. ficial alveoli were created by
making perforations (3 mm in A combination of panoramic and
MATERIALS AND METHODS diameter and 8.5 mm deep) in intraoral radiographs was used
the tibiae and radii. A total of 23 for preliminary evaluation of
Preparation of PRGF
implants (BTI Implant System, the intended implant sites. Bone
Blood was collected from human BTI, Vitoria-Gasteiz, Spain) were quality was assessed in preoper-
volunteers who had given their inserted in the holes in accordance ative scans (Denta PC). Implants
informed consent to the proce- with 2 protocols. Thirteen of the were placed at any site in the jaw
dure. The blood was deposited implants were made with a PRGF in bone of quality I, II, III, and IV.
in 3.8% w/v sodium citrate protocol (protocol A), which in- BTI implants with PRGF
1:9 v/v.10 volved covering the implant adsorbed onto the titanium acid-
Platelet-rich plasma was sep- surface with PRGF by simple etched surface were used. Im-
arated by centrifugation at 460 g adsorption and filling the alveo- plants were inserted following
for 8 minutes at room tempera- lus with PRGF before inserting a single-stage or other protocol
ture (PRGF System II, BTI, Vito- the implant. Ten of the implants as determined by the clinical
ria, Spain). The 0.5-mL plasma were made with a non-PRGF requirements, which included
Journal of Oral Implantology 73
OSSEOINTEGRATION WITH DYNAMIC IMPLANT SURFACE
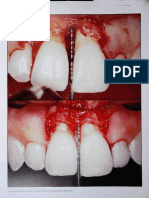
FIGURES 1 AND 2. FIGURE 1. Visualization of the interface between the plasma rich in growth factors and the titanium surface of the
implant (BTI, Vitoria, Spain). The surface boundary was directly visualized with an Electroscan 2020 environmental scanning
electron microscope without previous specimen preparation (magnification 36000). FIGURE 2. Visualization of the interface between
the titanium surface of the implant and the developed fibrin-rich clot that remains in close contact with the microroughened surface
of the BTI implant (Electroscan 2020, magnification 36000).
several surgical procedures, ridge RESULTS Figure 3a and b. Every biopsy of
augmentation, and sinus eleva- the implants made with PRGF
ESEM of implant surfaces
tion. The same operator per- revealed close contact between
coated with PRGF
formed all surgical procedures. the bone and implant. In Figure
The implant surface adsorbed the 3a, newly regenerated bone fills
Evaluation and criteria protein-rich material and was the middle and apical thirds of the
of success therefore bioactive, as is evident cavity. The cortical zone was in-
Implants were evaluated by mea- in Figure 1. After retraction, the distinguishable from the medul-
suring the resonance frequencies clot remained perfectly adhered lary zone in all the samples
of implants (Osstell). An implant to the bioactive surface (Figure 2), analyzed. In the implants made
was considered successful when producing a surface coated with
according to the non-PRGF con-
(1) no sign of failure appeared in a fibrin-rich clot. The associated
trol protocol, only the middle
panoramic and periapical radiog- growth factors remained in place
third is surrounded by cortical
raphy, (2) no pain or symptoms to promote bone consolidation
bone, and osseous contact is ab-
of infection were present, and (3) around the implant. Such a net-
sent in the apical third. Mac-
a reverse torque force of 20 N/cm work can facilitate the adhesion
could be applied with a torque and proliferation of osteoblasts at roscopic images of the same
wrench (BTI) when recording the titanium surface, a process biopsies are shown in Figure 3c
impressions. that may be favored by chemo- and d. Soft tissue was probably
tactic factors secreted by platelets. lost during biopsy in the con-
Statistical analysis Histochemical studies con- trol implants. Mean 6 SD BIC
Data of BIC were obtained by firmed the tight incorporation of percentages after histomorpho-
IAS 2000 software (Delta Sistemi, implants in newly formed bone metric analysis were 51.28% 6
Rome, Italy) and were expressed tissue in a total of 23 implants in 4.7% (n 13) for the PRGF pro-
as mean 6 SD. The statistical sig- artificial alveoli in the tibia or tocol and 21.89% 6 7.36% (n 10)
nificance between PRGF and non- radius of goats. Biopsies were for the non-PRGF control proto-
PRGF protocols was assessed by obtained after 8 weeks, and typi- col. It is evident that the implants
the Student t test. Values of P , cal histochemically stained sec- with a bioactive surface generated
.05 were considered significant. tions of the implants are shown in a significantly larger area of bone
74 Vol. XXXII / No. Two / 2006
Eduardo A. Anitua
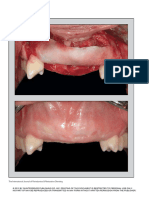
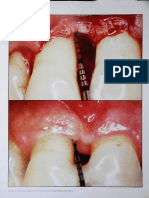
FIGURE 3. Analysis of the effect of the plasma rich in growth factors on bone regeneration around a titanium implant in an animal
model. Bone structure was examined histologically after 8 weeks. (A) A stained section shows the compact cortical structure of the
bone surrounding the entire implant. (B) A typical section from a control implant where soft tissue has been lost from the apical
portion during the biopsy. (C and D) Macroscopic views of similar treated and control implants, respectively.
contact at 2 months than did the 193 women (65.5%) and 72 smokers eighty-two implants (56%) were
control group (P , .01). (24.5%). Mean patient age was 55 placed in the maxilla and 609
years (range: 1984). Of 1391 im- (44%) in the mandible. In the
Implantation success in plants, 992 (71.3%) were placed in maxilla, most of the implants
patients treated with bioactive a 1-stage protocol, 111 (8%) were (n 518) were placed in posterior
surface implants placed in a 2-stage protocol, positions. In the mandible, poste-
A total of 1391 implants were and 288 (20.7%) were immedi- rior positions were also dominant
placed in 265 patients, including ately loaded. Seven hundred (n 520). Osseointegration failed
Journal of Oral Implantology 75
OSSEOINTEGRATION WITH DYNAMIC IMPLANT SURFACE
in 5 implants of 5 patients. All 5 fibronectin, and vitronectin to in- lets as a source of proteins for healing
implants were located in the teract with the implant surface. and tissue regeneration. Thromb Haemost.
2004;91:415.
maxilla: 3 in type IV bone, 1 in Extracellular matrix molecules 6. Sanchez M, Azofra J, Santisteban J,
an alveolar ridge expansion pro- can provide signals for gene et al. Plasma rich in growth factors to
cedure, and 1 in a totally eden- expression through integrin re- treat an articular cartilage avulsion: a
tulous patient. ceptors, so the interaction of local case report. Med Sci Sports Exer. 2003;35:
cells with the matrix could be 16481653.
expected to alter cell function.15 7. Aparicio C, Gil FJ, Planell JA, Engel
DISCUSSION E. Human-osteoblasts proliferation and
Studies in a goat model con- differentiation on grit-blasted and bio-
In light of available knowledge firmed that the PRGF layer active titanium for dental applications.
about the effect of growth factors enhanced ossification around im- J Mater Sci Mater Med. 2002;13:11051111.
on bone healing, new treatment plants. Our findings confirm and 8. Clark RAF. Fibrin and wound heal-
protocols have been developed. build on the results of other ing. Ann N Y Acad Sci. 2001;936:355367.
The gold standard implant treat- 9. Anitua E, Anda I. Un Nuevo
authors. Platelet concentrates Enfoque en la Regeneracion Osea. Anitua
ment protocol11 evolved as a con- have been found to promote bone E, ed. Vitoria, Spain: Puesta al Da
sequence of the introduction of healing after dental implants in Publicaciones; 2000.
new biological concepts in daily a pig model,16 as well as total 10. Nurden P, Savi P, Heilmann E, et
surgical practice. Our new un- tissue ingrowth into porous hy- al. An inherited bleeding disorder linked
derstanding of the healing pro- droxyapatite in a rat model17 or in to a defective interaction between ADP
and its receptor on platelets. J Clin Invest.
cess has led to the routine use of skull defects in rabbits.18 1995;95:16121622.
growth factors in oral clinical 11. Branemark P-I, Zarb GA, Al-
practice. Some of the many uses brektsson T, eds. Tissue-Integrated Prosthe-
of PRGF that have been devised CONCLUSION ses: Osseointegration in Clinical Dentistry.
include filling the alveolus after Chicago, Ill: Quintessence; 1985.
Osseointegration was enhanced
dental extraction,4 compacting 12. Anitua E. Gel di plasma rico di
by covering the implant surface piastrine. Implantol Orale. 2001;3:924.
bone grafts,12 or using them as
with PRGF before insertion into 13. Bessho K, Carnes DL, Cavin R,
biological membranes. We devel- Chen H-Y, Ong JL. BMP stimulation of
the alveolus. The clinical use of
oped a new application for PRGF bone response adjacent to titanium im-
this biologically active surface in
in which it is used to create plants in vivo. Clin Oral Implants Res.
oral implantology might improve 1999;10:212218.
a protein layer covering the im-
the prognosis. Long-term clinical 14. Yamada KM, Clark RAF. Provi-
plant surface. The rationale is that
studies are needed to validate sional matrix. In: Clark RAF, ed. Molecu-
such surfaces may stimulate lar and Cellular Biology of Wound Repair.
the findings of this study.
mechanisms of bone formation New York NY: Plenum; 1996:5193.
at the implant-bone interface, 15. Davis JR, Lofthouse RA,
thus modulating the healing pro- REFERENCES
Frondoza CG, Jinnah RH, Hungerford
cess. Other authors13 have re- JD. Does mechanical stimulation alter
1. Bostrom MPG, Yanf X, Koutras I. integrin expression by human osteo-
ported stimulation of bone
Biologics in bone healing. Curr Opin blasts? J Bone Joint Surg Br. 1999;81B:338.
response adjacent to implants 16. Zechner W, Tangi S, Tepper G,
Orthop. 2000;11:403412.
coated with bovine-purified bone 2. Lieberman JR, Daluiski A, Einhorn et al. Influence of platelet-rich plasma
morphogenetic protein. Th A. The role of growth factors in on osseous healing of dental implants: a
Platelets are activated and re- the repair of bone: biology and clinical histologic and histomorphometry study
lease a number of stimulatory applications. J Bone Joint Surg Am. 2002; in minipigs. Int J Oral Maxillofac Implants.
84-A:10321044. 2003;18:1522.
factors (eg, growth factors and 17. Siebrecht MA, De Rooij PP, Arm
3. Anitua E. Plasma rich in growth
other metabolites) that can pro- factors: preliminary results of use in the DM, Olsson ML, Aspenberg P. Platelet
mote the formation of bone, epi- preparation of future sites for implants. concentrate increases bone ingrowth into
thelium, and blood vessels. Fibrin Int J Oral Maxillofac Implants. 1999;14: porous hydroxyapatite. Orthopedics.
in conjunction with fibronectin 529535. 2002;25:169172.
4. Anitua E. The use of plasma-rich in 18. Kim ES, Choung PH. Platelet
acts as a provisional matrix14 for
growth factors (PRGF) in oral surgery. concentration and its effect on bone
the influx of local cells. These Pract Proced Aesthet Dent. 2001;13:487493. formation in calvarial defects: an exper-
migrating cells use integrin re- 5. Anitua E, Andia I, Ardanza B, imental study in rabbits. J Prosthet Dent.
ceptors that recognize fibrin, Nurden P, Nurden AT. Autologous plate- 2001;86:428433.
76 Vol. XXXII / No. Two / 2006
S-ar putea să vă placă și
- Zygomatic Implants: Optimization and InnovationDe la EverandZygomatic Implants: Optimization and InnovationJames ChowÎncă nu există evaluări
- Periosteal Pocket Flap For Horizontal Bone RegenerDocument10 paginiPeriosteal Pocket Flap For Horizontal Bone RegenerViorel Ion100% (1)
- RevisãosistematicaLousiana2019bom Pra Tec Mole e Quastionavel Pra OssoDocument11 paginiRevisãosistematicaLousiana2019bom Pra Tec Mole e Quastionavel Pra OssoGabriel Filipe Tosin ScivskiÎncă nu există evaluări
- 06.Long-Term Follow-Up On Soft and Hard TissueDocument8 pagini06.Long-Term Follow-Up On Soft and Hard Tissue친절Încă nu există evaluări
- 04 Urban 그리고 Monje - 2019 - Guided Bone Regeneration in Alveolar Bone ReconstrDocument8 pagini04 Urban 그리고 Monje - 2019 - Guided Bone Regeneration in Alveolar Bone Reconstrsupercool0120Încă nu există evaluări
- Keywords: Collagenated Heterologous Bone Graft, Demineralized Bone Matrix, Maxillary Sinus AugmentationDocument8 paginiKeywords: Collagenated Heterologous Bone Graft, Demineralized Bone Matrix, Maxillary Sinus AugmentationJesse Coelho SchardosimÎncă nu există evaluări
- 179-Article Text-302-1-10-20171121 PDFDocument5 pagini179-Article Text-302-1-10-20171121 PDFMichael XuÎncă nu există evaluări
- 99 IJPRD EMD TechDocument10 pagini99 IJPRD EMD Techinamboy7Încă nu există evaluări
- Expansion of The Zone of Keratinised Tissue For Healthy Implant Abutment Interface Using Deepithelized Amnion:Chorion AllograftDocument6 paginiExpansion of The Zone of Keratinised Tissue For Healthy Implant Abutment Interface Using Deepithelized Amnion:Chorion AllograftzethyhanumÎncă nu există evaluări
- Arunjaroensuk 2017Document11 paginiArunjaroensuk 2017everaldocruzÎncă nu există evaluări
- Guided BoneDocument6 paginiGuided BonealeegaleanoÎncă nu există evaluări
- Osseointegration Short Review PDFDocument5 paginiOsseointegration Short Review PDFkryptonites1234Încă nu există evaluări
- Guidedboneregeneration Inalveolarbone Reconstruction: Istvan A. Urban,, Alberto MonjeDocument8 paginiGuidedboneregeneration Inalveolarbone Reconstruction: Istvan A. Urban,, Alberto MonjeVanessa Iñiguez ArmijosÎncă nu există evaluări
- Rasperini 2013Document9 paginiRasperini 2013Alexa LoyaÎncă nu există evaluări
- Current Clinical Concepts in Regenerative Periodontal Therapy PDFDocument8 paginiCurrent Clinical Concepts in Regenerative Periodontal Therapy PDFFergy Christin MaitimuÎncă nu există evaluări
- Combined Therapy With Mineral Trioxide ADocument5 paginiCombined Therapy With Mineral Trioxide AMihaela TuculinaÎncă nu există evaluări
- 2019 Autogenous Soft Tissue Grafting For Periodontal and Peri-Implant Plastic Surgical ReconstructionDocument8 pagini2019 Autogenous Soft Tissue Grafting For Periodontal and Peri-Implant Plastic Surgical Reconstructionayu calisthaÎncă nu există evaluări
- Autogenous Soft Tissue Grafting For Periodontal and Peri-Implant Plastic Surgical ReconstructionDocument8 paginiAutogenous Soft Tissue Grafting For Periodontal and Peri-Implant Plastic Surgical ReconstructionjeeveshÎncă nu există evaluări
- Prosthetically Driven Zygomatic Implant TherapyDocument4 paginiProsthetically Driven Zygomatic Implant TherapyNikit DixitÎncă nu există evaluări
- Artículo 1 - OstectomíaDocument10 paginiArtículo 1 - OstectomíaEmilio RodríguezÎncă nu există evaluări
- Article About Lab Preparation of Implants For HistoDocument1 paginăArticle About Lab Preparation of Implants For HistodrjonduÎncă nu există evaluări
- The Efficiency of Using Advanced PRF-xenograft Mixture Around Immediate Implants in The Esthetic Zone: A Randomized Controlled Clinical TrialDocument6 paginiThe Efficiency of Using Advanced PRF-xenograft Mixture Around Immediate Implants in The Esthetic Zone: A Randomized Controlled Clinical TrialAnukriti SrivastaavaÎncă nu există evaluări
- Histologia MeniniDocument10 paginiHistologia Meninijuanita enriquezÎncă nu există evaluări
- Tissue Characteristics at Microthreaded Implants: An Experimental Study in DogsDocument7 paginiTissue Characteristics at Microthreaded Implants: An Experimental Study in Dogsapi-26419872Încă nu există evaluări
- Review Article: Guided Bone Regeneration: A Literature ReviewDocument16 paginiReview Article: Guided Bone Regeneration: A Literature ReviewMazenShbeebÎncă nu există evaluări
- Effect of Hyaluronic Acid On The Osseointegration of Dental ImplantsDocument5 paginiEffect of Hyaluronic Acid On The Osseointegration of Dental Implantsjaviers45Încă nu există evaluări
- Widyastuti 2018 IOP Conf. Ser. Earth Environ. Sci. 217 012033Document7 paginiWidyastuti 2018 IOP Conf. Ser. Earth Environ. Sci. 217 012033Khusnul Fatimah AzzahraÎncă nu există evaluări
- Windisch 등 - 2021 - Vertical-guided bone regeneration with a titaniumDocument12 paginiWindisch 등 - 2021 - Vertical-guided bone regeneration with a titaniumsupercool0120Încă nu există evaluări
- Holtzclaw2013 PDFDocument7 paginiHoltzclaw2013 PDFpallav GanataÎncă nu există evaluări
- Intaglio JPDDocument7 paginiIntaglio JPDEddie Ojeda HoffmannÎncă nu există evaluări
- SeptodontDocument26 paginiSeptodontAnonymous SgYG1NÎncă nu există evaluări
- Intraoral Versus Extraoral Cementation of Implant Supported Single Crowns Clinical, Biomarker, and Microbiological ComparisonsDocument10 paginiIntraoral Versus Extraoral Cementation of Implant Supported Single Crowns Clinical, Biomarker, and Microbiological ComparisonsBagis Emre GulÎncă nu există evaluări
- Jurnal Periapikal 2Document6 paginiJurnal Periapikal 2Rahmania AlikhlasÎncă nu există evaluări
- 01 Id 0000188379 46220 91Document8 pagini01 Id 0000188379 46220 91Alejandro FereñoÎncă nu există evaluări
- The Team ApproachDocument2 paginiThe Team ApproachThe SmileÎncă nu există evaluări
- 1 Position Paper RegeneracionDocument22 pagini1 Position Paper RegeneracionKatia AguilarÎncă nu există evaluări
- Srep 32045Document13 paginiSrep 32045NitikaÎncă nu există evaluări
- Immediate Prostheses On One Piece Trans Mucosal Implants in Flapless Surgical Procedures Case Series Report Part I Full Arch RehabilitationsDocument6 paginiImmediate Prostheses On One Piece Trans Mucosal Implants in Flapless Surgical Procedures Case Series Report Part I Full Arch RehabilitationsSalem Fathi BermawiÎncă nu există evaluări
- Ramus Onlay Bone Graft For Maxillary Alveolar Ridge Augmentation-A Case ReportDocument9 paginiRamus Onlay Bone Graft For Maxillary Alveolar Ridge Augmentation-A Case ReportIJAR JOURNALÎncă nu există evaluări
- Simion 2012)Document13 paginiSimion 2012)SergioÎncă nu există evaluări
- Tunnel Access For Guided Bone Regeneration in The Maxillary AnteriorDocument6 paginiTunnel Access For Guided Bone Regeneration in The Maxillary Anteriormax100% (1)
- Art Perfil de Emergencia Implantes PDFDocument7 paginiArt Perfil de Emergencia Implantes PDFdiego alenadro trujilloÎncă nu există evaluări
- Ab 2019Document7 paginiAb 2019Dome CárdenasÎncă nu există evaluări
- Guided Bone Regeneration of An Atrophic Mandible With A Heterologous Bone BlockDocument6 paginiGuided Bone Regeneration of An Atrophic Mandible With A Heterologous Bone BlockGabriela Marie CruzÎncă nu există evaluări
- A New Porcine-Derived Collagen Matrix To Thicken Peri-Implant Soft Tissues in High Aestethic Value AreasDocument1 paginăA New Porcine-Derived Collagen Matrix To Thicken Peri-Implant Soft Tissues in High Aestethic Value AreasEdoardo BiancoÎncă nu există evaluări
- Techniques On Vertical Ridge Augmentation IndicatiDocument30 paginiTechniques On Vertical Ridge Augmentation IndicatiFelix SchweppeÎncă nu există evaluări
- Mandibular Reconstruction With Tissue EngineeringDocument5 paginiMandibular Reconstruction With Tissue EngineeringtamisatnamÎncă nu există evaluări
- Histomorphometric Analysis of A Half Hydroxyapatite-Coated Implant in Humans: A Pilot StudyDocument7 paginiHistomorphometric Analysis of A Half Hydroxyapatite-Coated Implant in Humans: A Pilot Studyinamboy7Încă nu există evaluări
- Endodontic and Surgical Treatment of Chronic Apical Periodontitis A Randomized Clinical StudyDocument9 paginiEndodontic and Surgical Treatment of Chronic Apical Periodontitis A Randomized Clinical StudyexpertodentalboliviaÎncă nu există evaluări
- French 2019Document7 paginiFrench 2019Netra TaleleÎncă nu există evaluări
- Periodontal Regeneration Using Connective Tissue.17Document4 paginiPeriodontal Regeneration Using Connective Tissue.17Surya SharmaÎncă nu există evaluări
- Biomaterials: Contents Lists Available atDocument13 paginiBiomaterials: Contents Lists Available atMaria Beatriz Solis ValenciaÎncă nu există evaluări
- Implante Articulo 4Document8 paginiImplante Articulo 4alessandrachumbimuneÎncă nu există evaluări
- 10 1111@cid 12748Document8 pagini10 1111@cid 12748Behnam TaghaviÎncă nu există evaluări
- Articulo en Inglés 2Document8 paginiArticulo en Inglés 2Luis Ramirez BenaventeÎncă nu există evaluări
- Apiko 3Document4 paginiApiko 3Asri DamayantiÎncă nu există evaluări
- Simplified Papila Cortellini 1999Document13 paginiSimplified Papila Cortellini 1999Razvan SalageanÎncă nu există evaluări
- Periosteal Pocket Flap Technique. A Comparative StudyDocument10 paginiPeriosteal Pocket Flap Technique. A Comparative StudyosiglesiÎncă nu există evaluări
- DelFabro2021-Materiales para Sellado PostextracciónDocument19 paginiDelFabro2021-Materiales para Sellado PostextracciónMaria Gabriela ChapaÎncă nu există evaluări
- Tunkel Comparacion Tecnicas2013Document9 paginiTunkel Comparacion Tecnicas2013SergioÎncă nu există evaluări
- Orthodontics: A Case Study in The Tension Between Guild Standards and CapitalismDocument2 paginiOrthodontics: A Case Study in The Tension Between Guild Standards and CapitalismBagis Emre GulÎncă nu există evaluări
- 1999 115 2 125 132 MeehanDocument8 pagini1999 115 2 125 132 MeehanBagis Emre GulÎncă nu există evaluări
- Impact Resistance of Ceramic Brackets According To Ophthalmic Lenses StandardsDocument8 paginiImpact Resistance of Ceramic Brackets According To Ophthalmic Lenses StandardsBagis Emre GulÎncă nu există evaluări
- 1999 115 2 138 142 NakamuraDocument5 pagini1999 115 2 138 142 NakamuraBagis Emre GulÎncă nu există evaluări
- Class II, Division 1, Case With Multiple Treatment ChallengesDocument5 paginiClass II, Division 1, Case With Multiple Treatment ChallengesBagis Emre GulÎncă nu există evaluări
- Mand Incisor ExtDocument12 paginiMand Incisor ExtLanaÎncă nu există evaluări
- 1999 115 3 227 232 FernandesDocument6 pagini1999 115 3 227 232 FernandesBagis Emre GulÎncă nu există evaluări
- 1999 115 2 166 174 UmemoriDocument9 pagini1999 115 2 166 174 UmemoriBagis Emre GulÎncă nu există evaluări
- 1999 115 1 72 76 Jr.,-McNamaraDocument5 pagini1999 115 1 72 76 Jr.,-McNamaraBagis Emre GulÎncă nu există evaluări
- 1999 115 1 83 88 MiyawakiDocument6 pagini1999 115 1 83 88 MiyawakiBagis Emre GulÎncă nu există evaluări
- Craniofacial Adaptations Induced by Chincup Therapy in Class III PatientsDocument8 paginiCraniofacial Adaptations Induced by Chincup Therapy in Class III PatientsAlla MushkeyÎncă nu există evaluări
- A Longitudinal Cephalometric Study of Postorthodontic Craniofacial ChangesDocument6 paginiA Longitudinal Cephalometric Study of Postorthodontic Craniofacial ChangesBagis Emre GulÎncă nu există evaluări
- 1999 115 1 55 60 HansenDocument6 pagini1999 115 1 55 60 HansenBagis Emre GulÎncă nu există evaluări
- American Journal of Orthodontics and Dentofacial OrthopedicsDocument3 paginiAmerican Journal of Orthodontics and Dentofacial OrthopedicsBagis Emre GulÎncă nu există evaluări
- 10 11607@prd 3968Document8 pagini10 11607@prd 3968Bagis Emre GulÎncă nu există evaluări
- 1999 115 1 89 98 MarotoDocument10 pagini1999 115 1 89 98 MarotoAlla MushkeyÎncă nu există evaluări
- 1999 115 1 29 38 ChangDocument10 pagini1999 115 1 29 38 ChangAlla MushkeyÎncă nu există evaluări
- Shear Bond Strength of Composite, Glass Ionomer, and Acidic Primer Adhesive SystemsDocument5 paginiShear Bond Strength of Composite, Glass Ionomer, and Acidic Primer Adhesive SystemsRaluca GrigoreÎncă nu există evaluări
- Article PDFDocument12 paginiArticle PDFJames LinÎncă nu există evaluări
- 1999 115 1 39 51 ArticoloDocument13 pagini1999 115 1 39 51 ArticoloBagis Emre GulÎncă nu există evaluări
- 1999 115 1 13 23 NelsonDocument11 pagini1999 115 1 13 23 NelsonAlla MushkeyÎncă nu există evaluări
- The International Journal of Periodontics & Restorative DentistryDocument8 paginiThe International Journal of Periodontics & Restorative DentistryBagis Emre GulÎncă nu există evaluări
- 1999 115 1 1 12 Figueroa PDFDocument12 pagini1999 115 1 1 12 Figueroa PDFAlla MushkeyÎncă nu există evaluări
- Implantoplasty Enhancing Peri-Implant Bone Stability Over A 3-Year Follow-Up: A Case SeriesDocument8 paginiImplantoplasty Enhancing Peri-Implant Bone Stability Over A 3-Year Follow-Up: A Case SeriesBagis Emre GulÎncă nu există evaluări
- Physical-Chemical Analyses of Contaminations and Internal Holes in Dental Implants of Pure Commercial TitaniumDocument7 paginiPhysical-Chemical Analyses of Contaminations and Internal Holes in Dental Implants of Pure Commercial TitaniumBagis Emre GulÎncă nu există evaluări
- Inter-Occlusal Appliance For Implant Site Augmentation and Optimal Implant Positioning A Ten-Year Follow-Up Case ReportDocument7 paginiInter-Occlusal Appliance For Implant Site Augmentation and Optimal Implant Positioning A Ten-Year Follow-Up Case ReportBagis Emre GulÎncă nu există evaluări
- Editorial: Developing A Consensus: Abutment/Crown Design For Single-Tooth Implant RestorationsDocument1 paginăEditorial: Developing A Consensus: Abutment/Crown Design For Single-Tooth Implant RestorationsBagis Emre GulÎncă nu există evaluări
- The International Journal of Periodontics & Restorative DentistryDocument9 paginiThe International Journal of Periodontics & Restorative DentistryBagis Emre GulÎncă nu există evaluări
- Photoelastic Analysis of Dynamic Stress Distribution Around Short Implants Restored With Different MaterialsDocument6 paginiPhotoelastic Analysis of Dynamic Stress Distribution Around Short Implants Restored With Different MaterialsBagis Emre GulÎncă nu există evaluări
- In Vitro Analysis of The Fracture Resistance of CAD-CAM Monolithic Lithium Disilicate Molar Crowns With Different Occlusal ThicknessDocument7 paginiIn Vitro Analysis of The Fracture Resistance of CAD-CAM Monolithic Lithium Disilicate Molar Crowns With Different Occlusal ThicknessBagis Emre GulÎncă nu există evaluări
- Calder 2016 - Meta Analysis and Suggested Guidelines For Prevention of Venous Thromboembolism (VTE) in Foot and Ankle SurgeryDocument12 paginiCalder 2016 - Meta Analysis and Suggested Guidelines For Prevention of Venous Thromboembolism (VTE) in Foot and Ankle SurgeryNoel Yongshen LeeÎncă nu există evaluări
- Occlusion 26 / Orthodontic Courses by Indian Dental AcademyDocument77 paginiOcclusion 26 / Orthodontic Courses by Indian Dental Academyindian dental academy100% (1)
- Dental AssistantDocument3 paginiDental Assistantapi-78766098Încă nu există evaluări
- (SUS) Sabbagh Universal Spring2Document12 pagini(SUS) Sabbagh Universal Spring2Ali HmoudÎncă nu există evaluări
- Histology of PulpDocument61 paginiHistology of PulpLom SimloteÎncă nu există evaluări
- The Effects of Qigong On Reducing Stress and Anxiety and Enhancing Body Mind Well BeingDocument10 paginiThe Effects of Qigong On Reducing Stress and Anxiety and Enhancing Body Mind Well BeingSamo JaÎncă nu există evaluări
- Insomnia: Management of Underlying ProblemsDocument6 paginiInsomnia: Management of Underlying Problems7OrangesÎncă nu există evaluări
- History TakingDocument5 paginiHistory Takingolwethu.mpepandukuÎncă nu există evaluări
- Clinical Study of Postpartum Eclampsia: Dr. S. K. Rath, Dr. Swati AgrawalDocument3 paginiClinical Study of Postpartum Eclampsia: Dr. S. K. Rath, Dr. Swati AgrawalSulabh ShresthaÎncă nu există evaluări
- Head Nursing ToolDocument26 paginiHead Nursing ToolJeneva L. LauzonÎncă nu există evaluări
- Technical Specification: Proven To Reduce MRSA, E.coli & Salmonella by 99.9%Document2 paginiTechnical Specification: Proven To Reduce MRSA, E.coli & Salmonella by 99.9%abmanojÎncă nu există evaluări
- Lomba HHD IgdDocument34 paginiLomba HHD IgdIndra nugrahaÎncă nu există evaluări
- Kul Sistem CV Ut Prodi FarmasiDocument34 paginiKul Sistem CV Ut Prodi FarmasiMicho Zhii IntelÎncă nu există evaluări
- Biocon:: Launching A New Cancer Drug in IndiaDocument10 paginiBiocon:: Launching A New Cancer Drug in IndiaDeepak Jangid0% (1)
- US NCP-5 Owner Manual PN 21980-04 With Treatment Pants and Indications A PDFDocument103 paginiUS NCP-5 Owner Manual PN 21980-04 With Treatment Pants and Indications A PDFThanh Dao100% (1)
- NURS FPX 6016 Assessment 1 Adverse Event or Near-Miss AnalysisDocument6 paginiNURS FPX 6016 Assessment 1 Adverse Event or Near-Miss Analysisjoohnsmith070Încă nu există evaluări
- Baystate Med CTR Rapid Response Team Recordwith SBARDocument3 paginiBaystate Med CTR Rapid Response Team Recordwith SBARDanishyana DhiwaneoÎncă nu există evaluări
- About Arteriovenous Overview!!!Document7 paginiAbout Arteriovenous Overview!!!Cj UritaÎncă nu există evaluări
- 6665 PDFDocument4 pagini6665 PDFerindah puspowatiÎncă nu există evaluări
- IDA Maharashtra State Branch Dental Dialogue April-Jun 2012Document36 paginiIDA Maharashtra State Branch Dental Dialogue April-Jun 2012kkarkhanisÎncă nu există evaluări
- Sonography of Normal and Abnormal Thyroid and Parathyroid Glands PDFDocument15 paginiSonography of Normal and Abnormal Thyroid and Parathyroid Glands PDFJheyson Javier Barrios PereiraÎncă nu există evaluări
- SAAOL Heart Centre CaseDocument9 paginiSAAOL Heart Centre CaseMunem ChowdhuryÎncă nu există evaluări
- 2016 AVA NotesDocument7 pagini2016 AVA NotesPremÎncă nu există evaluări
- GERUND EXERCISES Finifabioariani 21000415201008Document3 paginiGERUND EXERCISES Finifabioariani 21000415201008k5hq5v7q2cÎncă nu există evaluări
- Cifras de Refer en CIA de Laboratorio - Harriet LaneDocument18 paginiCifras de Refer en CIA de Laboratorio - Harriet LaneLicea Bco JoseÎncă nu există evaluări
- Health Problems MyselfDocument54 paginiHealth Problems MyselfKrishnaveni Murugesh100% (2)
- Breech PresentationDocument27 paginiBreech PresentationNurkamilawati AristaÎncă nu există evaluări
- Ambulatory CareDocument11 paginiAmbulatory Caremitishahirlekar100% (1)
- PDO Thread Lift Treatment Los AngelesDocument8 paginiPDO Thread Lift Treatment Los AngelesSimmi Goyal0% (1)