Documente Academic
Documente Profesional
Documente Cultură
PV1211 Frantz CE
Încărcat de
Roman MamunDrepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
PV1211 Frantz CE
Încărcat de
Roman MamunDrepturi de autor:
Formate disponibile
3 CE Credits
Thermal Injury
Katie Frantz, DVM
Christopher G. Byers, DVM, DACVECC, DACVIM (SAIM)
MidWest Veterinary Specialty Hospital
Omaha, Nebraska
Abstract: This article addresses the pathophysiology of thermal burns, focusing on the complex inflammatory component, potential
complications, and treatment strategies.
T
hermal injury is a relatively uncommon presentation in vet-
erinary medicine. Contact with an electric heating pad, a hot Zone of hyperemia Epidermis
muffler of a motor vehicle, or an open flame is the most com-
mon inciting cause.1 Severe thermal injuries, particularly full- Zone of stasis Dermis
thickness burns exceeding 30% of total body surface area, provoke
a profound systemic inflammatory response characterized by Subcutaneous
Zone of tissue
leukocyte activation and plasma leakage in the microvasculature coagulation
of tissues or organs remote from the wound.2 Burns may be
caused by exposure to heat (thermal burns), electricity, chemicals,
or radiation.
Thermal Injury Characterization
Thermal injury is characterized by the depth of affected tissue3
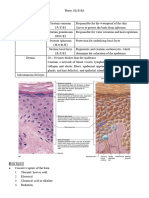
Figure 1. Jackson’s burn model.
(FIGURE 1). Superficial (first-degree) burns involve only the epi-
dermis. The skin is hyperemic, painful, and nonblistered and often
heals in 5 days without scarring. Partial-thickness (second-degree) cellular level, cytoskeletal components are disrupted, resulting in
burns involve the epidermis and the dermis and are further sub- increased membrane permeability. The cell membrane is extremely
categorized as superficial or deep partial-thickness burns. A super- vulnerable to thermal injury because its bilayer lipid component
ficial partial-thickness burn involves the epidermis and one-half is held together by forces of hydration. The disruption in cell
of the dermis. It is characterized by blisters, pain, blanching upon membrane permeability is speculated to be the most important
pressure, and intact hair and takes 2 to 3 weeks to heal. A deep pathophysiologic event leading to tissue death.4
partial-thickness burn leads to destruction of deep dermal layers The severity of a thermal injury depends on the temperature
and may be dry or moist and blistered; the area does not blanch, of the heat source, the size of the affected area, and the duration
and the hair falls out easily. Deep partial-thickness burns heal of contact. Jackson5 originally outlined three different zones evident
slowly with scarring and potential loss of function. Full-thickness on visual examination immediately following a burn injury: the
(third-degree) burns involve all dermal layers. The skin is often zone of coagulation, the zone of stasis, and the zone of hyperemia
dry and leathery and may appear white or charred. This type of (FIGURE 1).
burn results in loss of sensation and therefore may be nonpainful, The zone of coagulation is the area of a burn nearest to the heat
although the tissue has been irreversibly damaged. A third-degree source. This zone suffers the most damage, sustaining blood clotting
burn heals by contracture and epithelial migration or via excision and thrombosis of vessels. Tissue properties are irreversibly altered
and grafting. Third-degree burns may result in the formation of secondary to extensive protein denaturation.4 The zone of stasis
eschar, a hard, leathery product.3 surrounds the zone of coagulation and is characterized by decreased
blood flow (blood stasis). Edema forms in this zone due to vaso-
Local Response to Thermal Injury dilation and increased microvascular permeability. Excessive local
Thermal injury results in the disruption of cell membrane integrity, edema is followed by hypoperfusion, further exacerbating local
activation of cytokines, and cessation of local blood flow.4 At the tissue ischemia. The tissue in this zone is potentially salvageable
Vetlearn.com | December 2011 | Compendium: Continuing Education for Veterinarians® E1
©Copyright 2011 Vetstreet Inc. This document is for internal purposes only. Reprinting or posting on an external website without written permission from Vetlearn is a violation of copyright laws.
Thermal Injury
and is therefore the focus of most burn resuscitation therapies, may be in a state of compensated shock. Additionally, hypertension
which are aimed at restoring perfusion to the affected area to may be the result of pain, and appropriate analgesics should be
prevent irreversible damage.6 The zone of hyperemia is the administered (see Treatment of Thermal Injury, below).
peripheral area around the burn and is characterized by increased The hyperalgesia of a thermal injury is the result of a complex
blood flow. The tissue in this zone is likely to recover as long as inflammatory response.8 Numerous cytokines, including inter-
sepsis or prolonged hypotension do not ensue. leukin-1 (IL-1), interleukin-6 (IL-6), and TNF-α, have been evaluated
The body’s response to thermal injury may be described in and used as markers of the severity of thermal injury in people.8
four stages.7 Stage 1, the emergent phase, occurs within minutes to Hyperalgesic cytokines IL-1β, IL-6, and TNF-α are synthesized
hours of the initial injury and is characterized by a pain response, and released in response to injury,7 in addition to several other
catecholamine release, and subsequent tachycardia, tachypnea, and inflammatory mediators.8 The increase of these proinflammatory
mild hypertension. Stage 2, the fluid shift phase, lasts for 18 to 24 cytokines leads to pain, hypermetabolism, and tissue catabolism.8
hours. During this phase, damaged cells initiate the inflammatory The hypermetabolic response that follows thermal injury is
response. This local phenomenon results in a shift of fluid from characterized by an elevated body temperature, augmented oxygen
the intracellular space to the extracellular space, causing capillary and glucose consumption, and increased carbon dioxide production,
leakage and substantial edema. Stage 3, the hypermetabolic phase, glycogenolysis, proteolysis, and lipolysis.7 The elevation in body
may persist for days to weeks, and may be thought of as a period temperature is often mild and should be distinguished from fever
of profound increase in nutritional needs for proper tissue healing. secondary to infection, which is often more pronounced (>103.5°F)
Stage 4 is the resolution phase and results in scar formation with and may be accompanied by other changes, such as elevated white
a gradual return to normal tissue function. These stages typically blood cell count or an obvious source of infection. In people, the
follow a consecutive pattern, although some overlap between hypermetabolic response following thermal injury has been
stages may occur. reported to last up to 24 months.7 During this phase, there is loss of
lean body mass and bone density, muscle weakness, and delayed
Systemic Response to Thermal Injury wound healing.7
When a thermal injury affects more than 30% of total body surface
area (TBSA), the release of cytokines and other inflammatory Potential Complications of Thermal Injury
mediators at the site of the injury has a profound systemic effect.6 Insulin resistance associated with hepatocyte damage is common
The inflammatory response is initiated immediately after the burn in thermal injury patients. In human medicine, insulin adminis-
and may persist for several months,7 affecting the cardiovascular tration has been shown to improve survival and decrease the rate
system at many levels. Myocardial contractility is decreased, likely of infection.9 Insulin decreases the synthesis of proinflammatory
secondary to release of tumor necrosis factor-α (TNF-α) from cytokines and aids in the restoration of hepatic homeostasis by
injured tissue.6 Increased capillary permeability leads to loss of modifying damaged signaling pathways after thermal injury.9
intravascular proteins and fluid into the interstitial compartment. Hyperglycemia is common in burn patients and is secondary to
The combination of hypovolemia and negative inotropy results an increased rate of glucose production with impaired extraction
in hypotension and hypoperfusion. Inflammatory mediators such of glucose by the damaged tissues. Jeschke et al10 found that
as leukotrienes, histamine, and interleukin may induce broncho- patients with poor glycemic control had significantly higher rates
constriction, leading to acute lung injury and respiratory distress.6 of bacteremia, fungemia, and mortality. Interestingly, the current
All of these factors contribute to systemic inflammatory response practice of tight glycemic control in critically ill humans stems
syndrome (SIRS). The mainstay of treatment for patients with SIRS from early research evaluating glycemic control in thermal injury
consists of treatment of the underlying disease process and aggres- patients. Tight glycemic control remains a very controversial topic
sive supportive care (see Treatment of Thermal Injury, below). in both human and veterinary medicine and cannot be recom-
Hypoalbuminemia develops as a result of increased vascular mended as a therapy for veterinary patients at this time.
permeability and is exacerbated by up-regulation of acute-phase Adrenal insufficiency (AI), or critical illness–related corticos-
proteins and subsequent decreased hepatic production of albumin. teroid insufficiency, is a relatively uncommon complication of
Hypoalbuminemia perpetuates edema formation, hypotension, thermal injury in human patients.11 The development of AI in
and hypoperfusion. Colloid support with synthetic colloids such people appears to be associated with burns affecting a greater
as hetastarch is frequently necessary in these patients, and if hypo TBSA and older age.11 After a severe burn, a diagnosis of AI greatly
albuminemia is severe (<1.5 g/dL), the administration of canine increases the risk of death. AI after thermal injury results from
or human albumin solution should be considered. severe systemic inflammation leading to sepsis, thrombosis, and
Vasoconstrictive substances such as thromboxane A2/B2, pros- coagulopathy with hemorrhage into the adrenal glands.12 No studies
taglandins, cytokines, and reactive oxygen species produced at have examined the incidence of AI in veterinary burn patients.
the burn site may produce a normal or increased blood pressure Thus, routine monitoring of adrenal function in veterinary thermal
in thermal injury patients despite profound hypovolemia. A normal injury patients cannot be recommended at this time, but could
blood pressure should not preclude treating a thermal injury be considered in hypotensive patients that are not responding to
patient with aggressive intravenous fluid therapy, as the patient standard fluid resuscitation therapy.
Vetlearn.com | December 2011 | Compendium: Continuing Education for Veterinarians® E2
Thermal Injury
Sepsis is a major cause of death in burn patients. Sepsis may or hypovolemia secondary to fluid administration. Blood urea
result from wound infection, respiratory infection, catheter infection, nitrogen and creatinine should be assessed, along with urine specific
bacterial translocation from decreased gastrointestinal perfusion, gravity. Blood lactate should be measured, as inadequate tissue
and decreased mucosal integrity. Diagnostic criteria for sepsis perfusion may result in hyperlactatemia secondary to anaerobic
include fever, hypothermia (especially in cats), tachycardia, brady- metabolism. Moderate to marked elevations in blood lactate should
cardia (especially in cats), tachypnea, altered mental status, signifi- alert the clinician that more aggressive fluid therapy is warranted.
cant edema or positive fluid balance, hyperglycemia in the absence Additional parameters for monitoring patient fluid requirements
of diabetes, and hypoglycemia.1 Identification and treatment of include packed cell volume/total solids, electrolytes, and central
septic patients is discussed elsewhere in the veterinary literature. venous pressure (maintain between 3 and 7 cm H2O). Colloid
oncotic pressure (COP) should be measured if possible, and
Treatment of Thermal Injury colloid therapy (such as hetastarch) should be instituted if COP
The current practice of burn care in people is focused on antimi- is <15 mm Hg. Central venous pressure is regarded as the gold
crobial control of wound infection, enteral nutrition, analgesia, standard for practical assessment of intravascular volume in vet-
early surgery, initiation of anabolic steroid and β-blockade therapy, erinary patients. However, this measurement requires placement
and use of artificial/cultured skin where appropriate.13 The same of a central catheter, which may be risky in a patient that is pre-
level of care may be applied to veterinary patients. disposed to hypercoagulability.
In addition to replacing the tremendous volume of fluid lost with-
Acute Management in the first 24 hours, high fluid rates should be used to protect the
All veterinary burn patients should be provided with oxygen, intrave- renal tubules from myoglobin-induced injury and other waste prod-
nous fluids, and analgesia as well as assessed for shock and treated ucts from damaged tissue. Fluid therapy should be tailored to patient
accordingly. If inhalation injury is suspected, oxygen therapy should needs, and changes should be made based on patient monitoring.
be continued and fluid therapy should be more conservative to pre-
vent exacerbation of possible pulmonary capillary leak syndrome. Monitoring
The corneal epithelium is sensitive to temperature, and corneal Between days 2 and 6 after the injury, patients should be closely
ulcers are common secondary to thermal injury. Therefore, in monitored for anemia, immune dysfunction, SIRS, early burn
patients that have sustained burns from fires and were exposed to wound infection, and disseminated intravascular coagulation
smoke and high temperatures, the eyes should be treated with (DIC).1 Early laboratory changes associated with DIC include
lubricating and antibacterial ophthalmic medications. thrombocytopenia, prolonged PT, prolonged aPTT, hypofibrino-
Immediately following a thermal burn, the area should be genemia or hyperfibrinogenemia, low antithrombin levels, and
cooled with water or damp towels for 10 minutes to help prevent presence of fibrin degradation products or D-dimers. The diagnosis
further thermal injury in the absence of heat. Even once the heat and treatment of DIC are discussed in depth elsewhere.
is removed, tissue may continue to burn. However, cold water or
ice should be avoided, as they may cause vasoconstriction and Pain Management
increased wound depth.3 Due to the increased risk for hyperco- Inadequate pain management is detrimental to burn patients.
agulability in burn patients, jugular catheters should be avoided Immediate pain is due to stimulation of skin nociceptors, and
if possible. Patients with burns involving more than 20% of their thermal injury causes the release of chemical mediators that sensitize
TBSA may develop severe metabolic derangements, and appropriate active nociceptors at the site of injury.14 Burn patients may experience
diagnostic samples should be obtained once the patient is stabilized.1 both nociceptive and neuropathic pain. Continuous or repeated
Initial diagnostic tests should include a complete blood count, peripheral stimulation of nociceptive afferent fibers induces an
serum biochemical profile, urinalysis, prothrombin time (PT), increase in dorsal horn excitability via N-methyl-d-aspartate
activated partial thromboplastin time (aPTT), and orthogonal (NMDA) receptors, leading to increased sensitivity in the un-
thoracic radiography. In patients with burns involving >50% of damaged skin surrounding a burn.14 Peripheral and central sen-
their TBSA, the prognosis is poor to guarded, and euthanasia sitization contribute to the pathophysiology of burn pain; therefore,
should be discussed with the owner.1 analgesia should address both of these pain mechanisms.15
Analgesia should be provided with pure µ agonists such as
Fluid Therapy fentanyl (3 to 10 µg/kg/h CRI), hydromorphone (0.025 mg/kg/h
The greatest amount of fluid loss in thermal injury patients occurs CRI), and morphine (0.5 to 1 mg/kg SC q4h). Lidocaine (1.5 to 3
during the first 24 hours, secondary to increased microvascular mg/kg/h CRI) may be used as an adjunctive analgesic and as a
permeability. Patients receiving IV fluids should be monitored free radical scavenger in dogs and may be used cautiously in
closely for changes in body weight and abnormalities detected on cats.2,3 Ketamine (loading dose: 0.5 mg/kg IV; maintenance dose:
physical examination (skin turgor, heart rate and pulse quality, 2 to 5 µg/kg/min CRI) and gabapentin (7 to 10 mg/kg PO q12h)
mucous membrane color, and capillary refill time). If an indwelling are also particularly beneficial in canine and feline thermal
urinary catheter is in place, urine output can be compared with injury patients. Ketamine has been used for more than 40 years
fluid input to help guide fluid therapy and prevent hypervolemia in the treatment of burn pain in people. Ketamine inhibits
Vetlearn.com | December 2011 | Compendium: Continuing Education for Veterinarians® E3
Thermal Injury
Table 1. Systemic Antibiotics Commonly Used During Burn Wound Management1
Key Drug Drug Class Dosage Frequency Route Indications
Amoxicillin or Extended-spectrum 15–22 mg/kg q6–8h PO (amoxicillin), Infection, dirty wound
ampicillin penicillin
IV/IM (ampicillin)
Amoxicillin–clavulanic Extended-spectrum 13.75–20 mg/kg q8–12h PO Superficial wound
acid penicillin with
β-lactamase inhibitor
Amoxicillin– Extended-spectrum 13.75–20 mg/kg q8–12h IV, IM Superficial wounds
sulbactam pencillin with
β-lactamase inhibitor
Cefazolin Cephalosporin 22 mg/kg q6–8h IV, SC Infection, dirty wound
Enrofloxacin Fluoroquinolone 5–20 mg/kg q24h or divided q12h IV Infection, dirty wound
(do not exceed
5 mg/kg q24h in cats)
Metronidazole Antimicrobial 7–10 mg/kg q8–12h PO, IV Anaerobic infection,
dirty wound
γ-aminobutyric acid and may also block serotonin, norepineph- applying a bandage with a nonadherent, porous inner layer that
rine, and dopamine in the central nervous system. Ketamine can allows the passage of fluids and exudates and an absorbent gauze/
also inhibit NMDA receptors in the central nervous system and pad outer layer. Full-thickness burns should be excised at the end
decrease the wind-up effect.16 Gabapentin is an antihyperalgesic of the first week if possible. Before excision, conservative man-
drug that selectively affects central sensitization.17 In people, agement of burn wounds should entail hydrotherapy, removal of
gabapentin has been shown to reduce opioid consumption and necrotic tissue, topical therapy, and bandaging.1 This helps to
lower pain scores secondary to its ability to prevent central hype- prevent wound sepsis and SIRS as well as to decrease morbidity
ralgesia.17 Appropriate analgesia in thermal injury patients and mortality and down-regulate the hypermetabolic response.3
should be multimodal, incorporating antagonism of nociceptive Another option for more conservative management is enzymatic
and neuropathic pain pathways. debridement. This procedure entails the use of topical agents
such as trypsin-balsam of Peru castor oil to soften, loosen, and digest
Wound Management necrotic tissue. This facilitates easy removal of necrotic tissue with
Early excision and closure of the burn wound has been a major gentle lavage. Enzymatic debridement should only be considered in
advancement in treating human patients with severe thermal injuries the very early stages of burn management and must be discontinued
in the past 20 years.18 Although early wound closure is ideal, it should once a healthy granulation bed has formed.1 Full-thickness burns
not take place for the first 3 to 7 days until the full extent of the wound typically require skin grafts or flaps in order to achieve complete
is apparent.1 Wound care is best provided by first cleaning the area closure.
with a mild, water-based antiseptic such as povidone-iodine solution
or 0.25% chlorhexidine solution, and then applying silver sulfadiaz- Antibiotic Therapy
ine.3 Alternatively, aloe vera cream may be used for its antithrom- Significant thermal injuries induce a state of immunosuppression
boxane effects to prevent vasoconstriction and thromboembolic that predisposes patients to infectious complications. Most deaths
seeding of the microcirculation.1 Unpasteurized honey has also been in severely burned human patients are due to burn wound sepsis.19
used with favorable outcomes in thermal injury management. Honey Topical antibiotics are the antimicrobial of choice in thermal injury
reportedly decreases inflammatory edema, accelerates sloughing patients; systemic broad-spectrum antibiotics are not indicated
of necrotic tissue, and provides a good energy source for develop- unless there is evidence of immunosuppression, wound infection,
ment of granulation tissue.1 Other topical agents that may be used pneumonia, and/or sepsis. Examples of antibiotics commonly
in thermal injury wound management are mafenide acetate (a sul- used in the management of wound infections are listed in TABLE 1.
fonamide) and Polysporin (polymyxin B sulfate and bacitracin;
Johnson & Johnson).18 Sterile gloves should be worn at all times to Metabolic Management
protect the patient, and the wound should be cleaned on a daily Due to burn patients’ extreme state of catabolism, nutritional
basis until excision and closure can take place. support is vital for a positive outcome. Additionally, enteral
Provision of moisture to the affected area(s) following the nutrition may decrease the risk of translocation by promoting GI
application of topical agents is crucial and may be accomplished by motility.
Vetlearn.com | December 2011 | Compendium: Continuing Education for Veterinarians® E4
Thermal Injury
Several therapies have been developed in attempt to attenuate References
the hypermetabolic response to thermal injury. Pharmacologic 1. Thermal burn injury. In: Silverstein D, Hopper K, eds. Small Animal Critical Care
Medicine. Philadelphia, PA: Saunders; 2008.
interventions such as administration of growth hormone, insulin-
2. Breslin JW, Wu MH, Guo M, et al. Toll-like receptor 4 contributes to microvascular
like growth factor-1 (IGF-1), oxandrolone (an anabolic steroid), inflammation and barrier dysfunction in thermal injury. Shock 2007;29(3):349-355.
testosterone, insulin, and propranolol have been successfully 3. Jutkowitz AL. Care of the burned patient. Proc IVECCS 2005.
used to dampen the catabolic state that results from thermal injury 4. Orgill DP, Porter SA, Taylor HO. Heat injury to cells in perfused systems. Ann N Y Acad
in people.18 Many studies using propranolol and IGF-1 together Sci 2005;1066:106-118.
5. Jackson DM. The diagnosis of the depth of burning. Br J Surg 1953;40(164):588-596.
to address the hypermetabolic phase in human burn patients 6. Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ
have shown promise.9,10,18 Jeschke et al7 recently reported that 2004;328(7453):1427-1429.
propranolol administration attenuated lipolysis and the hepatic 7. Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe
acute-phase response; furthermore, β blockade decreased urinary burn injury. Ann Surg 2008;248(3):387-401.
8. Gauglitz G, Song J, Herndon DN, et al. Characterization of the inflammatory response
nitrogen loss, peripheral lipolysis, whole body urea production,
during acute and post-acute phases after severe burn. Shock 2008;30(5):503-507.
and resting energy exposure. These therapies are in the early 9. Gauglitz G, Herndon D, Jeschke M. Insulin resistance postburn: underlying mechanisms
phases of use in people, and it is possible that veterinarians might and current therapeutic strategies. J Burn Care Res 2008;29(5):683-694.
achieve the same beneficial results with propranolol, insulin, and 10. Jeschke MG, Boehning DF, Finnerty CC, et al. Effect of insulin on the inflammatory and
oxandrolone in patients in the future. However, no veterinary acute phase response after burn injury. Crit Care Med 2007;35(suppl 9):S519-S523.
11. Reiff D, Harkins C, McGwin, et al. Risk factors associated with adrenal insufficiency
studies evaluating the use of these drugs in thermal injury patients in severely injured burn patients. J Burn Care Res 2007;28(6):854-858.
have thus far been performed, and recommendation of their use 12. Fuchs P, Groger A, et al. Cortisol in severely burned patients: investigations on dis-
is premature at this time. turbance of the hypothalamic–pituitary–adrenal axis. Shock 2007;28(6):662-667.
13. Muller MJ, Pegg SP, Rule MR. Determinants of death following burn injury. Br J Surg
2001;88(4):583-587.
Conclusion 14. Latarjet J. The management of pain associated with dressing changes in patients with
Treatment of thermal injury is an active area of research in human burns. World Wide Wounds 2002. http://www.worldwidewounds.com/2002/november/
medicine. Novel treatment strategies continue to develop as Latarjet/Burn-Pain-At-Dressing-Changes.html. Accessed October 2011.
investigation continues into the inflammatory component of 15. Abdi S, Zhou Y. Management of pain after burn injury. Curr Opin Anesthesiol
2002;15(5):563-567.
burn pathophysiology. Although very little has been published in
16. Plumb DC. Plumb’s Veterinary Drug Handbook. 5th ed. Hoboken, NJ: Wiley-Blackwell;
the veterinary literature on this topic, much can be extrapolated 2005.
from human studies. Further research is warranted to investigate 17. Cuignet O, Pirson J, Soudon O, Zizi M. Effects of gabapentin on morphine consumption
a variety of novel therapies, particularly the use of oxandrolone, and pain in severely burned patients. Burns 2006;33(1):81-86.
propranolol, insulin, and IGF-1β in veterinary burn patients. 18. Gomez M, Wong DT, Stewart TE, et al. The FLAMES score accurately predicts mortality
risk in burn patients. J Trauma 2008;65(3):636-645.
Understanding the pathophysiology of thermal injury is vital to 19. Jeschke MG, Finnerty CC, Kulp GA, et al. Combination of recombinant human growth
developing effective treatment strategies and highlights the myriad hormone and propranolol decreases hypermetabolism and inflammation in severely
roles the immune system plays in critical illness. burned children. Pediatr Crit Care Med 2008;9(2):209-216.
Vetlearn.com | December 2011 | Compendium: Continuing Education for Veterinarians® E5
Thermal Injury
This article qualifies for 3 contact hours of continuing education credit from the Auburn University College of Veterinary Medicine. CE tests
must be taken online at Vetlearn.com; test results and CE certificates are available immediately. Those who wish to apply this credit to
3 CE Credits fulfill state relicensure requirements should consult their respective state authorities regarding the applicability of this program.
1. Pain response, catecholamine release, and mild hyperten- 6. For cytokines and other inflammatory mediators released
sion are characteristics of the _______ phase in the body’s at the site of injury to have a systemic effect, more than
response to thermal injury. ____ of the TBSA must be burned.
a. emergent a. 10%
b. fluid shift b. 30%
c. hypermetabolic c. 66%
d. resolution d. 75%
2. Which is speculated to be the most important pathophysi- 7. A full-thickness burn is characterized by
ologic event leading to tissue death after thermal injury? a. blanching upon pressure.
a. disruption in cell membrane permeability b. destruction of deep dermal layers.
b. temperature of the heat source causing the burn c. presence of blisters and pain.
c. inhibition of DNA synthesis and transcription d. hyperemic, painful skin.
d. ischemia
8. Which is not a potential complication of thermal injury?
3. Jackson’s burn model includes the a. sepsis
a. zone of coagulation. b. hyperadrenocorticism
b. zone of stasis. c. hyperglycemia
c. zone of hyperemia. d. adrenal insufficiency
d. all of the above
9. Burn patients experience the greatest amount of fluid loss
4. Which zone in Jackson’s burn model sustains the most during the first _____ after injury.
damage? a. 4 hours
a. zone of stasis b. 24 hours
b. zone of hyperemia c. 48 hours
c. zone of coagulation d. 72 hours
d. zone of necrosis
10. Which analgesics are recommended for IV administration
5. The zone of hyperemia is characterized by in burn patients?
a. increased blood flow. a. pure µ agonists
b. decreased blood flow. b. lidocaine
c. increased microvascular permeability. c. ketamine
d. clotted blood and thrombosis of blood vessels. d. all of the above
Vetlearn.com | December 2011 | Compendium: Continuing Education for Veterinarians® E6
©Copyright 2011 Vetstreet Inc. This document is for internal purposes only. Reprinting or posting on an external website without written permission from Vetlearn is a violation of copyright laws.
S-ar putea să vă placă și
- Introduction To BurnsDocument47 paginiIntroduction To BurnsanushavergheseÎncă nu există evaluări
- Thermal Burns: PrinciplesDocument11 paginiThermal Burns: PrinciplesHafiz KhairunÎncă nu există evaluări
- Pathophysiology of Burns: Dr. Shiara Ortiz-Pujols Burn Fellow NC Jaycee Burn CenterDocument75 paginiPathophysiology of Burns: Dr. Shiara Ortiz-Pujols Burn Fellow NC Jaycee Burn CenterJohnBedaLatawanMalecdanÎncă nu există evaluări
- 6 BurnsDocument4 pagini6 BurnsMoon KillerÎncă nu există evaluări
- Gran QuemadoDocument11 paginiGran QuemadoLisbeth Gaete CórdovaÎncă nu există evaluări
- Burns: Dr. Iye D. Otigba-KolawoleDocument37 paginiBurns: Dr. Iye D. Otigba-KolawoleMaryÎncă nu există evaluări
- Burn Terapi Topikal 1Document75 paginiBurn Terapi Topikal 1Mohamad Rizki DwikaneÎncă nu există evaluări
- BURN NoteDocument3 paginiBURN NoteClarence BravioÎncă nu există evaluări
- Management and Treatment of Burn WoundDocument5 paginiManagement and Treatment of Burn WoundTaqi MehdiÎncă nu există evaluări
- Ringer's Lactated (RL) Solution 4 ML/KG/% Burn For AdultsDocument3 paginiRinger's Lactated (RL) Solution 4 ML/KG/% Burn For AdultsCharmae NaveaÎncă nu există evaluări
- Burns: Samir Jabaiti, MD, FRCS University of JordanDocument7 paginiBurns: Samir Jabaiti, MD, FRCS University of JordanSuhaeb AlmarafyÎncă nu există evaluări
- BurnDocument47 paginiBurnJoanna EdenÎncă nu există evaluări
- burnsDocument26 paginiburnsgemergencycareÎncă nu există evaluări
- Burn Injury PDFDocument82 paginiBurn Injury PDFFirras Salsabila100% (1)
- BURNSDocument69 paginiBURNS57- Kalanidhi mÎncă nu există evaluări
- Nursing Care of Patients With BURNDocument21 paginiNursing Care of Patients With BURNShuciee ImoedtsÎncă nu există evaluări
- Burn management guideDocument36 paginiBurn management guideAshok SabalÎncă nu există evaluări
- Burns 2Document34 paginiBurns 2Vaibhavi JainÎncă nu există evaluări
- Burn InjuryDocument80 paginiBurn InjuryQueena Neysa CalcarinaÎncă nu există evaluări
- BurnDocument47 paginiBurnVanessa Yvonne GurtizaÎncă nu există evaluări
- ABC of Burns Pathophysiology and Types of BurnsDocument4 paginiABC of Burns Pathophysiology and Types of BurnsMaria BudnicÎncă nu există evaluări
- Pathogenesis of Burn Injury (Initial and DelayedDocument3 paginiPathogenesis of Burn Injury (Initial and DelayedKusuma RamadhaniÎncă nu există evaluări
- BurnsDocument16 paginiBurnsSalman KhanÎncă nu există evaluări
- Acute Biologic Crisis - Hand OutDocument48 paginiAcute Biologic Crisis - Hand OutLouis Carlos RoderosÎncă nu există evaluări
- Classification of BurnsDocument4 paginiClassification of BurnsErtania NirmalaÎncă nu există evaluări
- The White Army-BurnsDocument69 paginiThe White Army-BurnsVishnu PriyaÎncă nu există evaluări
- Pamantasan NG Lungsod NG Maynila: (University of The City of Manila)Document23 paginiPamantasan NG Lungsod NG Maynila: (University of The City of Manila)Em Hernandez AranaÎncă nu există evaluări
- Burns: Major and Minor BurnsDocument25 paginiBurns: Major and Minor BurnsTrisha TannerÎncă nu există evaluări
- BurnsDocument3 paginiBurnsShaz Zrin100% (1)
- Environmental and Nutritional Factor in Disease: by Dr. Theresia Indah B, DRG., M.KesDocument48 paginiEnvironmental and Nutritional Factor in Disease: by Dr. Theresia Indah B, DRG., M.KesJay MÎncă nu există evaluări
- Classification of BurnsDocument16 paginiClassification of BurnsMauricio SvÎncă nu există evaluări
- Types of Burns ArticleDocument7 paginiTypes of Burns ArticleSeltri 'ceti' SeptianiÎncă nu există evaluări
- General Information 3.fluid-Remobilization PhaseDocument4 paginiGeneral Information 3.fluid-Remobilization Phasejulie-pearl-632950% (2)
- Añosa Caballo - First Aid For Burns HandoutDocument21 paginiAñosa Caballo - First Aid For Burns HandoutMa. Ferimi Gleam BajadoÎncă nu există evaluări
- Advances A. BackgroundDocument24 paginiAdvances A. Backgroundalistia andiniÎncă nu există evaluări
- Chapter 10: Burns and Frostbite: Assessment and Treatment of Bums Bum Deformities Other Types of Burns FrostbiteDocument8 paginiChapter 10: Burns and Frostbite: Assessment and Treatment of Bums Bum Deformities Other Types of Burns FrostbitepoddataÎncă nu există evaluări
- Burns: James Taclin C. Banez M.D., FPSGS, FPCSDocument48 paginiBurns: James Taclin C. Banez M.D., FPSGS, FPCSCodillia CheongÎncă nu există evaluări
- Rehabilitation Methods For The Burn Injured Individual PDFDocument25 paginiRehabilitation Methods For The Burn Injured Individual PDFNestor BalboaÎncă nu există evaluări
- Ccap - BurnsDocument6 paginiCcap - BurnsAkshadhar KonetiÎncă nu există evaluări
- Case Discussion-Severe Thermal Burn InjuryDocument9 paginiCase Discussion-Severe Thermal Burn InjuryTrixÎncă nu există evaluări
- Problem 2: By: Varla S. G (405090215)Document122 paginiProblem 2: By: Varla S. G (405090215)varlavarleyÎncă nu există evaluări
- Types of BurnsDocument10 paginiTypes of BurnsMamata BeheraÎncă nu există evaluări
- Thermal Injuries Treatment and ManagementDocument43 paginiThermal Injuries Treatment and ManagementSuyanto TonyÎncă nu există evaluări
- Burns and ShockDocument13 paginiBurns and Shockjames garciaÎncă nu există evaluări
- BURNSDocument93 paginiBURNSRobin Mathew0% (1)
- Burn PathophysiologyDocument1 paginăBurn Pathophysiologymitishree6Încă nu există evaluări
- Burn Injury. Frostbite. Elrctrotrauma: Department of General SurgeryDocument135 paginiBurn Injury. Frostbite. Elrctrotrauma: Department of General SurgeryIbrahim AlmahmoudÎncă nu există evaluări
- Region 5 Burn Training Expanded ModuleDocument17 paginiRegion 5 Burn Training Expanded ModuleTin MagbanuaÎncă nu există evaluări
- Definition:: Is Defined As A Wound Caused by Exogenous Agent Leading To Coagulative Necrosis of The TissueDocument16 paginiDefinition:: Is Defined As A Wound Caused by Exogenous Agent Leading To Coagulative Necrosis of The TissueMoad Al-TalouliÎncă nu există evaluări
- Burns FinalDocument84 paginiBurns FinalAsna FasilÎncă nu există evaluări
- Burns: Pathophysiology Types Degree PercentageDocument19 paginiBurns: Pathophysiology Types Degree PercentageanuzÎncă nu există evaluări
- Forensic Medicine Guide on Burn InjuriesDocument15 paginiForensic Medicine Guide on Burn InjuriesSonam JamdarÎncă nu există evaluări
- Konsep Luka BakarDocument6 paginiKonsep Luka BakarDikha PutraÎncă nu există evaluări
- Basic Concepts of Combustion InjuriesDocument6 paginiBasic Concepts of Combustion InjuriesFitriÎncă nu există evaluări
- Picu Protocols-Dr - Mohan Shenoy - 2.8.2010Document117 paginiPicu Protocols-Dr - Mohan Shenoy - 2.8.2010Aimhigh_PPMÎncă nu există evaluări
- 12 BurnsDocument8 pagini12 Burns楊畯凱Încă nu există evaluări
- Standard of Care: Inpatient Physical Therapy Management of Patients With Burns ICD 9 CodesDocument14 paginiStandard of Care: Inpatient Physical Therapy Management of Patients With Burns ICD 9 CodesalizzxÎncă nu există evaluări
- 1 s2.0 S0263931921002404 MainDocument8 pagini1 s2.0 S0263931921002404 MainMarina UlfaÎncă nu există evaluări
- Sexually Related Homicide1Document5 paginiSexually Related Homicide1Roman MamunÎncă nu există evaluări
- DYSBARISMDocument13 paginiDYSBARISMRoman MamunÎncă nu există evaluări
- Deoxyribonucleic Acid (DNA) Identification Act 2014.Document4 paginiDeoxyribonucleic Acid (DNA) Identification Act 2014.Roman Mamun100% (1)
- Brain MappingDocument8 paginiBrain MappingRoman Mamun100% (1)
- Custodial Deaths: Medicolegal Aspects of Death in CustodyDocument6 paginiCustodial Deaths: Medicolegal Aspects of Death in CustodyRoman MamunÎncă nu există evaluări
- Collection and Preservation of Blood Evidence From Crime ScenesDocument17 paginiCollection and Preservation of Blood Evidence From Crime ScenesRoman Mamun100% (1)
- During The Pakistan Regime The Factories ActDocument10 paginiDuring The Pakistan Regime The Factories ActRoman MamunÎncă nu există evaluări
- Information and Communication Technology ActDocument9 paginiInformation and Communication Technology ActRoman MamunÎncă nu există evaluări
- Suicidal BombingDocument11 paginiSuicidal BombingRoman MamunÎncă nu există evaluări
- Bundle of Bones FNLDocument9 paginiBundle of Bones FNLRoman MamunÎncă nu există evaluări
- The Identification of Human Remains Is Important For Both Legal and Humanitarian ReasonsDocument4 paginiThe Identification of Human Remains Is Important For Both Legal and Humanitarian ReasonsRoman MamunÎncă nu există evaluări
- Death Investigation With Compromised Human RemainsDocument14 paginiDeath Investigation With Compromised Human RemainsRoman MamunÎncă nu există evaluări
- Estimating Gestational Age in Forensic MedicineDocument23 paginiEstimating Gestational Age in Forensic MedicineRoman Mamun100% (1)
- Dna FingerprintingDocument3 paginiDna FingerprintingkriedÎncă nu există evaluări
- Abortion and MTP Act and Medicolegal ImportanceDocument64 paginiAbortion and MTP Act and Medicolegal ImportanceRoman MamunÎncă nu există evaluări
- Potential Terrorist Agents (Bioterrorism)Document33 paginiPotential Terrorist Agents (Bioterrorism)Roman MamunÎncă nu există evaluări
- Sports Related Head Injuries..Document31 paginiSports Related Head Injuries..Roman MamunÎncă nu există evaluări
- Fatal Fire Investigations in Forensic MedicineDocument8 paginiFatal Fire Investigations in Forensic MedicineRoman MamunÎncă nu există evaluări
- 6747803Document15 pagini6747803Roman MamunÎncă nu există evaluări
- Short-Term and Long-Term Physical Effects of Exposure To CS SprayDocument3 paginiShort-Term and Long-Term Physical Effects of Exposure To CS SprayRoman MamunÎncă nu există evaluări
- Article 1 160 en PDFDocument6 paginiArticle 1 160 en PDFRoman MamunÎncă nu există evaluări
- Law and Ethics of Abortion Nov2014 PDFDocument22 paginiLaw and Ethics of Abortion Nov2014 PDFRoman MamunÎncă nu există evaluări
- Psychological Autopsy FNLDocument6 paginiPsychological Autopsy FNLRoman MamunÎncă nu există evaluări
- BJBMS 10 282 PDFDocument5 paginiBJBMS 10 282 PDFRoman MamunÎncă nu există evaluări
- Intra Operative DeathsDocument4 paginiIntra Operative DeathsRoman MamunÎncă nu există evaluări
- Information and Communication Technology ActDocument9 paginiInformation and Communication Technology ActRoman MamunÎncă nu există evaluări
- TH e Relationship Between Human Leukocyte Antigens (HLA) and Renal Cell CarcinomaDocument5 paginiTH e Relationship Between Human Leukocyte Antigens (HLA) and Renal Cell CarcinomaRoman MamunÎncă nu există evaluări
- Pulmonary Air EmbolismDocument33 paginiPulmonary Air EmbolismRoman MamunÎncă nu există evaluări
- Bundle of Bones FNLDocument9 paginiBundle of Bones FNLRoman MamunÎncă nu există evaluări
- Anesthetic DeathDocument14 paginiAnesthetic DeathRoman MamunÎncă nu există evaluări
- Biology Curriculum MapDocument2 paginiBiology Curriculum Mapapi-644434957Încă nu există evaluări
- Salivation PDFDocument5 paginiSalivation PDFmehdi mafakheri100% (1)
- AntihypertensivesDocument35 paginiAntihypertensivesjanuajipatriaÎncă nu există evaluări
- Basics of DentistryDocument65 paginiBasics of DentistryHiba Shah100% (1)
- Managing Hatch Rate and Diseases in Catfish EggsDocument6 paginiManaging Hatch Rate and Diseases in Catfish Eggsapi-3737467Încă nu există evaluări
- Blue Legs - A New Long-COVID Symptom How To Test For and Treat It - Docx #@Document27 paginiBlue Legs - A New Long-COVID Symptom How To Test For and Treat It - Docx #@Dan TudorÎncă nu există evaluări
- Feasibility of Muntingia Calabura as WineDocument19 paginiFeasibility of Muntingia Calabura as WineKhen Raselle BaculioÎncă nu există evaluări
- Biological Science Reviewer QuestionsDocument14 paginiBiological Science Reviewer QuestionsRavian Mhe BitonÎncă nu există evaluări
- Managing Cardiac Patients in the ICUDocument26 paginiManaging Cardiac Patients in the ICUvamshidh100% (2)
- CHAPTER II Open BurningDocument6 paginiCHAPTER II Open Burningjedric_14100% (1)
- Renal Vegetarian NutritionDocument2 paginiRenal Vegetarian NutritionSg Balaji100% (1)
- Cannabis Use and Disorder - Epidemiology, Comorbidity, Health Consequences, and Medico-Legal Status - UpToDateDocument34 paginiCannabis Use and Disorder - Epidemiology, Comorbidity, Health Consequences, and Medico-Legal Status - UpToDateAnonymous kvI7zBNÎncă nu există evaluări
- Guidelines For Distt Hospitals - Indian Public Health StandardsDocument124 paginiGuidelines For Distt Hospitals - Indian Public Health StandardsTejinder SinghÎncă nu există evaluări
- Faecal AnalysisDocument83 paginiFaecal AnalysisJoseph SabidoÎncă nu există evaluări
- Hydrocephalus IDocument52 paginiHydrocephalus IVlad Alexandra50% (2)
- Group 2 Research G8Document24 paginiGroup 2 Research G8aurasaliseÎncă nu există evaluări
- Govorno Jezicki Poremecaji Razvojnog Doba Speech and Language Disorders at Developmental AgeDocument259 paginiGovorno Jezicki Poremecaji Razvojnog Doba Speech and Language Disorders at Developmental AgeVeljko BorovinaÎncă nu există evaluări
- Plant Based Diet A Way To Healthier Life: September 2020Document9 paginiPlant Based Diet A Way To Healthier Life: September 2020MihryazdÎncă nu există evaluări
- Clinical Procedures For Medical Assistants 9th Edition Bonewit Test BankDocument12 paginiClinical Procedures For Medical Assistants 9th Edition Bonewit Test Bankcarriejordanwboriaqmfn100% (16)
- Radiology Report-R5016547Document4 paginiRadiology Report-R5016547Rajeev SÎncă nu există evaluări
- Yes, aside from the educational SMS intervention, the groups were treated equally as they both received routine trainingDocument54 paginiYes, aside from the educational SMS intervention, the groups were treated equally as they both received routine trainingYogiÎncă nu există evaluări
- A 55-Year-Old Woman With Shock and Labile Blood PressureDocument11 paginiA 55-Year-Old Woman With Shock and Labile Blood PressureMr. LÎncă nu există evaluări
- 2023061-21 Seminar On The Development and Application of Mobile PaymentDocument3 pagini2023061-21 Seminar On The Development and Application of Mobile PaymentMunkhuu NasaaÎncă nu există evaluări
- Chiropractic Treatment Techniques-2016Document9 paginiChiropractic Treatment Techniques-2016Dr. Mateen ShaikhÎncă nu există evaluări
- Phet Natural SelectionDocument6 paginiPhet Natural Selectionapi-315485944Încă nu există evaluări
- 272 Moobs Adv 26th January 2012Document5 pagini272 Moobs Adv 26th January 2012MatesDonSantosÎncă nu există evaluări
- Earthfall As Told by ArodenDocument6 paginiEarthfall As Told by ArodenKevin FunkÎncă nu există evaluări
- Mark Scheme Unit f211 Cells Exchange and Transport June PDFDocument17 paginiMark Scheme Unit f211 Cells Exchange and Transport June PDFAngkelova ChristinaÎncă nu există evaluări
- Congenital SyphilisDocument28 paginiCongenital SyphilisMeena Koushal100% (4)
- Pharmacology ReviewerDocument21 paginiPharmacology ReviewerCzairalene QuinzonÎncă nu există evaluări
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDe la EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionEvaluare: 4 din 5 stele4/5 (402)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDe la EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityEvaluare: 3.5 din 5 stele3.5/5 (2)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisDe la EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisEvaluare: 4 din 5 stele4/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDe la EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedEvaluare: 5 din 5 stele5/5 (78)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDe la EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityEvaluare: 4 din 5 stele4/5 (13)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDe la EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeÎncă nu există evaluări
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDe la EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsEvaluare: 3.5 din 5 stele3.5/5 (3)
- Techniques Exercises And Tricks For Memory ImprovementDe la EverandTechniques Exercises And Tricks For Memory ImprovementEvaluare: 4.5 din 5 stele4.5/5 (40)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDe la EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsEvaluare: 5 din 5 stele5/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsDe la EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsEvaluare: 4.5 din 5 stele4.5/5 (169)
- The Obesity Code: Unlocking the Secrets of Weight LossDe la EverandThe Obesity Code: Unlocking the Secrets of Weight LossEvaluare: 5 din 5 stele5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesDe la EverandThe Ultimate Guide To Memory Improvement TechniquesEvaluare: 5 din 5 stele5/5 (34)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingDe la EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingEvaluare: 5 din 5 stele5/5 (4)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDe la EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaEvaluare: 4.5 din 5 stele4.5/5 (266)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDe la EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsÎncă nu există evaluări
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDe la EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisEvaluare: 5 din 5 stele5/5 (8)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingDe la EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingEvaluare: 3.5 din 5 stele3.5/5 (33)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.De la EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Evaluare: 4.5 din 5 stele4.5/5 (110)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisDe la EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisEvaluare: 5 din 5 stele5/5 (3)
- The Happiness Trap: How to Stop Struggling and Start LivingDe la EverandThe Happiness Trap: How to Stop Struggling and Start LivingEvaluare: 4 din 5 stele4/5 (1)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDe la EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeEvaluare: 4.5 din 5 stele4.5/5 (253)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsDe la EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsÎncă nu există evaluări
- The Tennis Partner: A Doctor's Story of Friendship and LossDe la EverandThe Tennis Partner: A Doctor's Story of Friendship and LossEvaluare: 4.5 din 5 stele4.5/5 (4)