Documente Academic
Documente Profesional
Documente Cultură
Lecture 4
Încărcat de
angelTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Lecture 4
Încărcat de
angelDrepturi de autor:
Formate disponibile
Proteomics & Genomics Dr.
Vikash Kumar Dubey
Lecture 4: Ion exchange Chromatography –II
Note: This lecture is continuation of previous lecture. Before starting this one
should have clear understanding of relationship of charge on protein and pH
of buffer explained in last class.
Anion Exchange Chromatography: Anion exchange chromatography exploits difference
in surface negative charge of protein or other molecules for separation. Anion exchange
matrix has positively charged functional group. Few common examples of anion exchanger
functional groups are as follows:
As explained in cation exchange chromatography, solid support with these functional groups
can be prepared with various beads. They differ in few properties like flow rate etc. Anion
exchangers based on dextran (Sephadex), agarose (Sepharose) or cross-linked cellulose
(Sephacel) are few common options. Anion exchange Chromatography are performed using
buffers at pH between 7 and 10 and running a gradient from a solution containing just this
buffer to a solution containing this buffer with 1M NaCl. The surface charge of the molecule
(proteins, nucleic acids etc) which bind will be net negative, thus to get binding of a specific
IIT Guwahati Page 1 of 4
Proteomics & Genomics Dr. Vikash Kumar Dubey
protein one should perform purification above the pI of that protein.
The salt in the solution competes for the binding to the column matrix and releases the
protein from its bound state at a given concentration. Proteins separate because the amount of
salt needed to release the protein varies with the external charge of the protein.
Method:
The amount of sample applied on the column is determined by the dimensions of column and
capacity of exchanger used. After packing, column should be washed extensively with
distilled water and then equilibrated with starting buffer. Once the molecules are bound, the
column is washed to equilibrate it in your starting buffer, which should be of low ionic
strength, then the bound molecules are eluted off using a gradient of a second buffer which
steadily increases the ionic strength of the eluent solution. Alternatively, the pH of the eluent
buffer can be modified as to give your protein or the matrix a charge at which they will not
interact and your molecule of interest elutes from the resin. Generally gradient elution is
more common than the isocratic elution. Continuous or stepwise pH and ionic strength
gradient may be used but continuous gradient gives better results in comparison to stepwise.
Generally for cation exchanger both pH and ionic gradient increases whereas with an anion
exchanger the pH gradient decreases and the ionic strength increases.
Anion ( Cl-) is attached to a positively charged matrix. Negatively charged protein competes
with anion and binds to resin. A competing anion concentration (NaCl gradient) replaces the
protein and elution takes place. Protein with less negative charge gets eluted early while those
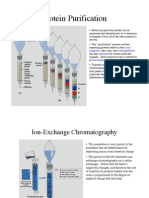
with more negative charge get eluted late (Fig.1 and Fig. 2)
IIT Guwahati Page 2 of 4
Proteomics & Genomics Dr. Vikash Kumar Dubey
Figure 1: Expermental set-up are similar as in case of cation and anion exchange
chromatography
IIT Guwahati Page 3 of 4
Proteomics & Genomics Dr. Vikash Kumar Dubey
Figure 2: A typical chromatogram for an enzyme purification. First protein peak is showing
enzyme activity while the second peak is not active. Thus, enzyme is purified/partially
purified in the first peak. Unwanted proteins are separated in second peak. Purity of the
enzyme in first peak may be checked (we shall discuss methods in coming classes). In case of
partial purification, fractions covering first peak may be pooled for next step of purification.
Quiz:
Explain how ion exchange chromatography may be used for
concentrating a protein?
IIT Guwahati Page 4 of 4
S-ar putea să vă placă și
- Clinical Biochemistry and Metabolic Medicine 2012Document441 paginiClinical Biochemistry and Metabolic Medicine 2012RedaGaafar100% (3)
- Clinical Biochemistry and Metabolic Medicine 2012Document441 paginiClinical Biochemistry and Metabolic Medicine 2012RedaGaafar100% (3)
- CH 5 Ky Thua Protein-Môn hóa sinh họcDocument83 paginiCH 5 Ky Thua Protein-Môn hóa sinh họcNam NguyenHoangÎncă nu există evaluări
- Molecular Cell Biology 7th Edition Lodish Test BankDocument8 paginiMolecular Cell Biology 7th Edition Lodish Test BankLaurenThompsonfpniz100% (15)
- s15 Miller Chap 3b LectureDocument25 paginis15 Miller Chap 3b LectureDorice Clement100% (1)
- 02 Protein IsolationDocument14 pagini02 Protein IsolationAntonio Calleja IIÎncă nu există evaluări
- Laudon - Mis16 - PPT - ch11 - KL - CE (Updated Content For 2021) - Managing Knowledge and Artificial IntelligenceDocument45 paginiLaudon - Mis16 - PPT - ch11 - KL - CE (Updated Content For 2021) - Managing Knowledge and Artificial IntelligenceSandaru RathnayakeÎncă nu există evaluări
- Protein Extraction and QuantificationDocument6 paginiProtein Extraction and QuantificationWÎncă nu există evaluări
- Biochem Chapter 5 Notes. Protein PurificationDocument14 paginiBiochem Chapter 5 Notes. Protein PurificationOAÎncă nu există evaluări
- DocxDocument7 paginiDocxrobomunkey12345Încă nu există evaluări
- Analysis of Protein ConformationDocument36 paginiAnalysis of Protein ConformationNikka Mia AbadiesÎncă nu există evaluări
- Protein Chromatography: Ion Exchange Chromatography AimsDocument4 paginiProtein Chromatography: Ion Exchange Chromatography AimskapilphysioÎncă nu există evaluări
- Elphor Chrom AnswersDocument3 paginiElphor Chrom AnswersAnonymous b14ATJtÎncă nu există evaluări
- CHEM 160 Module 3 Resource 5Document7 paginiCHEM 160 Module 3 Resource 5meyaÎncă nu există evaluări
- GE IEXcolumnsDocument8 paginiGE IEXcolumnsRiri SyaviraÎncă nu există evaluări
- Unit 4 Topic 4. Ion Exchange, Affinity - Theory, Instrumentation and Applications.Document25 paginiUnit 4 Topic 4. Ion Exchange, Affinity - Theory, Instrumentation and Applications.ashra sindhikkaaÎncă nu există evaluări
- Purification of Protiens: Mohan CC M.SC, Biochemistry 1 SemesterDocument24 paginiPurification of Protiens: Mohan CC M.SC, Biochemistry 1 SemesterMelwin MejanÎncă nu există evaluări
- Iex of Biopharmaceuticals 1234Document15 paginiIex of Biopharmaceuticals 1234aa385864Încă nu există evaluări
- Protein Purification: Ionic Properties Hydrophobicity Affinities FractionationDocument11 paginiProtein Purification: Ionic Properties Hydrophobicity Affinities FractionationNas KataÎncă nu există evaluări
- Ion Exchange ChromatographyDocument3 paginiIon Exchange ChromatographysherfudeenÎncă nu există evaluări
- Ion Exchange Chromatography-IECDocument33 paginiIon Exchange Chromatography-IECnindiya20Încă nu există evaluări
- Ion Exchange Chromatography: Cationic Exchangers Possess Negatively Charged Group, and TheseDocument6 paginiIon Exchange Chromatography: Cationic Exchangers Possess Negatively Charged Group, and TheseJylla AngwayÎncă nu există evaluări
- Determination of Protein Molecular Weight: Gel Filtration ChromatographyDocument4 paginiDetermination of Protein Molecular Weight: Gel Filtration ChromatographyDINA PRATIWIÎncă nu există evaluări
- Biochemistry LN06-2Document9 paginiBiochemistry LN06-2Rahaf Al-muhtasebÎncă nu există evaluări
- Labjournal1 For NursingDocument2 paginiLabjournal1 For NursingDanielle MatelÎncă nu există evaluări
- Advanced Analytical Chemistry 1 Separation Technique, ChromatographyDocument18 paginiAdvanced Analytical Chemistry 1 Separation Technique, ChromatographyOmar DoskyÎncă nu există evaluări
- Isolation/Puri Fication of Proteins: GlossaryDocument9 paginiIsolation/Puri Fication of Proteins: GlossaryDiny HazitaÎncă nu există evaluări
- AssignmentDocument4 paginiAssignmentsajidajavaid640Încă nu există evaluări
- Protein PurificationDocument7 paginiProtein PurificationArchana BorahÎncă nu există evaluări
- CHROMATOGRAPHY2Document33 paginiCHROMATOGRAPHY2Nurfatihah ZulkifliÎncă nu există evaluări
- Visualizing Molecules in Living CellsDocument42 paginiVisualizing Molecules in Living CellsmskikiÎncă nu există evaluări
- Nguyễn Thị Ngọc Linh - enzymes assignment 2Document2 paginiNguyễn Thị Ngọc Linh - enzymes assignment 2LinhNguyeÎncă nu există evaluări
- Chembio 2 - Week 5Document4 paginiChembio 2 - Week 5EJÎncă nu există evaluări
- BiochemistryDocument7 paginiBiochemistrymalaver528Încă nu există evaluări
- Techniques To Separate Amino Acids and ProteinsDocument38 paginiTechniques To Separate Amino Acids and ProteinsDawlat SalamaÎncă nu există evaluări
- Org - Unit 6Document63 paginiOrg - Unit 6lijyohannesmekonnen7Încă nu există evaluări
- Chromatographic Protein Refolding/Renaturation: R 2018 Elsevier Inc. All Rights ReservedDocument17 paginiChromatographic Protein Refolding/Renaturation: R 2018 Elsevier Inc. All Rights ReservedMai LinhÎncă nu există evaluări
- BMB 21082Document8 paginiBMB 21082mjkoÎncă nu există evaluări
- Pollock 2007Document11 paginiPollock 2007Carolina TabascoÎncă nu există evaluări
- Tissue Engineering 3Document12 paginiTissue Engineering 3Vidit NarayanÎncă nu există evaluări
- Purification of ProteinsDocument24 paginiPurification of ProteinsDhansukh PatelÎncă nu există evaluări
- Anajali ChromatographyDocument10 paginiAnajali ChromatographyAbhishek BarnwalÎncă nu există evaluări
- Electrophoresis - Cholesterol - Bile AcidsDocument13 paginiElectrophoresis - Cholesterol - Bile Acidsmohammad ahmedÎncă nu există evaluări
- Purification of Proteins Using Ion Exchange Chromatography and Gel Filtration Chromatography PDFDocument4 paginiPurification of Proteins Using Ion Exchange Chromatography and Gel Filtration Chromatography PDFIza SalvadorÎncă nu există evaluări
- CH 5 Lecture SlidesDocument83 paginiCH 5 Lecture SlidesUyên Trần NhưÎncă nu există evaluări
- MBT31 - Proteomics NotesDocument40 paginiMBT31 - Proteomics Notesprb80730Încă nu există evaluări
- The Purification and Analysis of Proteins: The Gray Tree FrogDocument27 paginiThe Purification and Analysis of Proteins: The Gray Tree FrogThomas JonesÎncă nu există evaluări
- MLS 425 Chemical Pathology I Lecture NoteDocument55 paginiMLS 425 Chemical Pathology I Lecture NoteMayowa Ogunmola100% (1)
- BDP - Tutorial 3Document3 paginiBDP - Tutorial 3Johnny LimÎncă nu există evaluări
- BCH 706 Lec 6 CHR Type Ion Exchange - 7591Document40 paginiBCH 706 Lec 6 CHR Type Ion Exchange - 7591zshanali6236Încă nu există evaluări
- Lecture 22 HandoutDocument13 paginiLecture 22 HandoutPragya PandeyÎncă nu există evaluări
- Principles of ChromatographyDocument8 paginiPrinciples of ChromatographylordniklausÎncă nu există evaluări
- Protein Membrane Overlay AssayDocument4 paginiProtein Membrane Overlay AssayZain BaderÎncă nu există evaluări
- CHEM 5 03 Protein DenaturationPurificationCharacterizationDocument19 paginiCHEM 5 03 Protein DenaturationPurificationCharacterizationGeorge GomezÎncă nu există evaluări
- Paper 1 Ion Exchange ChromatographyDocument18 paginiPaper 1 Ion Exchange Chromatographysamruddhi patilÎncă nu există evaluări
- D I F F e R e N T L e V e L o F Different Levels of Structural Hierarchy in ProteinsDocument14 paginiD I F F e R e N T L e V e L o F Different Levels of Structural Hierarchy in ProteinsRivaldo MewohÎncă nu există evaluări
- Ion Exchange ChromatographyDocument9 paginiIon Exchange Chromatographylamolymonika02Încă nu există evaluări
- Ion Exchange ChromatographyDocument10 paginiIon Exchange ChromatographyJUDE serpesÎncă nu există evaluări
- Cell Structure and Function Pretest: The Organelle Has A Phospholipid MembraneDocument28 paginiCell Structure and Function Pretest: The Organelle Has A Phospholipid MembranelayanhaliloÎncă nu există evaluări
- Proteomics Lab ReportDocument4 paginiProteomics Lab Reportnursah.aslanÎncă nu există evaluări
- I. Title of Experiment: Determining The Value of Protein in ADocument29 paginiI. Title of Experiment: Determining The Value of Protein in AAnggraini Nugroho PÎncă nu există evaluări
- PDF PDFDocument9 paginiPDF PDFCindy Nona100% (2)
- Test 2: Electrolytes/renal/acid Base: Clinical Biochemistry and Metabolic MedicineDocument6 paginiTest 2: Electrolytes/renal/acid Base: Clinical Biochemistry and Metabolic MedicineangelÎncă nu există evaluări
- Transition Metal Organometallic Compounds & Catalysis: French Chemist L. C. Cadet 1760 As Me DicacodylDocument41 paginiTransition Metal Organometallic Compounds & Catalysis: French Chemist L. C. Cadet 1760 As Me Dicacodylangel0% (1)
- SKF Shaft Alignment Tool TKSA 41Document2 paginiSKF Shaft Alignment Tool TKSA 41Dwiki RamadhaniÎncă nu există evaluări
- Lotus Exige Technical InformationDocument2 paginiLotus Exige Technical InformationDave LeyÎncă nu există evaluări
- Sanskrit Lessons: �丘��恆� � by Bhikshuni Heng HsienDocument4 paginiSanskrit Lessons: �丘��恆� � by Bhikshuni Heng HsiendysphunctionalÎncă nu există evaluări
- Contigency Plan On Class SuspensionDocument4 paginiContigency Plan On Class SuspensionAnjaneth Balingit-PerezÎncă nu există evaluări
- Atom SDDocument5 paginiAtom SDatomsa shiferaÎncă nu există evaluări
- Uts Cmo Module 5Document31 paginiUts Cmo Module 5Ceelinah EsparazÎncă nu există evaluări
- Beer Pilkhani DistilleryDocument44 paginiBeer Pilkhani DistillerySunil Vicky VohraÎncă nu există evaluări
- Controlled DemolitionDocument3 paginiControlled DemolitionJim FrancoÎncă nu există evaluări
- 7 ElevenDocument80 pagini7 ElevenakashÎncă nu există evaluări
- Pontevedra 1 Ok Action PlanDocument5 paginiPontevedra 1 Ok Action PlanGemma Carnecer Mongcal50% (2)
- Slides - SARSDocument191 paginiSlides - SARSCedric PoolÎncă nu există evaluări
- Nse 2Document5 paginiNse 2dhaval gohelÎncă nu există evaluări
- Plant Report Template Class 81Document2 paginiPlant Report Template Class 81Kamran KhanÎncă nu există evaluări
- Integrator Windup and How To Avoid ItDocument6 paginiIntegrator Windup and How To Avoid ItHermogensÎncă nu există evaluări
- Assignment RoadDocument14 paginiAssignment RoadEsya ImanÎncă nu există evaluări
- Assignment-For-Final of-Supply-Chain - Management of Courses PSC 545 & 565 PDFDocument18 paginiAssignment-For-Final of-Supply-Chain - Management of Courses PSC 545 & 565 PDFRAKIB HOWLADERÎncă nu există evaluări
- Marieb ch3dDocument20 paginiMarieb ch3dapi-229554503Încă nu există evaluări
- How To Be A Better StudentDocument2 paginiHow To Be A Better Studentct fatima100% (1)
- Bathinda - Wikipedia, The Free EncyclopediaDocument4 paginiBathinda - Wikipedia, The Free EncyclopediaBhuwan GargÎncă nu există evaluări
- Malling DemallingDocument25 paginiMalling DemallingVijay KumarÎncă nu există evaluări
- Individual Daily Log and Accomplishment Report: Date and Actual Time Logs Actual AccomplishmentsDocument3 paginiIndividual Daily Log and Accomplishment Report: Date and Actual Time Logs Actual AccomplishmentsMarian SalazarÎncă nu există evaluări
- EDB Postgres Failover Manager Guide v2.1Document86 paginiEDB Postgres Failover Manager Guide v2.1Anggia MauritianaÎncă nu există evaluări
- Auto Turn-Off For Water Pump With Four Different Time SlotsDocument3 paginiAuto Turn-Off For Water Pump With Four Different Time SlotsKethavath Sakrunaik K100% (1)
- CH 15Document58 paginiCH 15Chala1989Încă nu există evaluări
- Rs2-Seamanship (Inc Anchoring, Mooring, Berthing, Pilot Ladder)Document19 paginiRs2-Seamanship (Inc Anchoring, Mooring, Berthing, Pilot Ladder)Mdpn. Salvador67% (3)
- Assembly InstructionsDocument4 paginiAssembly InstructionsAghzuiÎncă nu există evaluări
- AppcDocument71 paginiAppcTomy lee youngÎncă nu există evaluări
- Compact 1.8" Height Standardized Installation 9 Months To Flight Powerful and LightweightDocument2 paginiCompact 1.8" Height Standardized Installation 9 Months To Flight Powerful and LightweightStanley Ochieng' OumaÎncă nu există evaluări
- Energy-Roles-In-Ecosystems-Notes-7 12bDocument10 paginiEnergy-Roles-In-Ecosystems-Notes-7 12bapi-218158367Încă nu există evaluări