Documente Academic
Documente Profesional
Documente Cultură
Copper Bullet Lab
Încărcat de
api-314065532Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Copper Bullet Lab
Încărcat de
api-314065532Drepturi de autor:
Formate disponibile
Anna Chiprean
Haberman
AP Chemistry
March 16 2018
Determination of Copper in Brass Lab
Purpose:
The goal of this experiment is to determine the spectroscopically of the amount of
copper in brass shot.
Procedure:
Create .4 M Cu (NO3)2 (stock solution) out of water and copper nitrate. Dissolve the Cu
(NO3)2 in H20 and add it to a volumetric flask. Determine the value of each diluted solution,
making sure that one cuvette is filled with 10 mL of water and one cuvette is filled with 10 mL
stock solution. M1V1=M2V2 is used to find the rest of your diluted solution contents. Use the
spectrometer to measure the absorbance of you solutions. Fill the cuvettes ¾ of the way full and
record absorbance. Graph your results. To dissolve the brass bullet, determine the amount of
nitric acid. Do this step under a fume hood. Determine the amount of copper present in the brass
sample. Use the spectrometer to determine the absorbance.
Data:
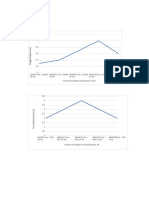
Calibration Chart for Cu(NO3)2
1.4
1.2 y = 2.9862x
R² = 0.9969
1
Absorbance
0.8
0.6
0.4
0.2
0
0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45
Concentration (M)
Solution Concentration Absorption Stock/Water
1 0.4 M 1.190 10 mL Stock Solution
2 0.2 M 0.582 5 mL Stock Solution
+ 5 mL H20
3 0.1 M 0.333 2.5 mL Stock
Solution + 7.5 mL
H20
4 0.05 M 0.158 1.25 mL Stock
Solution + 8.75 ml
H20
5 0.025 M 0.113 0.625 mL Stock
Solution + 9.375ml
H20
6 0.00 M 0.000 10 mL H20
S-ar putea să vă placă și
- A F (Concentratie) : 0.6 F (X) 0.107621917808219 X + 0.007476712328767 R 0.984180901225002Document2 paginiA F (Concentratie) : 0.6 F (X) 0.107621917808219 X + 0.007476712328767 R 0.984180901225002Nastase DamianÎncă nu există evaluări
- Example 1.1 Example 1.1 Example 1.1 Example 1.1 Example 1.1 Solution Solution Solution Solution SolutionDocument1 paginăExample 1.1 Example 1.1 Example 1.1 Example 1.1 Example 1.1 Solution Solution Solution Solution Solutionjamal khanÎncă nu există evaluări
- Name: Orio, Psyber V. Date: October 11, 2020 Degree/Year: BSED SCIENCE 1B NIGHT ScoreDocument4 paginiName: Orio, Psyber V. Date: October 11, 2020 Degree/Year: BSED SCIENCE 1B NIGHT ScoreChanie Baguio Pitogo100% (1)
- Tubo Solucion Patron Solucion Problema Solucion Salina Absorbancia ConclusionDocument2 paginiTubo Solucion Patron Solucion Problema Solucion Salina Absorbancia ConclusionYovanniAbadMoreiraÎncă nu există evaluări
- Informe AnalisisDocument3 paginiInforme AnalisisJesus Daniel OrtizÎncă nu există evaluări
- PP Molarity and DilutionsDocument15 paginiPP Molarity and DilutionsLuisa Gardênia FariasÎncă nu există evaluări
- Worksheet 2 - Chapter 07 (SOLUTION AND SUSPENSION)Document2 paginiWorksheet 2 - Chapter 07 (SOLUTION AND SUSPENSION)Aisha AnwarÎncă nu există evaluări
- Effect of NaOH and acid volume on copper sulfate and HCl reactionsDocument2 paginiEffect of NaOH and acid volume on copper sulfate and HCl reactionsJariyah AmiliaÎncă nu există evaluări
- pH and Buffer CalculationsDocument3 paginipH and Buffer CalculationsRegine Coeli Menta LansanganÎncă nu există evaluări
- Lab Experiment 4Document10 paginiLab Experiment 4Nor FazilahÎncă nu există evaluări
- H-060 Thermo. Titr. Application Note No.: Title: Standardization of 0.1M Perchloric Acid in Glacial Acetic AcidDocument3 paginiH-060 Thermo. Titr. Application Note No.: Title: Standardization of 0.1M Perchloric Acid in Glacial Acetic AcidGunjan KalyaniÎncă nu există evaluări
- Calibration Curve for Total Iron ContentDocument17 paginiCalibration Curve for Total Iron Content510418106 ARITRYAGHOSHÎncă nu există evaluări
- Lab Report (Spectroscopy)Document6 paginiLab Report (Spectroscopy)Levison Kasenga100% (2)
- Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFDocument3 paginiMolarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFrevie100% (1)
- Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFDocument3 paginiMolarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFGerald KamulanjeÎncă nu există evaluări
- Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFDocument3 paginiMolarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12 PDFZeferinix100% (2)
- Normality and Molarity 3 PDFDocument3 paginiNormality and Molarity 3 PDFMayra FlorÎncă nu există evaluări
- Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12Document3 paginiMolarity, Molality, Normality, and Mass Percent Worksheet II Answer Key 11-12Md HossainÎncă nu există evaluări
- Analytical Chemistry First LaboratoryDocument6 paginiAnalytical Chemistry First LaboratoryMELANIE ANTOLINÎncă nu există evaluări
- Practical 1 - Spectrophotometry TechniquesDocument13 paginiPractical 1 - Spectrophotometry TechniquesDhanen DranÎncă nu există evaluări
- Measuring glucose concentration using a spectrophotometerDocument2 paginiMeasuring glucose concentration using a spectrophotometerSebastian SmytheÎncă nu există evaluări
- Courbe D'etalonnageDocument1 paginăCourbe D'etalonnageSara LovaÎncă nu există evaluări
- Lab Report A2Document7 paginiLab Report A2SumayyahÎncă nu există evaluări
- Report Organic Chemistry 18125292 Liu Ni QuynhDocument8 paginiReport Organic Chemistry 18125292 Liu Ni QuynhLe HieuÎncă nu există evaluări
- EXP 5 KoloidDocument12 paginiEXP 5 KoloidLau Yong HuiÎncă nu există evaluări
- T2 Stoichiometry ET4Document24 paginiT2 Stoichiometry ET4qishzz pqazzÎncă nu există evaluări
- V V C C: Mass Ion ConcentratDocument2 paginiV V C C: Mass Ion ConcentratJoshua RuizÎncă nu există evaluări
- Results and Calculation: 3 - (Aq) - (Aq) + (Aq) 3 - (Aq) 2 (Aq)Document20 paginiResults and Calculation: 3 - (Aq) - (Aq) + (Aq) 3 - (Aq) 2 (Aq)myzna_husna_90788547Încă nu există evaluări
- Determining Ethanoic Acid Content in VinegarDocument9 paginiDetermining Ethanoic Acid Content in Vinegarmhd sssyamilÎncă nu există evaluări
- Determining Calcium CarbonateDocument12 paginiDetermining Calcium CarbonateCassyÎncă nu există evaluări
- Medias: Yeast Mannitol AgarDocument7 paginiMedias: Yeast Mannitol AgarKhadijaÎncă nu există evaluări
- CaCO3 Content in EggshellDocument12 paginiCaCO3 Content in Eggshellmhd sssyamilÎncă nu există evaluări
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Document7 paginiFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterÎncă nu există evaluări
- Solution Concentration - Unit 4 ChemDocument32 paginiSolution Concentration - Unit 4 ChemSiskaWahyuniÎncă nu există evaluări
- OBS! For All Solutions, Use Sterilized Distilled Deionized Water!Document1 paginăOBS! For All Solutions, Use Sterilized Distilled Deionized Water!Ingeniero AnonimoÎncă nu există evaluări
- Alat Haspeng AndatDocument13 paginiAlat Haspeng AndatHakamÎncă nu există evaluări
- A. Perhitunganpada Proses Pembuatan Arang Aktif 1. Pembuatan Larutan H PO 10%Document15 paginiA. Perhitunganpada Proses Pembuatan Arang Aktif 1. Pembuatan Larutan H PO 10%saidin fahmi fadillahÎncă nu există evaluări
- Chem 262 Lab#2 ReportDocument2 paginiChem 262 Lab#2 ReportAhmedÎncă nu există evaluări
- ch14 PDFDocument17 paginich14 PDFMyke AguinaldoÎncă nu există evaluări
- Lab Report 4 - Dissolved Oxygen Content of Water: Winkler MethodDocument6 paginiLab Report 4 - Dissolved Oxygen Content of Water: Winkler MethodATHALIAH JENINE TABUCLIN BANTUGÎncă nu există evaluări
- Determination of Nitrate in Wastewater Using Sodium SalicylateDocument13 paginiDetermination of Nitrate in Wastewater Using Sodium SalicylateJuan ManuelÎncă nu există evaluări
- Serial Dilutions AnalysisDocument6 paginiSerial Dilutions AnalysisJasni PagarautomaticÎncă nu există evaluări
- Feo Determination in Rocks and Minerals: by Wet ChemistryDocument5 paginiFeo Determination in Rocks and Minerals: by Wet ChemistryCésar VargasÎncă nu există evaluări
- Practical 14 Iron Wool by Redox TitrationDocument4 paginiPractical 14 Iron Wool by Redox TitrationAngnes PoliskaÎncă nu există evaluări
- Chm580 Experiment 1Document9 paginiChm580 Experiment 1ohhiÎncă nu există evaluări
- Introduction To General Organic and Biochemistry 10Th Edition Bettelheim Solutions Manual Full Chapter PDFDocument33 paginiIntroduction To General Organic and Biochemistry 10Th Edition Bettelheim Solutions Manual Full Chapter PDFarthur.hendricks257100% (13)
- Molarity - Molality and DilutionsDocument13 paginiMolarity - Molality and DilutionsMuhammad AhmedÎncă nu există evaluări
- Bab Iv.1 HasilDocument6 paginiBab Iv.1 HasilCitra KencanaÎncă nu există evaluări
- TITLEDocument2 paginiTITLETishonna DouglasÎncă nu există evaluări
- Spectrophotometric Study of Complexes by Job's Method: Report of The Minor UGC Project EntitledDocument19 paginiSpectrophotometric Study of Complexes by Job's Method: Report of The Minor UGC Project EntitledM Irfan Khan100% (1)
- Exp 4result Discussion For FaDocument4 paginiExp 4result Discussion For FanasuhaÎncă nu există evaluări
- Density LabDocument6 paginiDensity LabAlex GalangÎncă nu există evaluări
- Determinarea CromuluiDocument2 paginiDeterminarea Cromuluiramafares47Încă nu există evaluări
- Lab Report AASDocument8 paginiLab Report AASMohamad Saiful Mohd RaffiahÎncă nu există evaluări
- 6th ChapDocument15 pagini6th ChapAYESHA MUMTAZÎncă nu există evaluări
- chm421 Exp 2 FixedDocument8 paginichm421 Exp 2 Fixedhannannnjihh2002Încă nu există evaluări
- Pengujian Air LimbahDocument6 paginiPengujian Air LimbahMuhammad Ilham RabbaniÎncă nu există evaluări
- Experiment 5 - Data TreatmentDocument6 paginiExperiment 5 - Data TreatmentShawn Ann SilanÎncă nu există evaluări
- Dye Lab 1Document1 paginăDye Lab 1api-314065532Încă nu există evaluări
- Care For CreationDocument7 paginiCare For Creationapi-314065532Încă nu există evaluări
- Lindberg KidnappingDocument6 paginiLindberg Kidnappingapi-314065532Încă nu există evaluări
- Anna ChipreanDocument6 paginiAnna Chipreanapi-314065532Încă nu există evaluări
- Relic PaperDocument7 paginiRelic Paperapi-314065532Încă nu există evaluări
- Sacramentality in LiteratureDocument4 paginiSacramentality in Literatureapi-314065532Încă nu există evaluări
- Anna Chiprean - in Class Chart TestDocument2 paginiAnna Chiprean - in Class Chart Testapi-314065532Încă nu există evaluări
- Religion Reflection 1Document3 paginiReligion Reflection 1api-314065532Încă nu există evaluări
- Walk The LineDocument3 paginiWalk The Lineapi-314065532Încă nu există evaluări
- Annotaited BibliographyDocument7 paginiAnnotaited Bibliographyapi-314065532Încă nu există evaluări
- Light Project 1Document16 paginiLight Project 1api-314065532Încă nu există evaluări
- Grubbs Lab ReportDocument2 paginiGrubbs Lab Reportapi-314065532Încă nu există evaluări
- VanessaDocument4 paginiVanessaapi-314065532Încă nu există evaluări
- Vanessa ParadisDocument5 paginiVanessa Paradisapi-314065532Încă nu există evaluări
- Indian PowerpointDocument8 paginiIndian Powerpointapi-314065532Încă nu există evaluări
- Napoleon BonaparteDocument5 paginiNapoleon Bonaparteapi-314065532Încă nu există evaluări
- ST LuciaDocument7 paginiST Luciaapi-314065532Încă nu există evaluări
- Anna ResumeDocument1 paginăAnna Resumeapi-314065532Încă nu există evaluări
- NBDocument4 paginiNBapi-314065532Încă nu există evaluări
- Publication 3Document12 paginiPublication 3api-314065532Încă nu există evaluări
- Anna ChipreanDocument4 paginiAnna Chipreanapi-314065532Încă nu există evaluări
- Math HistoryDocument3 paginiMath Historyapi-314065532Încă nu există evaluări
- Hamlet SoundtrackDocument11 paginiHamlet Soundtrackapi-314065532Încă nu există evaluări
- Les MiserablesDocument3 paginiLes Miserablesapi-314065532Încă nu există evaluări
- Annas InfernoDocument3 paginiAnnas Infernoapi-314065532Încă nu există evaluări
- Publication 2Document3 paginiPublication 2api-314065532Încă nu există evaluări
- Religion ProjectDocument7 paginiReligion Projectapi-314065532Încă nu există evaluări
- Violence in VersaillesDocument2 paginiViolence in Versaillesapi-314065532Încă nu există evaluări
- NMRDocument173 paginiNMRঋ ত্বিকÎncă nu există evaluări
- Deep Eutectic Solvents Syntheses, Properties and ApplicationsDocument39 paginiDeep Eutectic Solvents Syntheses, Properties and ApplicationsJulio Cesar Almeida100% (1)
- FP 17 32754 06Document3 paginiFP 17 32754 06Murugan RaghuÎncă nu există evaluări
- Intro CsamtDocument4 paginiIntro CsamtJoshLeighÎncă nu există evaluări
- Seismic Tremor Reveals Active Trans-Crustal Magmatic System Beneath Kamchatka VolcanoesDocument10 paginiSeismic Tremor Reveals Active Trans-Crustal Magmatic System Beneath Kamchatka VolcanoesAbel SanchezÎncă nu există evaluări
- 2007 LifengWangDocument211 pagini2007 LifengWangYonny Ayala EspinelÎncă nu există evaluări
- Eneria Product List: Design Conditions Fuel Gas DataDocument1 paginăEneria Product List: Design Conditions Fuel Gas DataPocola AdrianÎncă nu există evaluări
- Type 6010, 6011, and Whisper Disk Inline Diffusers: Bulletin 80.1:6010Document6 paginiType 6010, 6011, and Whisper Disk Inline Diffusers: Bulletin 80.1:6010Datt NguyenÎncă nu există evaluări
- Pressure Drop in An Axial Turbine System, Case Study of The Suction LineDocument6 paginiPressure Drop in An Axial Turbine System, Case Study of The Suction LineInternational Journal of Innovative Science and Research TechnologyÎncă nu există evaluări
- Electrochemistry AssignmentDocument3 paginiElectrochemistry AssignmentSulekha SharmaÎncă nu există evaluări
- Wps For Smaw06-001 (Pipe 6g CS)Document10 paginiWps For Smaw06-001 (Pipe 6g CS)walitedisonÎncă nu există evaluări
- SRP Lesson Plan 1 Basic - External (Nerf Gun)Document6 paginiSRP Lesson Plan 1 Basic - External (Nerf Gun)Ahmed shabanÎncă nu există evaluări
- Qip Ice 31 Stirling EnginesDocument20 paginiQip Ice 31 Stirling EnginesChetanPrajapatiÎncă nu există evaluări
- Computational Fluid Dynamics 2006 Proceedings of The Fourth International Conference On Computational Fluid Dynamics ICCFD4 Ghent Belgium 10 14 JDocument914 paginiComputational Fluid Dynamics 2006 Proceedings of The Fourth International Conference On Computational Fluid Dynamics ICCFD4 Ghent Belgium 10 14 JMarcos AquinoÎncă nu există evaluări
- Iso 10456 2007 en PDFDocument11 paginiIso 10456 2007 en PDFClaudia Carhuani25% (4)
- HOSTAFORM® C 27021 - POM - Unfilled: DescriptionDocument5 paginiHOSTAFORM® C 27021 - POM - Unfilled: Descriptionrahul vermaÎncă nu există evaluări
- Slide3 2017 - Quick StartDocument18 paginiSlide3 2017 - Quick StartJordana FurmanÎncă nu există evaluări
- Heat Transfer FundamentalsDocument44 paginiHeat Transfer FundamentalsSamuel Jade RoxasÎncă nu există evaluări
- Reinforced Masonry Engineering Handbook .6th - Ed.secDocument647 paginiReinforced Masonry Engineering Handbook .6th - Ed.secEc Ef100% (2)
- Hermetic CAN&CNFDocument20 paginiHermetic CAN&CNFkikechuÎncă nu există evaluări
- Precipitation Modelling in Ni-AlloysDocument35 paginiPrecipitation Modelling in Ni-AlloysИлья ЧекинÎncă nu există evaluări
- Structural Loads GuideDocument22 paginiStructural Loads GuideOGUZ DAYIÎncă nu există evaluări
- Phase Equilibrium: Phases, Components, and Degrees of FreedomDocument69 paginiPhase Equilibrium: Phases, Components, and Degrees of FreedomSyahirah FazialÎncă nu există evaluări
- Proof Hi PDFDocument29 paginiProof Hi PDF孙中体Încă nu există evaluări
- Fermi Dirac StatisticsDocument15 paginiFermi Dirac StatisticsRiya SalujaÎncă nu există evaluări
- Galvanic Cells, The Nernst EquationDocument2 paginiGalvanic Cells, The Nernst Equationanon_86967897Încă nu există evaluări
- Efunda - Plate Calculator - Clamped Circular Plate With Uniformly Distributed Loading-12.06.2017Document2 paginiEfunda - Plate Calculator - Clamped Circular Plate With Uniformly Distributed Loading-12.06.2017vinay1999Încă nu există evaluări
- Lecture Sheet PDFDocument65 paginiLecture Sheet PDFFaruk abdullahÎncă nu există evaluări
- Catalysis Science & Technology: Accepted ManuscriptDocument11 paginiCatalysis Science & Technology: Accepted Manuscriptumesh2329Încă nu există evaluări
- lc140 EngDocument2 paginilc140 EnganassÎncă nu există evaluări