Documente Academic
Documente Profesional
Documente Cultură
Lesson Plan: Lesson: Acid-Base Titration
Încărcat de
MarcTnnDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Lesson Plan: Lesson: Acid-Base Titration
Încărcat de
MarcTnnDrepturi de autor:
Formate disponibile
Lesson Plan
Lesson : Acid-Base Titration
Aim :
To study indicators and the different types of acid-base titrations.
Learning Outcomes :
At the end of the lesson, students will be able to:
1. state the use of an acid-base indicator.
2. define the pH range of an indicator.
3. select suitable indicators for a particular acid-base titration.
Assumed prior knowledge :
Students should already be familiar with:
1. the concept of acids and bases.
2. the concept of chemical equilibrium and Le Chatelier’s principle.
3. the concept of pH.
Underlying Principles
1. Making the invisible, visible.
2. Enabling students to know what to look for.
Time taken to complete the activities : 80 minutes
Differentiation
Questions in the student notes are designed to enable all students to complete the activity.
The pop-up answers are provided for the students to view when they have considered their
responses. Worksheet questions include questions that require recall, understanding and
application of the new concepts learned.
© 2003 Ministry of Education Malaysia. All Rights Reserved. Page 1 of 4
Development of Lesson :
No. Steps Strategy Resources
1 Set Induction. Teacher to quiz students to ensure that
(Ascertaining prior they have the necessary background
knowledge and knowledge.
introducing lesson
topic for the day).
2 Student Activity Teacher to use Activity 1 page 1 to • Courseware
introduce the concept of indicators to
students. Teacher to go through the rest
of Activity 1 with students.
• Activity 1 : Acid-base indicators
Students are shown why the colour of an
acid-base indicator changes in acidic and
basic conditions. They get to view the
colours of some indicators at different pH
values.
• Activity 2 : Acid-base titration
Students get to view the change in the
pH during the titration of :
- a strong acid with a strong base
- a weak acid with a strong base
- a weak base with a strong acid
- a weak acid with a weak base.
Students also get to learn the concept of
equivalence point and the end-point of a
titration.
3 Evaluation • Students to answer questions in the • Worksheet
student worksheet on their own.
4 Extension activity • Students to go through the extension • Websites
activities on their own. • Reference
books.
© 2003 Ministry of Education Malaysia. All Rights Reserved. Page 2 of 4
Worksheet answers
1. Acid-base indicators
1.1 a. Addition of KOH will reduce the concentration of hydrogen ions, hence the
equilibrium will shift to the right.
b. Red.
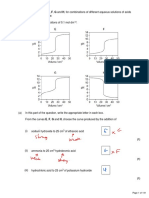
2. Acid-base titration
2.1 a.
13
12
11
10
pH 9
pH
4
0 10 20 30 40of 50 3 60 70
Volume Y/cm
V
b. At equivalence point, 50.0 cm3 of X required 50.00 cm3 of Y for complete
reaction.
(MV)
=1
(MV)
M x 50.0 = 0.10 x 50.0
∴ M = 0.10 mol dm-3
The concentration of Y is 0.10 mol dm-3.
c. X is a weak acid and Y is a strong base.
This is because the pH of the equivalence point is more than 7 and there is a
steep rise in pH near the equivalence point.
© 2003 Ministry of Education Malaysia. All Rights Reserved. Page 3 of 4
2.2 a. NH3/HCl
Methyl orange
b. K2CO3/HCl, NH3/HCl
Phenolphthalein and methyl orange
c. Ba(OH)2/HCl
Any indicator
d. KOH/CH3CH2COOH
Phenolphthalein
2.3
pH
13 KOH + HNO3
11
9
7 NH3 + HNO3
5
3
30.0 Volume of HNO3/cm3
As indicated in the graph:
KOH + HNO3 NH3 + HNO3
i. pH = 13 pH = 11
ii. pH = 7 pH < 7
iii. 30.00 cm3 30.00 cm3
iv. pH < 3 pH < 3
© 2003 Ministry of Education Malaysia. All Rights Reserved. Page 4 of 4
S-ar putea să vă placă și
- Lesson 40Document5 paginiLesson 40MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Introduction To Ionic EquilibriumDocument3 paginiLesson Plan: Lesson: Introduction To Ionic EquilibriumMarcTnnÎncă nu există evaluări
- Lesson 29Document3 paginiLesson 29MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: The Base Dissociation ConstantDocument4 paginiLesson Plan: Lesson: The Base Dissociation ConstantMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Shape of MoleculesDocument7 paginiLesson Plan: Lesson: Shape of MoleculesMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: K and K For Heterogeneous SystemDocument4 paginiLesson Plan: Lesson: K and K For Heterogeneous SystemMarcTnnÎncă nu există evaluări
- Lesson 3Document5 paginiLesson 3MarcTnnÎncă nu există evaluări
- Lesson 50Document3 paginiLesson 50MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: For Homogeneous SystemsDocument7 paginiLesson Plan: Lesson: For Homogeneous SystemsMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: For Homogeneous SystemDocument5 paginiLesson Plan: Lesson: For Homogeneous SystemMarcTnnÎncă nu există evaluări
- Lesson Plan:: 80 MinutesDocument7 paginiLesson Plan:: 80 MinutesMarcTnnÎncă nu există evaluări
- Lesson 41Document4 paginiLesson 41MarcTnn100% (1)
- Lesson 21Document5 paginiLesson 21MarcTnnÎncă nu există evaluări
- Lesson 42Document4 paginiLesson 42MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Le Chatelier's Principle (II)Document4 paginiLesson Plan: Lesson: Le Chatelier's Principle (II)MarcTnnÎncă nu există evaluări
- Chem Sem 1 Q &A PDFDocument9 paginiChem Sem 1 Q &A PDFevacuate clashÎncă nu există evaluări
- STPM Physics Chapter 18 Alternating Current CircuitsDocument2 paginiSTPM Physics Chapter 18 Alternating Current CircuitsChris Lau100% (1)
- STPM 2023 SEM 2 Mock AnsDocument2 paginiSTPM 2023 SEM 2 Mock AnsHannah KaienÎncă nu există evaluări
- Physics Coursework 2016/2017 STPMDocument13 paginiPhysics Coursework 2016/2017 STPMShi JieÎncă nu există evaluări
- Experiment 1 Chemistry STPM Practical (2011)Document2 paginiExperiment 1 Chemistry STPM Practical (2011)Fu HongÎncă nu există evaluări
- Proposal For Chemistry Project STPM 2016Document2 paginiProposal For Chemistry Project STPM 2016Voon Keat Nicholas Thoo100% (1)
- 2 Electrochemistry (Semester 2)Document49 pagini2 Electrochemistry (Semester 2)Esther Ngieng100% (1)
- 962 Chemistry (PPU - STPM) Semester 3 Topics-SyllabusDocument13 pagini962 Chemistry (PPU - STPM) Semester 3 Topics-SyllabusJosh, LRTÎncă nu există evaluări
- STPM Chemistry Form 6 NotesDocument5 paginiSTPM Chemistry Form 6 NotesAfz Min100% (3)
- Topic 13 Transition Element ExeciseDocument7 paginiTopic 13 Transition Element Execise复融陈Încă nu există evaluări
- STPM Chemistry Form 6Document5 paginiSTPM Chemistry Form 6BabasChong100% (1)
- Chapter 1 Limit N ContinuityDocument9 paginiChapter 1 Limit N Continuityelidawati85100% (1)
- STPM Physics Chapter 13 Capacitors PDFDocument1 paginăSTPM Physics Chapter 13 Capacitors PDFChris LauÎncă nu există evaluări
- Chemical Bonding HybridisationDocument7 paginiChemical Bonding HybridisationCherry T CYÎncă nu există evaluări
- Physics STPM Sem 2 DefinitionDocument2 paginiPhysics STPM Sem 2 DefinitionBen100% (4)
- Lesson Plan: Lesson: D-Block Elements (I)Document3 paginiLesson Plan: Lesson: D-Block Elements (I)MarcTnnÎncă nu există evaluări
- Chemistry Form 6 Sem 3 Chapter 2Document52 paginiChemistry Form 6 Sem 3 Chapter 2Yuzamrah Awang NohÎncă nu există evaluări
- STPM Physics Experiment 8 Earth S Magnetic Field Second Term PDFDocument2 paginiSTPM Physics Experiment 8 Earth S Magnetic Field Second Term PDFVishalinie RamanÎncă nu există evaluări
- STPM Physics Sem 3 Definition ListDocument4 paginiSTPM Physics Sem 3 Definition ListredroseÎncă nu există evaluări
- STPM Physics Chapter 17 Electromagnetic InductionDocument5 paginiSTPM Physics Chapter 17 Electromagnetic InductionChris Lau100% (1)
- Marking Scheme For Term 2 Trial Exam, STPM 2019 (Gbs Melaka) Section A (45 Marks)Document7 paginiMarking Scheme For Term 2 Trial Exam, STPM 2019 (Gbs Melaka) Section A (45 Marks)Michelles JimÎncă nu există evaluări
- Chemistry Project STPM 2016Document13 paginiChemistry Project STPM 2016Sabri Bri EDÎncă nu există evaluări
- STPM Sem 3 Chemistry Note - Chapter AlkanesDocument21 paginiSTPM Sem 3 Chemistry Note - Chapter AlkanesSTPMBAHARU100% (3)
- Experiment 2: RedoxDocument1 paginăExperiment 2: RedoxFu HongÎncă nu există evaluări
- Physics Report DraftDocument19 paginiPhysics Report DraftViola Voon Li WeiÎncă nu există evaluări
- Chemistry Form 6 Sem 3 Chapter 1Document47 paginiChemistry Form 6 Sem 3 Chapter 1Yuzamrah Awang Noh50% (2)
- Lesson Plan: Some of Their PropertiesDocument3 paginiLesson Plan: Some of Their PropertiesMarcTnnÎncă nu există evaluări
- STPM Past Year Objectives Question 1999 2015 PDFDocument28 paginiSTPM Past Year Objectives Question 1999 2015 PDFNora ShikinÎncă nu există evaluări
- Uppp2 Sem 1 2017Document9 paginiUppp2 Sem 1 2017WWZÎncă nu există evaluări
- STPM Physics Chapter 14 Electric CurrentDocument1 paginăSTPM Physics Chapter 14 Electric CurrentChris LauÎncă nu există evaluări
- Anderson STPM Trial Chemistry P2 With AnswerDocument23 paginiAnderson STPM Trial Chemistry P2 With Answerlsueyin100% (1)
- STPM Chemistry 2002 - Paper 1Document16 paginiSTPM Chemistry 2002 - Paper 1Steve_Sam93Încă nu există evaluări
- STPM Chemistry 2004 - Paper 2Document12 paginiSTPM Chemistry 2004 - Paper 2Steve_Sam93100% (2)
- Sem 1 2022 Manual ChemistryDocument9 paginiSem 1 2022 Manual ChemistryVZYFVVZHVMÎncă nu există evaluări
- Electrochemistry - Cont Module 4 STPMDocument10 paginiElectrochemistry - Cont Module 4 STPMPavithiranÎncă nu există evaluări
- IPTA Cut Off Point For PHYSICS STPM 2011 / 2012 University EntryDocument12 paginiIPTA Cut Off Point For PHYSICS STPM 2011 / 2012 University EntrySKÎncă nu există evaluări
- Chemistry Project STPM 2016Document13 paginiChemistry Project STPM 2016Sabri Bri EDÎncă nu există evaluări
- Chemistry 2008 STPMDocument21 paginiChemistry 2008 STPMtecklee89100% (20)
- Lesson Plan: Lesson: PH and pOHDocument4 paginiLesson Plan: Lesson: PH and pOHMarcTnnÎncă nu există evaluări
- Lesson Plan Acid N BaseDocument3 paginiLesson Plan Acid N BasedediyanÎncă nu există evaluări
- Lesson Plan 10th ClassPS Acids Bases and SaltsDocument5 paginiLesson Plan 10th ClassPS Acids Bases and SaltsHIRAL SOLANKIÎncă nu există evaluări
- Acids and Bases Lesson PlanDocument4 paginiAcids and Bases Lesson PlanCarlos SotoÎncă nu există evaluări
- Day 8 - Lesson Plan - Acids, Bases, and PHDocument2 paginiDay 8 - Lesson Plan - Acids, Bases, and PHJennifer DequinaÎncă nu există evaluări
- Lesson Plan: Lesson: Heat Energy ChangeDocument4 paginiLesson Plan: Lesson: Heat Energy ChangeMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Uses of ElectrolysisDocument3 paginiLesson Plan: Lesson: Uses of ElectrolysisMarcTnnÎncă nu există evaluări
- Dun DownloadDocument1 paginăDun DownloadMarcTnnÎncă nu există evaluări
- Lesson 50Document3 paginiLesson 50MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Standard Cell PotentialDocument4 paginiLesson Plan: Lesson: Standard Cell PotentialMarcTnnÎncă nu există evaluări
- Lesson PlanDocument4 paginiLesson PlanMarcTnnÎncă nu există evaluări
- Lesson PlanDocument4 paginiLesson PlanMarcTnnÎncă nu există evaluări
- Lesson 42Document4 paginiLesson 42MarcTnnÎncă nu există evaluări
- Lesson PlanDocument4 paginiLesson PlanMarcTnnÎncă nu există evaluări
- Lesson 45Document4 paginiLesson 45MarcTnnÎncă nu există evaluări
- Lesson 41Document4 paginiLesson 41MarcTnn100% (1)
- Lesson Plan: Lesson: Colligative Properties of SolutionsDocument3 paginiLesson Plan: Lesson: Colligative Properties of SolutionsMarcTnnÎncă nu există evaluări
- Lesson 49Document3 paginiLesson 49MarcTnnÎncă nu există evaluări
- Lesson 48Document3 paginiLesson 48MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Le Chatelier's Principle (I)Document4 paginiLesson Plan: Lesson: Le Chatelier's Principle (I)MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Le Chatelier's Principle (II)Document4 paginiLesson Plan: Lesson: Le Chatelier's Principle (II)MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: K and K For Heterogeneous SystemDocument4 paginiLesson Plan: Lesson: K and K For Heterogeneous SystemMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: PH and pOHDocument4 paginiLesson Plan: Lesson: PH and pOHMarcTnnÎncă nu există evaluări
- Lesson 28Document5 paginiLesson 28MarcTnnÎncă nu există evaluări
- Lesson 21Document5 paginiLesson 21MarcTnnÎncă nu există evaluări
- Lesson 27Document4 paginiLesson 27MarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: Zero Order ReactionDocument4 paginiLesson Plan: Lesson: Zero Order ReactionMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: For Homogeneous SystemsDocument7 paginiLesson Plan: Lesson: For Homogeneous SystemsMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: For Homogeneous SystemDocument5 paginiLesson Plan: Lesson: For Homogeneous SystemMarcTnnÎncă nu există evaluări
- Lesson Plan: Lesson: First and Second Order ReactionDocument6 paginiLesson Plan: Lesson: First and Second Order ReactionMarcTnnÎncă nu există evaluări
- Lesson 20Document4 paginiLesson 20MarcTnnÎncă nu există evaluări
- Determine An Equilibrium ConstantDocument13 paginiDetermine An Equilibrium ConstantMeMeMelol100% (2)
- Acids A2ppDocument141 paginiAcids A2ppDishaÎncă nu există evaluări
- Ionic Equilibrium - CPP-1 To 5 - CRSDocument5 paginiIonic Equilibrium - CPP-1 To 5 - CRSFake GamerÎncă nu există evaluări
- Experiment 3Document6 paginiExperiment 3Concepcion R. AquinoÎncă nu există evaluări
- Ch16 StudentsDocument29 paginiCh16 Studentsqvk8yy9pxcÎncă nu există evaluări
- Chem Lab ReportDocument18 paginiChem Lab Reportapi-514523338Încă nu există evaluări
- Honors Chemistry QuizDocument4 paginiHonors Chemistry QuizmariaÎncă nu există evaluări
- CHM 111 General Chemistry I: Department of Chemistry University of Medical Sciences Ondo, Ondo State, NigeriaDocument16 paginiCHM 111 General Chemistry I: Department of Chemistry University of Medical Sciences Ondo, Ondo State, Nigeriaomelle AbubakarÎncă nu există evaluări
- Class 10 Chapter 2 Science Notes Acids, Bases, and SaltsDocument18 paginiClass 10 Chapter 2 Science Notes Acids, Bases, and SaltsJapani TutorÎncă nu există evaluări
- Acid Base WebquestDocument3 paginiAcid Base WebquestDimmu BorgirÎncă nu există evaluări
- Chemistery Notes 10th FDocument65 paginiChemistery Notes 10th Frizwan15Încă nu există evaluări
- Learn Vibrant Coaching Institute Gyanpur: Class 7 Science Acids Bases and Salts McqsDocument13 paginiLearn Vibrant Coaching Institute Gyanpur: Class 7 Science Acids Bases and Salts McqsDarsh SinghÎncă nu există evaluări
- Vivegaa School Cbse: Class 7 - Science Worksheet CHAPTER - 5: Acids Bases and SaltsDocument2 paginiVivegaa School Cbse: Class 7 - Science Worksheet CHAPTER - 5: Acids Bases and SaltsPRADEEP SHANJAN100% (1)
- 04 (2) HJBHDocument3 pagini04 (2) HJBHncubepharmaÎncă nu există evaluări
- Acid Base Chemistry ShortDocument27 paginiAcid Base Chemistry ShortJianÎncă nu există evaluări
- Acids, Bases and SaltsDocument5 paginiAcids, Bases and SaltsIsa ShahidÎncă nu există evaluări
- Biophysical ChemistryDocument5 paginiBiophysical Chemistryarsene wenÎncă nu există evaluări
- General Chemistry 2 WorksheetDocument26 paginiGeneral Chemistry 2 WorksheetDayle CortezÎncă nu există evaluări
- Analysis (Module 6)Document74 paginiAnalysis (Module 6)vishal sharmaÎncă nu există evaluări
- TitrationsDocument7 paginiTitrationsPrathamesh ParmarÎncă nu există evaluări
- Atomic ResearchDocument21 paginiAtomic ResearchPrashant DoundÎncă nu există evaluări
- Ebook Chemistry 10Th Edition Whitten Solutions Manual Full Chapter PDFDocument37 paginiEbook Chemistry 10Th Edition Whitten Solutions Manual Full Chapter PDFJaniceMarqueznxed100% (13)
- ch18 PDFDocument45 paginich18 PDFHafidz RafiqiÎncă nu există evaluări
- AlkalinityDocument2 paginiAlkalinityRushiÎncă nu există evaluări
- Acids BasesDocument17 paginiAcids BasesAkshay NilawarÎncă nu există evaluări
- Acid Base Theory AnswersDocument4 paginiAcid Base Theory AnswersPasha TanÎncă nu există evaluări
- Questions 1 - 20 PDFDocument2 paginiQuestions 1 - 20 PDFUjjawal kumarÎncă nu există evaluări
- Lab Experiment 3 Ka Determination Through PH TitrationDocument4 paginiLab Experiment 3 Ka Determination Through PH TitrationxmusiqaÎncă nu există evaluări
- Equilibrium DPP 014728Document11 paginiEquilibrium DPP 014728Yash MalviyaÎncă nu există evaluări
- Module 2 Learning Tasks and AssessmnetsDocument5 paginiModule 2 Learning Tasks and AssessmnetsEssielve BatistilÎncă nu există evaluări