Documente Academic
Documente Profesional
Documente Cultură
Gohlke 2005
Încărcat de
yulia fatma nstTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Gohlke 2005
Încărcat de
yulia fatma nstDrepturi de autor:
Formate disponibile
0021-972X/05/$15.
00/0 The Journal of Clinical Endocrinology & Metabolism 90(4):2270 –2274
Printed in U.S.A. Copyright © 2005 by The Endocrine Society
doi: 10.1210/jc.2004-1192
Fetal Insulin-Like Growth Factor (IGF)-I, IGF-II, and
Ghrelin in Association with Birth Weight and Postnatal
Growth in Monozygotic Twins with Discordant Growth
Bettina C. Gohlke, Agnes Huber, Kurt Hecher, Rolf Fimmers, Peter Bartmann, and Christian L. Roth
Departments of Pediatrics (B.C.G., C.L.R.), Statistics (R.F.), and Neonatology (P.B.), University of Bonn, 53113 Bonn,
Germany; and Department of Obstetrics and Fetal Medicine (A.H., K.H.), University Clinic Hamburg-Eppendorf,
20246 Hamburg, Germany
Objective: To investigate the relative contribution of genetic IGF-binding protein-2 levels (r ⴝ ⴚ0.68; P < 0.001) but with
(fetal) vs. environmental (maternal/placental) factors on neither ⌬ IGF-II nor ⌬ ghrelin. There was a strong intertwin
growth, we studied monozygotic twins with intertwin birth correlation for all hormones. By comparing the growth in the
weight difference. first year, we found an overall reduction of the relative weight
Patients and Methods: Twenty-seven twins (15 with discor- difference between the twins of 57%. ANOVA was used to cal-
dant growth) who have been treated for severe twin-to-twin culate factors for prediction of postnatal catch-up growth.
transfusion syndrome by laser coagulation were studied. Besides the birth weight difference (R2 ⴝ 0.84; P < 0.0001), only
Cord blood samples were analyzed for IGF-I, IGF-II, IGF-bind- ghrelin was of prognostic value for postnatal catch-up growth
ing protein-2, and ghrelin. Intertwin difference (⌬) of birth (R2 ⴝ 0.94; P ⴝ 0.0035).
weight was correlated to ⌬ of the parameters analyzed. The ⌬ Conclusion: These data confirm the importance of IGF-I in
weight after 1 yr was correlated with ⌬ birth weight and all contrast to IGF-II for fetal weight. Additionally, ghrelin seems
hormones. to be involved in fetal and probably postnatal growth. (J Clin
Results: The ⌬ birth weight was positively correlated with Endocrinol Metab 90: 2270 –2274, 2005)
⌬ IGF-I (r ⴝ 0.66; P < 0.0002) and negatively correlated with ⌬
I N MONOCHORIONIC TWIN pregnancies, there are pla-
cental vascular communications between the two fetuses.
In 15% of such pregnancies, there is an imbalance in net flow
placental transport or in fetal hormone function. Our study
population recruited only monozygotic (MZ) but hemody-
namically dichorionic twins with discordant birth weight.
between the twins resulting in the twin-twin-transfusion MZ twinning is a powerful clinical model because of the
syndrome (TTTS). The donor becomes hypovolemic, hypo- identical genetical background, the same maternal nutrition,
tensive, and oliguric, which leads to oligo/anhydramnios and only the individual differences for the fetal environment.
and growth restriction. The recipient, who usually is appro- Therefore, they are an excellent model to study the interac-
priate for gestational age, becomes hyervolemic, hyperten- tion between genetic and environmental factors and their
sive, and polyuric, leading to polyhydramnios and conges- effects on fetal growth (5, 6).
tive heart failure (1). With recent advancements in diagnostic The IGFs are involved in the regulation of growth during
and therapeutic modalities in fetal medicine, the survival rate pregnancy as well as in early embryonic and fetal develop-
of chronic TTTS has improved from less than 10 to 68% (2– 4). ment. In most studies, cord blood IGF-I but not IGF-II con-
Endoscopic coagulation of the vascular anastomoses respon- centrations correlate with birth weight (7–11). In contrast,
sible for fetofetal transfusion (3, 4) performed during the others have shown normal IGF-I (12, 13) and reduced IGF-II
second trimenon is a causal treatment option. levels in singleton intrauterine growth retardation fetuses
Intrauterine growth is regulated by fetal as well as ma- (14, 15). There are only a few studies in twins with interpair
ternal and environmental factors. Little is known of their variation in birth weight. Studies in concordant twins sug-
relative contribution and importance because singleton stud- gest that fetal circulating IGF-I levels may be genetically
ies are limited in this regard. Several studies of singleton determined (16).
pregnancies have shown that various hormones are associ- Ghrelin is a peptide predominantly produced by the stom-
ated with birth weight. However, singleton studies fail to ach, hypothalamus, and adipose tissue (17, 18). It displays
address the question of whether alterations in fetal nutrition strong GH-releasing action (19). In addition, ghrelin is also
leading to impaired birth weight are due to disturbance in actively synthesized by the placenta (20) and can be detected
in cord blood at 30 wk gestational age, which indicates that
First Published Online February 1, 2005 it may play a role in fetal development (21–23). Its various
Abbreviations: ⌬, Differences; AGA, appropriate birth weight; endocrine and nonendocrine actions have been subjects of
IGFBP, IGF binding protein; MZ, monozygotic; SGA, small for gesta-
tional age; TTTS, twin-twin-transfusion syndrome. recent scientific work (24). Previous studies indicate that
JCEM is published monthly by The Endocrine Society (http://www.
ghrelin levels are influenced by acute and chronic feeding
endo-society.org), the foremost professional society serving the en- state, with increase by fasting and energy restriction (25, 26)
docrine community. but decrease by food intake and glucose (27–29).
2270
Downloaded from https://academic.oup.com/jcem/article-abstract/90/4/2270/2836881
by guest
on 22 July 2018
Gohlke et al. • Fetal IGF-I, -II, and Ghrelin in Monozygotic Twins J Clin Endocrinol Metab, April 2005, 90(4):2270 –2274 2271
In the present study, we measured IGF-I, IGF-II, IGF bind- cent) and ⌬ of the hormones. The percent growth discordance was
ing protein (IGFBP)-2, and ghrelin concentrations in cord defined as the difference in birth weight expressed as a proportion of the
birth weight of the larger twin. Intertwin difference of IGF-I, IGF-II,
blood in MZ monochorionic twins and examined its rela- IGFBP-2, and ghrelin was expressed in ⌬. Statistical analysis was per-
tionship to birth weight and growth after 1 yr of life. formed by the SAS system (SAS Institute, Cary, NC). Multiple linear
regression analysis was used to calculate the predictors for weight dif-
Patients and Methods ference at birth and after 1 yr.
Patients
Results
Twenty-seven women with monochorionic MZ twin pregnancies and
TTTS were studied. The diagnosis of TTTS was made by the combination Comparison of anthropometric data and cord hormone
of single monochorionic placenta, polyhydramnios and oligohydram- levels between the discordant SGA-AGA (n ⫽ 15) and con-
nios, stuck-twin, and an initial diagnosis before 25 wk gestation. These cordant AGA-AGA (n ⫽ 12) groups is shown (Table 1). The
fetuses were treated by means of laser coagulation between 17 and 25
wk gestation. All the fetuses were delivered in the local referring
birth weight of the AGA twin in the discordant group was
hospital. comparable with that of the concordant twin pairs.
Fifteen twin pairs presented with discordant growth [birth weight
difference ⬎ 15%, one twin being small for gestational age (SGA, birth IGF-I
weight ⬍ ⫺2 sd for gestational age), the other appropriate birth weight
(AGA)], and 12 twin pairs were concordant concerning their growth In the discordant group, fetal IGF-I concentrations in SGA
(AGA-AGA). All twins were measured regularly at 2, 4, 6, and 12 months twins were significantly lower than those in the AGA cotwin
of age. Nineteen twin pairs who were older than 1 yr were analyzed (P ⬎ 0.01) (Table 1). No difference was observed in the
concerning their postnatal growth.
concordant group (P ⫽ 0.76). Spearman correlation coeffi-
Collection of samples cient (r) between ⌬ birth weight and ⌬ IGF-I was 0.66 (P ⬍
0.001) (Fig. 1A).
Fetal cord blood was obtained from each twin from the umbilical
venous blood from clamped segment of cord at the birth. The samples
were centrifuged, and serum was stored at ⫺70 C until a batch assay was IGF-II
performed. Informed consent for collection of cord blood samples was Fetal IGF-II levels in recipient and donor twins in both
obtained from all parents. The study protocol was approved by the local
ethics committee. groups were comparable (P ⫽ 0.79 for discordant twins, P ⫽
0.4 for concordant twins; Table 1). Spearman correlation co-
Immunoassays efficient (r) ⌬ birth weight to ⌬ IGF-II difference was ⫺0.08
(P ⫽ 0.71; Fig. 1B).
All hormone concentrations were measured by radioimmunometric
assays using commercially available kits (Mediagnost, Reutlingen, Ger-
many). IGF-I (nanograms per milliliter) was measured by RIA after IGFBP-2
employing excess IGF-II to saturate IGFBPs. The detection limit was 0.02
ng/ml. IGF-II (nanograms per milliliter) was measured after separation In the discordant group, fetal IGFBP-2 concentrations in
from IGFBP by acid chromatography according to Blum and Breier (30). SGA twins were significantly higher than those in the AGA
The detection limit was 0.1 ng/ml. The sensitivity of the IGFBP-2 assay cotwin (P ⬍ 0.01; Table 1). Spearman correlation coefficient
was 0.2 ng/ml. Interassay variances were 7.4 (IGF-I), 7.9 (IGF-II), and (r) of ⌬ birth weight to ⌬ IGFBP-2 was ⫺0.68 (P ⬍ 0.001). The
9.6% (IGFBP-2), respectively. Intraassay variances were 5.6 (IGF-I), 5.4
(IGF-II), and 8.5% (IGFBP-2), respectively. Immunoreactive ghrelin con-
⌬ IGFBP-2 levels were significantly correlated to ⌬ IGF-I (r ⫽
centrations were measured in duplicate using a commercial RIA (Linco ⫺0.5; P ⫽ 0.01) but not to ⌬ IGF-II.
Research, Inc., St. Charles, MO). The antibody used in the assay is a
rabbit polyclonal antibody against full-length octanoylated human Ghrelin
ghrelin. Intra- and interassay coefficients of variation were 3.3 and
17.8%, respectively. Spearman correlation coefficient showed a negative but
not significant correlation between ⌬ birth weight and ⌬
Statistical analysis ghrelin levels (r ⫽ ⫺0.39, P ⫽ 0.10).
Clinical data and hormone concentrations are expressed as medians
and ranges. Delta values (⌬) indicate differences between the twins. For Correlations among study hormones and between twin pairs
parametric data, the paired t test was used to compare values within
twin pairs and Student’s t test between groups. Spearman correlation We examined univariate correlations among study hor-
was calculated between relative intertwin birth weight difference (per- mones and found IGF-I to be negatively correlated to
TABLE 1. Median and range of study anthropometric indices and hormones in the discordant and in the concordant group
Discordant group (n ⫽ 15) Concordant group (n ⫽ 12)
SGA-donor AGA-recipient P AGA-donor AGA-recipient P
Gestational age at 34 (31–38) 36 (32–37)
delivery (wk)
Birth weight (g) 1600 (710 –2280) 2130 (1330 –2900) ⬍0.01 2040 (1410 –2890) 2110 (1410 –3060) 0.76
IGF-I (ng/ml) 35 (6 – 81) [19.5] 65 (25–93) [20.9] ⬍0.002 43 (13–70) [16.4] 49 (9 –58) [15.6] 0.79
IGF-II (ng/ml) 296 (165– 415) [49] 302 (198 – 406) [63.3] 0.79 298 (250 – 454) [58.2] 291 (205–398) [49.2] 0.4
IGFBP-2 (ng/ml) 1250 (566 –2684) [696.1] 855 (513–1332) [263.2] ⬍0.01 827 (650 –3457) [1195.7] 873 (563–3457) [738.4] 0.54
Ghrelin (ng/ml) 1109 (660 –1701) [338.8] 1060 (870 –1916) [270] 0.06 1109 (836 –2696) [626] 1055 (921–3234) [830] 0.43
Data expressed as median (range) [SD].
Downloaded from https://academic.oup.com/jcem/article-abstract/90/4/2270/2836881
by guest
on 22 July 2018
2272 J Clin Endocrinol Metab, April 2005, 90(4):2270 –2274 Gohlke et al. • Fetal IGF-I, -II, and Ghrelin in Monozygotic Twins
TABLE 2. Spearman correlation coefficient between the various
study hormones
⌬ Birth
⌬ IGF-I ⌬ IGF-II ⌬ IGFBP-2 ⌬ Ghrelin
weight
⌬ Birth weight 1.000 0.67 ⫺0.08 ⫺0.68 ⫺0.39
P ⫽ 0.0002 P ⫽ 0.71 P ⫽ 0.001
⌬ IGF-I 1.000 0.26 ⫺0.53 ⫺0.17
P ⫽ 0.2 P ⫽ 0.015
⌬ IGF-II 1.000 ⫺0.14 0.05
P ⫽ 0.81
⌬ Ghrelin 1.000
twins with discordant weight at birth did not show a sig-
nificant reduction of relative weight difference.
ANOVA revealed a strong correlation between relative
weight difference, at the age of 1 yr to (⌬ 1 yr) and relative
birth weight difference (R2 ⫽ 0.84; P ⬍ 0.0001) (Fig. 4). Step-
wise regression showed that the only factor with a further
association to weight difference at 1 yr was ⌬ ghrelin level
(R2 ⫽ 0.94; P ⬍ 0.0035).
FIG. 1. A, Correlation between percent birth weight difference and
intertwin difference of IGF-I (⌬ IGF-I) levels in cord blood (n ⫽ 27) (r ⫽
0.66; P ⬍ 0.001). Correlation line and 95% confidence interval are
shown. B, Correlation between percent birth weight difference and
intertwin difference of IGF-II (⌬ IGF-II) levels in cord blood (n ⫽ 27)
(r ⫽ ⫺0.08; P ⫽ 0.71). Correlation line and 95% confidence interval
are shown.
IGFBP-2 (r ⫽ ⫺0.53; P ⬍ 0.01). There was no correlation
among the other hormones at birth. Table 2 shows Spearman
correlation coefficients among all measured hormones.
For all hormones a highly significant intertwin correlation
was found (IGF-I and IGF-II are shown, Fig. 2, A and B).
Longitudinal data
Nineteen twin pairs were analyzed concerning their post-
natal weight and length development, and an overall reduc- FIG. 2. Correlation for IGF-I levels (r ⫽ 0.49; P ⬍ 0.009) (A) and
tion of 57.1% of relative weight difference was observed. IGF-II levels (r ⫽ 0.58; P ⬍ 0.0014) (B) among all 27 twin pairs is
Single data are shown in Fig. 3. Three of the nine pairs of shown.
Downloaded from https://academic.oup.com/jcem/article-abstract/90/4/2270/2836881
by guest
on 22 July 2018
Gohlke et al. • Fetal IGF-I, -II, and Ghrelin in Monozygotic Twins J Clin Endocrinol Metab, April 2005, 90(4):2270 –2274 2273
regulation of fetal growth, and its mechanism appears to be,
at least in part, through an endocrine action. There is also
evidence that the weight difference between twins suffering
from chronic TTTS cannot be explained only by the transfer
of blood and nutrients along the placental vascular anasto-
moses. Although all our patients had a laser coagulation of
the connecting vessels, more than half of the twins presented
with discordant birth weight.
In addition, we found a strong intertwin correlation for
IGF-I for all twin pairs, confirming the underlying genetic
influence on the IGF-I level (31).
In IGF-II a strong intertwin correlation was found, but it
was not related to fetal weight or length in our study pop-
ulation. IGF-II may play a role as a fetal somatomedin during
earlier periods of human intrauterine life.
IGFBP-2 is one of the major IGFBPs in the fetus (32). It is
known to correlate negatively to birth weight, indicating an
antagonistic regulatory mechanism for the effect of IGF. Al-
ternatively, IGFBP-2 levels might be up-regulated in the
presence of low IGF-I levels. In disease states it has been
shown that if IGF-I levels were suppressed, IGFBP-2 levels
were elevated (33).
FIG. 3. Relative weight difference of all twin pairs at birth, 2 months,
4 months, 6 months, and 1 yr. Circles represent the pairs of twins with Umbilical cord blood concentrations of ghrelin were in-
concordant birth weight (⬍10%, n ⫽ 10), triangles those with discor- versely related to birth weight. This is in accordance with
dant birth weight (difference ⬎ 15%, n ⫽ 9) very recent data demonstrating that cord ghrelin concentra-
tions were higher in SGA neonates, compared with AGA/
Discussion
large-for-gestational-age neonates (22, 23, 34). A similar in-
Based on our data of IGF-I, IGF-II, IGFBP-2, and ghrelin in verse relationship is found in patients with anorexia nervosa
venous cord blood from MZ monochorionic twins with birth having higher and obese patients having lower ghrelin levels
weight difference, the following conclusions can be drawn. than controls (25, 26, 29). We cannot add information about
In accordance with most published data (7–11) and in change of ghrelin concentrations throughout gestation due to
contrast to Bajoria et al. (5), we found a strong correlation of our small patient numbers.
IGF-I levels and birth weight. Among the group of discor- The source(s) of circulating ghrelin in fetuses remains un-
dant twins, the normally grown twin, in all but one case, had clear. It could originate from the placenta (20), the stomach
significantly higher cord serum IGF-I levels than the growth- (17), or other fetal organ tissues (35) that are known to syn-
restricted cotwin. There was no significant intertwin differ- thesize ghrelin during early fetal life. Our study population
ence in the cord blood IGF-I levels in the concordant twin recruited only MZ but hemodynamically dichorionic twins
pairs. These data demonstrate that IGF-I is important in the after laser coagulation. We suggest that the SGA fetus itself
had developed a compensatory mechanism to the negative
energy balance, and the increased ghrelin level would be an
indication of an adaptive response.
The most important predictor for catch-up growth be-
tween the discordant twin pairs was the relative weight
difference at birth, a greater difference at birth being asso-
ciated with greater weight difference after 1 yr. Although
IGF-I was positively associated with birth weight, it was not
of additional prognostic value for the weight development
during the first year of life. Further information was received
only by including intertwin difference of ghrelin. SGA twins
with a high ghrelin concentration had a better chance for
catching up of weight after 1 yr. We suggest that higher
ghrelin concentrations remained high throughout the first
year of life, leading to an increased appetite and different
satiety regulation in those patients followed by rapid post-
natal catch-up growth. This is in accordance with recent
findings of Iniguez et al. (36). They observed a significantly
FIG. 4. Correlation between weight difference at 1 yr and birth
smaller glucose-induced drop in ghrelin concentrations in
weight difference only of all twins (R2 ⫽ 0.84, P ⬍ 0.0001). Correlation 1-yr-old infants born SGA who had experienced catch-up
line and 95% confidence interval are shown. growth, compared with those who had not, and proposed
Downloaded from https://academic.oup.com/jcem/article-abstract/90/4/2270/2836881
by guest
on 22 July 2018
2274 J Clin Endocrinol Metab, April 2005, 90(4):2270 –2274 Gohlke et al. • Fetal IGF-I, -II, and Ghrelin in Monozygotic Twins
that higher postprandial ghrelin concentrations may have M 1996 Insulin-like growth factors (IGFs) and IGF binding proteins-1, -2, and
-3 in newborn serum: relationship to feto-placental growth at term. Early Hum
resulted in greater weight gain early in life. Dev 46:15–26
In conclusion, our data confirm the importance of IGF-I vs. 14. Gluckman PD, Johnson Barrett JJ, Butler JH, Edgar BW, Gunn TR 1983
IGF-II for fetal weight development, although IGF-I levels Studies of insulin-like growth factor-I and -II by specific radioligand assays in
umbilical cord blood. Clin Endocrinol (Oxf) 19:405– 413
were significantly higher in the AGA twin, compared with 15. Giudice LC, de Zegher F, Gargosky SE, Dsupin BA, de las Fuentes L, Crytal
the SGA cotwin, whereas these levels were similar in the RA, Hintz RL, Rosenfeld RG 1995 Insulin-like growth factors and their bind-
AGA twin pairs. Furthermore, this demonstrates the strong ing proteins in the term and preterm human fetuses and neonates with normal
and extremes of intrauterine growth. J Clin Endocrinol Metab 80:1548 –1555
contribution of the uterine environment for IGF-I. However, 16. Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M 1991
a significant intertwin correlation was found for both growth Serum insulin-like growth factors and insulin-like growth factor binding pro-
teins in the human fetus. Relationship with growth in normal subjects and in
factors, showing also the genetic influence on IGF-I and -II. subjects with intrauterine growth retardation. Pediatr Res 29:219 –225
Furthermore, our data show that ghrelin is negatively cor- 17. Kojima M, Hosoda H, Data Y, Nakazato M, Matsuo H, Kangawa K 1999
related to birth weight difference with higher levels in the Ghrelin is a growth-hormone releasing acylated peptide from the stomach.
Nature 402:656 – 660
lighter twin. Birth weight difference was the strongest predictor 18. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K,
for weight difference after 1 yr, but prediction of catch-up Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature
growth was dependent on ghrelin levels with high cord blood 409:194 –198
19. Christou H, Connors JM, Zikojima M, Hosoda H, Kangawa K 2001 Purifi-
ghrelin associated with improved catch-up growth than lower cation and distribution of ghrelin: the natural endogenous ligand for growth
levels. These data confirm the importance of both genetic and hormone secretagogue receptor. Horm Res 56:93–97
intrauterine factors in early human development. 20. Gualillo O, Caminos E, Blanco M, Garcia-Caballero, Kojima M, Kangawa K,
Dieguez C, Casanueva FF 2001 Ghrelin a novel placental-derived hormone.
Endocrinology 142:788 –794
Acknowledgments 21. Chanoine JP, Yeung LPK, Wong ACK 2003 Umbilical cord ghrelin concen-
trations in Asian and Caucasian neonates. Horm Res 60:116 –120
The authors are grateful to E. Maslak for her excellent technical 22. Farquhar J, Heiman M, Wong AC, Wach R, Chessex P, Chanoine JP 2003
assistance in the laboratory. Elevated umbilical cord ghrelin concentrations in small for gestational age
neonates. J Clin Endocrinol Metab 88:4324 – 4327
23. Sorino-Guillén L, Barrios V, Chowen JA, Sanchez I, Vila S, Quero J, Argente
Received June 22, 2004. Accepted January 21, 2005. J 2004 Ghrelin levels from fetal life through early adulthood: relationship with
Address all correspondence and requests for reprints to: Dr. B. endocrine and metabolic and anthropometric measures. J Pediatr 144:30 –35
Gohlke, Zentrum für Kinderheilkunde, der Universität Bonn, Adenauer- 24. Broglio F, Gottero C, Arvat E, Ghigo E 2003 Endocrine and non-endocrine
allee 119, 53113 Bonn, Germany. E-mail: gohlke-bonn@t-online.de. actions of ghrelin. Horm Res 59:109 –117
25. Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczney C, Riepl RL, Heiman
References ML, Lehnert P, Ficher M, Tschop M 2001 Weight gain decreases elevated
plasma ghrelin concentrations in patients with anorexia nervosa. Eur J Endo-
1. Kingdom JCP, Bajoria R 1998 Fetal assessment and management of labour in crinol 145:669 – 673
twin pregnancy. Recent Adv Obstet Gynecol 20:65– 80 26. Sorino-Guillén L, Barrios V, Campos-Barros A, Argente J 2004 Ghrelin levels
2. Bajoria R, Kingdom JCP 1998 Chorionic plate vascular anatomy determines in obesity and anorexia nervosa: effect of weight reduction or recuperation.
the efficacy of amnioreduction therapy for twin-twin transfusion syndrome. J Pediatr 144:36 – 42
Hum Reprod 13:1709 –1713 27. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S,
3. Ville Y, Hyett J, Hecher K, Nicolaides K 1995 Preliminary experience with Hosoda H, Kangawea K, Matsrkura S 2002 Plasma ghrelin levels in lean and
endoscopic laser surgery for severe twin-twin transfusion syndrome. N Engl obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol
J Med 332:224 –227 Metab 87:240 –244
4. Hecher K, Diehl W, Zikulnig L, Vetter M, Hackeloer BJ 2000 Endoscopic laser 28. Broglio F, Arvat E, Benso A, Gottero C, Mucioli G, Papotti M, van der Lely
coagulation of placental anastomoses in 200 pregnancies with severe mid- AJ, Deghenenghi R, Ghigo E 2001 Ghrelin, a natural GH secretagogue pro-
trimester twin-to-twin transfusion syndrome. Eur J Obstet Gynecol Reprod duced by the stomach, induces hyperglycemia and reduces insulin secretion
Biol 92:135–139 in humans. J Clin Endocrinol Metab 86:5083–5086
5. Bajoria R, Hancock M, Ward S, D’Souza SW, Sooranna SR 2000 Discordant 29. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML
amino acid profiles in monochorionic twins with twin-twin transfusion syn- 2001 Circulating ghrelin levels are decreased in human obesity. Diabetes 50:
drome. Pediatr Res 48:821– 828 707–709
6. Bajoria R, Gibson M, Ward S, Sooranna S, Neilson J, Westwood M 2001 30. Blum WF, Breier BH 1994 Radioimmunoassays for IGFs and IGFBPs. Growth
Placental regulation of insulin like growth factor axis in monochorionic twins Regul 4 (Suppl 1):11–19
with chronic twin-twin transfusion syndrome. J Clin Endocrinol Metab 86: 31. Verhaeghe J, Loos R, Vlietinck R, van Herck E, van Bree R, de Schutter A-M
3150 –3156 1996 C-peptide, insulin-like growth factors I and II, and insulin-like growth
7. Baker J, Liu JP, Robertson EJ, Efstratiadis A 1993 Role of insulin-like growth factor binding protein-1 in cord serum of twins: genetic versus environmental
factors in embryonic and postnatal growth. Cell 175:73– 82 regulation. Am J Obstet Gynecol 1175:1180 –1183
8. Powell Braxton L, Hollingshead P, Warburton C 1993 IGF-I is required for 32. Gallaher BW, Breier BH, Oliver MH, Harding JE, Gluckman PD 1992 On-
normal embryonic growth in mice. Genes Dev 7:2609 –2617 togenetic differences in the nutritional regulation of circulating IGF binding
9. De Chiara TM, Efstratiadis A, Robertson EJ 1990 A growth deficiency phe- proteins in sheep plasma. Acta Endocrinol 126:49 –54
notype in heterozygous mice carrying an insulin-like growth factor II gene 33. Clemmons DA, Snyder DK, Busby Jr WH 1991 Variables controlling the
disrupted by targeting. Nature 345:78 – 80 secretion of insulin-like growth factor binding protein-2 in normal human
10. Woods KA, Camacho Hubner C, Savage MO, Clark AJ 1996 Intrauterine subjects. J Clin Endocrinol Metab 73:727–733
growth retardation and postnatal growth failure associated with deletion of the 34. Kitamura S, Yokota I, Hosoda H, Kotani Y, Matsuda J, Naito E, Ito M,
insulin-like growth factor I gene. N Engl J Med 335:1363–1367 Kangawa K, Kuroda Y 2003 Ghrelin concentrations in cord blood and neonatal
11. Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D 2000 Size at birth blood: relation to fetal growth and energy balance. J Clin Endocrinol Metab
and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, 88:5473–5477
IGF-binding protein 1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose- 35. Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P,
6-phosphate receptor in term human infants. ASPAC Study Team. J Clin Bhattacharya S, Carpenter R, Grossman AB, Korbonits M 2002 The tissue
Endocrinol Metab 85:4266 – 4269 distribution of mRNA of ghrelin and subtypes of its receptor, GHS-R, in
12. Spencer JA, Chang TC, Jones J, Robson SC, Preece MA 1995 Third trimester humans. J Clin Endocrinol Metab 87:2988
fetal growth and umbilical venous blood concentrations of IGF-I, IGFBP-1, and 36. Iniguez G, Ong K, Pena V, Avila A, Dunger D, Mericq V 2002 Fasting and
growth hormone at term. Arch Dis Child 173:F87–F90 post-glucose ghrelin levels in SGA infants: relationship with size and weight
13. Osario M, Torres J, Moya F Pezzullo J, Salafia C, Baxter R, Schwander J, Fant gain at one year of age. J Clin Endocrinol Metab 87:5830 –5833
JCEM is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the
endocrine community.
Downloaded from https://academic.oup.com/jcem/article-abstract/90/4/2270/2836881
by guest
on 22 July 2018
S-ar putea să vă placă și
- JC 2016-3452Document8 paginiJC 2016-3452Zoel NikonianÎncă nu există evaluări
- IGF-1, leptin, cortisol levels in cord, maternal serum of SGA, AGA newbornsDocument7 paginiIGF-1, leptin, cortisol levels in cord, maternal serum of SGA, AGA newbornsyulia fatma nstÎncă nu există evaluări
- Altered Angiogenesis As A Common Mechanism Underlying Preterm Birth, Small For Gestational Age, and Stillbirth in Women Living With HIVDocument17 paginiAltered Angiogenesis As A Common Mechanism Underlying Preterm Birth, Small For Gestational Age, and Stillbirth in Women Living With HIVMuhammad Pebri Nilhakim ZavbmÎncă nu există evaluări
- Ultrasound Diagnosis of MacrosomiaDocument4 paginiUltrasound Diagnosis of MacrosomiaIndah 15Încă nu există evaluări
- Genetic Origin of Low Birth WeightDocument7 paginiGenetic Origin of Low Birth WeightCorina CiobanÎncă nu există evaluări
- A Lower Maternal Cortisol-to-Cortisone Ratio Precedes Clinical Diagnosis of Preterm and Term Preeclampsia by Many WeeksDocument12 paginiA Lower Maternal Cortisol-to-Cortisone Ratio Precedes Clinical Diagnosis of Preterm and Term Preeclampsia by Many WeeksMichael HusainÎncă nu există evaluări
- Small for Gestational Age Preterm Neonates Exhibit Defective GH:IGF1 Signaling PathwayDocument8 paginiSmall for Gestational Age Preterm Neonates Exhibit Defective GH:IGF1 Signaling PathwayTimothy supitÎncă nu există evaluări
- GLP-1 and IGF-I Levels Are Elevated in Late Infancy in Low Birthweight Infants, Independently of GLP-1 Receptor Polymorphisms and Neonatal NutritionDocument14 paginiGLP-1 and IGF-I Levels Are Elevated in Late Infancy in Low Birthweight Infants, Independently of GLP-1 Receptor Polymorphisms and Neonatal NutritionmarciamariareisÎncă nu există evaluări
- Insulin-Like Growth Factor Axis Biomarkers and Gestational Diabetes Mellitus: A Systematic Review and Meta-AnalysisDocument12 paginiInsulin-Like Growth Factor Axis Biomarkers and Gestational Diabetes Mellitus: A Systematic Review and Meta-AnalysisBala ChandiranÎncă nu există evaluări
- Children Born Small For Gestational Age Differential Diagnosis, Molecular Genetic Evaluation, and ImplicationsDocument44 paginiChildren Born Small For Gestational Age Differential Diagnosis, Molecular Genetic Evaluation, and ImplicationsErika AvilaÎncă nu există evaluări
- Anemia During PregnnacyDocument9 paginiAnemia During PregnnacytantriÎncă nu există evaluări
- Changes in Maternal Hemoglobin During Pregnancy and Birth OutcomesDocument10 paginiChanges in Maternal Hemoglobin During Pregnancy and Birth OutcomesNimas Ayu Lestari NÎncă nu există evaluări
- alcoholicoseigf1Document10 paginialcoholicoseigf1JOSE PORTILLOÎncă nu există evaluări
- Moderate To Severe, But Not Mild, Maternal Anemia Is Associated With Increased Risk of Small-for-Gestational-Age OutcomesDocument5 paginiModerate To Severe, But Not Mild, Maternal Anemia Is Associated With Increased Risk of Small-for-Gestational-Age OutcomesBonaventura Ahardiansyah BoÎncă nu există evaluări
- Antenatal Maternal HypoxiaDocument6 paginiAntenatal Maternal HypoxiaGilang Hadi FÎncă nu există evaluări
- IGF MilkDocument11 paginiIGF MilktalaÎncă nu există evaluări
- Senapati2018 Article SuperovulationAltersTheExpressDocument10 paginiSenapati2018 Article SuperovulationAltersTheExpressInneke NoerÎncă nu există evaluări
- Identification of IUGR via Cord Blood and Neonatal Serum BiomarkersDocument1 paginăIdentification of IUGR via Cord Blood and Neonatal Serum BiomarkersWijitha VarenniÎncă nu există evaluări
- The Interactive Effect of Prepregnancy Overweight/Obesity and Isolated Maternal Hypothyroxinemia On MacrosomiaDocument8 paginiThe Interactive Effect of Prepregnancy Overweight/Obesity and Isolated Maternal Hypothyroxinemia On MacrosomiaAndres GallegosÎncă nu există evaluări
- Should High BMI Be A Reason For IVF Treatment Denial?Document5 paginiShould High BMI Be A Reason For IVF Treatment Denial?Carlos ParraÎncă nu există evaluări
- 18-American Journal of Reproductive Immunology 2019 SistiDocument5 pagini18-American Journal of Reproductive Immunology 2019 SistiSara PaccosiÎncă nu există evaluări
- Pharmaceutical Sciences: Anemia in Pregnancy A Cause of Low Neonatal Birth WeightDocument4 paginiPharmaceutical Sciences: Anemia in Pregnancy A Cause of Low Neonatal Birth WeightiajpsÎncă nu există evaluări
- Jurnal Hiperemesis GravidarumDocument6 paginiJurnal Hiperemesis GravidarumArief Tirtana PutraÎncă nu există evaluări
- 1346 FullDocument6 pagini1346 FullYbc Berm CruzÎncă nu există evaluări
- Piis 0002937819305873Document8 paginiPiis 0002937819305873Riris RaudyaÎncă nu există evaluări
- Maternal Risk Factors for HypospadiasDocument5 paginiMaternal Risk Factors for HypospadiasHamdan Yuwafi NaimÎncă nu există evaluări
- 10253890.2021(1)Document12 pagini10253890.2021(1)diego.battiatoÎncă nu există evaluări
- Expressions of VEGF-A and VEGFR-2 in Placentae From GDM PregnanciesDocument9 paginiExpressions of VEGF-A and VEGFR-2 in Placentae From GDM PregnanciesMayra PereiraÎncă nu există evaluări
- Growth and Growth Hormone - An Overview PDFDocument3 paginiGrowth and Growth Hormone - An Overview PDFFajar Yuniftiadi0% (1)
- Sirichotiyakul 2016Document5 paginiSirichotiyakul 2016Atul GargÎncă nu există evaluări
- Intrauterine Growth RestrictionDocument8 paginiIntrauterine Growth RestrictionAgung SentosaÎncă nu există evaluări
- 2021 - Voerman - Ped Obesity - Associations of Maternal & Infant Metabolite Profiles With Fetal Growth & Odds Adverse OutcomesDocument11 pagini2021 - Voerman - Ped Obesity - Associations of Maternal & Infant Metabolite Profiles With Fetal Growth & Odds Adverse OutcomespaletaÎncă nu există evaluări
- 21-Archives of Gynecology and Obstetrics - 2019 - SistiDocument5 pagini21-Archives of Gynecology and Obstetrics - 2019 - SistiSara PaccosiÎncă nu există evaluări
- Increasing Fetal Ovine Number Per Gestation Alters Fetal Plasma Clinical Chemistry ValuesDocument12 paginiIncreasing Fetal Ovine Number Per Gestation Alters Fetal Plasma Clinical Chemistry ValuesHuang GlivecÎncă nu există evaluări
- Correlations Between Parameters of Glycaemic Variability and Foetal Growth Neonatal Hypoglycaemia and Hyperbilirubinemia in Women With Gestational diabetesPLoS ONEDocument14 paginiCorrelations Between Parameters of Glycaemic Variability and Foetal Growth Neonatal Hypoglycaemia and Hyperbilirubinemia in Women With Gestational diabetesPLoS ONEVicente Fasce OlmosÎncă nu există evaluări
- IGF1 and Cognitive FunctionDocument8 paginiIGF1 and Cognitive FunctionTanri LindawatiÎncă nu există evaluări
- Differences in angiogenic factors and adipocytokines between early and late pre-eclampsiaDocument7 paginiDifferences in angiogenic factors and adipocytokines between early and late pre-eclampsiaNi Wayan Ana PsÎncă nu există evaluări
- Kim - Taiwan J Obstet Gynecol - 2017Document5 paginiKim - Taiwan J Obstet Gynecol - 2017Sayedur RahmanÎncă nu există evaluări
- Anthropometric Markers Are Poor Predictors of Androgen Levels in Obese Adolescent Girls With PCOS 2017 Journal of Pediatric and Adolescent GynecologyDocument1 paginăAnthropometric Markers Are Poor Predictors of Androgen Levels in Obese Adolescent Girls With PCOS 2017 Journal of Pediatric and Adolescent GynecologyfujimeisterÎncă nu există evaluări
- Original ContributionDocument10 paginiOriginal ContributionzzakieÎncă nu există evaluări
- Obesidad MujeresDocument18 paginiObesidad MujeresJuli MuñozÎncă nu există evaluări
- Middle East Fertility Society Journal: Maryam Eftekhar, Elham Naghshineh, Nosrat Neghab, Robabe HosseinisadatDocument4 paginiMiddle East Fertility Society Journal: Maryam Eftekhar, Elham Naghshineh, Nosrat Neghab, Robabe HosseinisadatM AbhiÎncă nu există evaluări
- Role of VEGF in Preeclampsia PathophysiologyDocument7 paginiRole of VEGF in Preeclampsia PathophysiologyLintang SuroyaÎncă nu există evaluări
- Can Placental Growth Factor in Maternal Circulation Identify Fetuses With Placental Intrauterine Growth RestrictionDocument7 paginiCan Placental Growth Factor in Maternal Circulation Identify Fetuses With Placental Intrauterine Growth RestrictionagusÎncă nu există evaluări
- Association Between Intrahepatic Cholestasis in Pregnancy and Gestational Diabetes Mellitus. A Retrospective AnalysisDocument6 paginiAssociation Between Intrahepatic Cholestasis in Pregnancy and Gestational Diabetes Mellitus. A Retrospective AnalysisDr.Karima EldarhobiÎncă nu există evaluări
- Gestational Diabetes Mellitus Is Associated With Adverse Outcomes in Twin PregnanciesDocument8 paginiGestational Diabetes Mellitus Is Associated With Adverse Outcomes in Twin PregnanciesDwi OktaviliaÎncă nu există evaluări
- Sistema Inmune Fetal y Factores Angiogénicos. Diferencias Según Sexo Fetal PDFDocument12 paginiSistema Inmune Fetal y Factores Angiogénicos. Diferencias Según Sexo Fetal PDFVictor AyalaÎncă nu există evaluări
- Genes: Modulation of Placental Gene Expression in Small-for-Gestational-Age InfantsDocument23 paginiGenes: Modulation of Placental Gene Expression in Small-for-Gestational-Age InfantsLuciani Magdalena Palacios GarcíaÎncă nu există evaluări
- (20834594 - Anthropological Review) Relationship Between Type and Weight of Placenta and Neonate Birth Weight in Twin PregnancyDocument10 pagini(20834594 - Anthropological Review) Relationship Between Type and Weight of Placenta and Neonate Birth Weight in Twin PregnancyAndreÎncă nu există evaluări
- Andersen Et Al 2019Document14 paginiAndersen Et Al 2019Emil AndersenÎncă nu există evaluări
- Muest RaDocument13 paginiMuest RaBruno LinoÎncă nu există evaluări
- 1 s2.0 S0301211510005671 MainDocument4 pagini1 s2.0 S0301211510005671 MainTeodora OnofreiÎncă nu există evaluări
- $116 SMFM AbstractsDocument1 pagină$116 SMFM AbstractsSheila Regina TizaÎncă nu există evaluări
- Inguinal Hernia in Preterm Infants ( 32-Week Gestation) : Original ArticleDocument7 paginiInguinal Hernia in Preterm Infants ( 32-Week Gestation) : Original ArticleBlank SpaceÎncă nu există evaluări
- Selective IUGRDocument6 paginiSelective IUGRMeycha Da FhonsaÎncă nu există evaluări
- Pregnancy Outcomes in Familial Hypercholesterolemia: Epidemiology and PreventionDocument10 paginiPregnancy Outcomes in Familial Hypercholesterolemia: Epidemiology and PreventionsenkonenÎncă nu există evaluări
- Body composition and hormones in infants born smallDocument5 paginiBody composition and hormones in infants born smallLuana BarrosÎncă nu există evaluări
- Complementary and Alternative Medical Lab Testing Part 10: ObstetricsDe la EverandComplementary and Alternative Medical Lab Testing Part 10: ObstetricsÎncă nu există evaluări
- Maternal Underweight and Risk of Preterm BirthDocument7 paginiMaternal Underweight and Risk of Preterm BirthSurraya JamshaidÎncă nu există evaluări
- Infants of Diabetic Mothers: Risks and ManagementDocument29 paginiInfants of Diabetic Mothers: Risks and ManagementMochammad Arsyi GÎncă nu există evaluări
- Antepartal Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor Binding Protein 2 Concentrations Are Indicative of Ketosis in Dairy CowsDocument11 paginiAntepartal Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor Binding Protein 2 Concentrations Are Indicative of Ketosis in Dairy Cowsyulia fatma nstÎncă nu există evaluări
- Psychoneuroendocrinology: Mariska Bot, Yuri Milaneschi, Brenda W.J.H. Penninx, Madeleine L. DrentDocument8 paginiPsychoneuroendocrinology: Mariska Bot, Yuri Milaneschi, Brenda W.J.H. Penninx, Madeleine L. Drentyulia fatma nstÎncă nu există evaluări
- Combined iron and zinc supplementation improves hematologic status of pregnant womenDocument10 paginiCombined iron and zinc supplementation improves hematologic status of pregnant womenyulia fatma nstÎncă nu există evaluări
- Growth and Growth Hormone - Insulin Like Growth Factor - I (GH-IGF-I) Axis in Chronic AnemiasDocument11 paginiGrowth and Growth Hormone - Insulin Like Growth Factor - I (GH-IGF-I) Axis in Chronic Anemiasyulia fatma nstÎncă nu există evaluări
- Joslin Diabetes Center and Joslin Clinic Guideline For Detection and Management of Diabetes in PregnancyDocument10 paginiJoslin Diabetes Center and Joslin Clinic Guideline For Detection and Management of Diabetes in Pregnancyyulia fatma nstÎncă nu există evaluări
- Metabolic Consequences of Pregnancy-Associated Plasma Protein-A Deficiency in Mice: Exploring Possible Relationship To The Longevity PhenotypeDocument7 paginiMetabolic Consequences of Pregnancy-Associated Plasma Protein-A Deficiency in Mice: Exploring Possible Relationship To The Longevity Phenotypeyulia fatma nstÎncă nu există evaluări
- IGF-1 Therapy in Children With Liver Dysfunction: Anum Akbar, Ume Salmah AhmadDocument5 paginiIGF-1 Therapy in Children With Liver Dysfunction: Anum Akbar, Ume Salmah Ahmadyulia fatma nstÎncă nu există evaluări
- Nutrients: Effects of Vitamin D Supplementation On IGF-1 and Calcitriol: A Randomized-Controlled TrialDocument10 paginiNutrients: Effects of Vitamin D Supplementation On IGF-1 and Calcitriol: A Randomized-Controlled Trialyulia fatma nstÎncă nu există evaluări
- Serum IGF-1 and IGFBP-3 Levels in Healthy Children Between 0 and 6 Years of AgeDocument6 paginiSerum IGF-1 and IGFBP-3 Levels in Healthy Children Between 0 and 6 Years of Ageyulia fatma nstÎncă nu există evaluări
- PDF Hosted at The Radboud Repository of The Radboud University NijmegenDocument15 paginiPDF Hosted at The Radboud Repository of The Radboud University Nijmegenyulia fatma nstÎncă nu există evaluări
- Insulin-Like Growth Factor 1 and Growth Rate in Nestlings of A Wild Passerine BirdDocument8 paginiInsulin-Like Growth Factor 1 and Growth Rate in Nestlings of A Wild Passerine Birdyulia fatma nstÎncă nu există evaluări
- FullDocument37 paginiFullyulia fatma nstÎncă nu există evaluări
- JID-01437-AU Placentalmalaria Igf v1Document40 paginiJID-01437-AU Placentalmalaria Igf v1yulia fatma nstÎncă nu există evaluări
- Role of Insulin Like Growth Factor in Polycystic Ovary SyndromeDocument7 paginiRole of Insulin Like Growth Factor in Polycystic Ovary Syndromeyulia fatma nstÎncă nu există evaluări
- DOCTORS CENTER WELLNESS Blood Markers27 Clinical SignificanceDocument16 paginiDOCTORS CENTER WELLNESS Blood Markers27 Clinical Significanceyulia fatma nstÎncă nu există evaluări
- Thesis Charlotte IacobaeusDocument76 paginiThesis Charlotte Iacobaeusyulia fatma nstÎncă nu există evaluări
- DOCTORS CENTER WELLNESS Blood Markers27 Clinical SignificanceDocument16 paginiDOCTORS CENTER WELLNESS Blood Markers27 Clinical Significanceyulia fatma nstÎncă nu există evaluări
- J. Biol. Chem.-2013-Kjaer-Sorensen-9982-92Document12 paginiJ. Biol. Chem.-2013-Kjaer-Sorensen-9982-92yulia fatma nstÎncă nu există evaluări
- GrowthHormoneDeficiency Dwarfism PDFDocument4 paginiGrowthHormoneDeficiency Dwarfism PDFyulia fatma nstÎncă nu există evaluări
- The Insulin-Like Growth Factor SystemDocument13 paginiThe Insulin-Like Growth Factor Systemyulia fatma nstÎncă nu există evaluări
- Determinants of Growth Hormone Resistance in MalnutritionDocument14 paginiDeterminants of Growth Hormone Resistance in Malnutritionyulia fatma nstÎncă nu există evaluări
- Placental Growth Hormone (GH), GH-Binding Protein Mcintry 2000 PDFDocument8 paginiPlacental Growth Hormone (GH), GH-Binding Protein Mcintry 2000 PDFyulia fatma nstÎncă nu există evaluări
- Fontana Et Al 2008Document7 paginiFontana Et Al 2008yulia fatma nstÎncă nu există evaluări
- IGF-1 and Atherothrombosis Relevance To Pathophysiology and TherapyDocument8 paginiIGF-1 and Atherothrombosis Relevance To Pathophysiology and Therapyyulia fatma nstÎncă nu există evaluări
- Introduction - Prof SudigdoDocument49 paginiIntroduction - Prof Sudigdoyulia fatma nstÎncă nu există evaluări
- Determinants of GH Resistance in PDFDocument9 paginiDeterminants of GH Resistance in PDFyulia fatma nstÎncă nu există evaluări
- Nej M 199310213291701Document6 paginiNej M 199310213291701yulia fatma nstÎncă nu există evaluări
- Jurnal DesiDocument11 paginiJurnal Desiyulia fatma nstÎncă nu există evaluări
- PethotelDocument11 paginiPethotelkarimakki0% (1)
- Don't Say Forever Victoria Falls SequenceDocument9 paginiDon't Say Forever Victoria Falls SequenceMaha GolestanehÎncă nu există evaluări
- Mosaic - TRD1 - U2 - EP CorregidoDocument6 paginiMosaic - TRD1 - U2 - EP CorregidoSonia Daniela Ayuso FrutosÎncă nu există evaluări
- Passion and Deceit Part VI PDFDocument20 paginiPassion and Deceit Part VI PDFno2meÎncă nu există evaluări
- Silk Road Journal 6-2Document69 paginiSilk Road Journal 6-2Ciro Lo MuzioÎncă nu există evaluări
- Newsletter 1013 PDFDocument4 paginiNewsletter 1013 PDFMountLockyerÎncă nu există evaluări
- Rapid Assessment SheetDocument5 paginiRapid Assessment SheetElise HowardÎncă nu există evaluări
- Status of Poultry Industry in Sri LankaDocument21 paginiStatus of Poultry Industry in Sri LankaDickson MahanamaÎncă nu există evaluări
- CH 12 Word ListDocument3 paginiCH 12 Word ListtigertiaÎncă nu există evaluări
- Animal Trafficking Lesson PlanDocument4 paginiAnimal Trafficking Lesson Plankurucz barbaraÎncă nu există evaluări
- Checklist of Bats From Iraq-Mammalian Biology 2020Document14 paginiChecklist of Bats From Iraq-Mammalian Biology 2020Adil DalafÎncă nu există evaluări
- Bai Darfo Registered Pet Shops As of June 30 2023Document6 paginiBai Darfo Registered Pet Shops As of June 30 2023itshardtogetauserÎncă nu există evaluări
- Honey Bee Complaint As Community Policing For Scouting Against PCA Act, 1960: Restoring 5 FreedomsDocument31 paginiHoney Bee Complaint As Community Policing For Scouting Against PCA Act, 1960: Restoring 5 FreedomsNaresh KadyanÎncă nu există evaluări
- SchistosomiasisDocument3 paginiSchistosomiasisBeRnAlieÎncă nu există evaluări
- Mage The Awakening - Intruders - Encounters With The Abyss PDFDocument228 paginiMage The Awakening - Intruders - Encounters With The Abyss PDFJohnRichardWuethrich100% (2)
- THE STAG - Class NotesDocument3 paginiTHE STAG - Class Notesmishka100% (1)
- Legend 1985Document205 paginiLegend 1985EruditeHoboÎncă nu există evaluări
- Double Click 1 UNIT 1Document6 paginiDouble Click 1 UNIT 1matiw ledesma0% (1)
- What Is HomeoprophylaxisDocument6 paginiWhat Is HomeoprophylaxiswrestlerloverÎncă nu există evaluări
- 2013 Skin and Wound Infections - StudentDocument35 pagini2013 Skin and Wound Infections - Studentmicroperadeniya0% (1)
- Soal Bhs Inggris Kls 11Document15 paginiSoal Bhs Inggris Kls 11CahyonoÎncă nu există evaluări
- Fables Grade 2Document4 paginiFables Grade 2angeliÎncă nu există evaluări
- Reading and Wrting Performance TaskDocument13 paginiReading and Wrting Performance TaskjefeljoevillacortaÎncă nu există evaluări
- MeerkatDocument4 paginiMeerkatCont JocÎncă nu există evaluări
- Surfactant Composition and Function: Joanna Floros, PH.DDocument21 paginiSurfactant Composition and Function: Joanna Floros, PH.DΜαρία-Άννα ΚιρμπάκηÎncă nu există evaluări
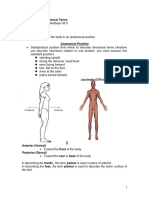
- Anatomical TermsDocument8 paginiAnatomical TermsDAGUMAN, FIONA DEI L.Încă nu există evaluări
- Human and Animal ConsciousnessDocument23 paginiHuman and Animal ConsciousnessNoelle Leslie Dela CruzÎncă nu există evaluări
- Ujian Masuk Program Pascasarjana: Jangan Dibuka Dulu. Tunggu PetunjukDocument21 paginiUjian Masuk Program Pascasarjana: Jangan Dibuka Dulu. Tunggu PetunjukLalu SuhaimiÎncă nu există evaluări
- June 1, (Thursday) 2017Document10 paginiJune 1, (Thursday) 2017BS Central, Inc. "The Buzz"Încă nu există evaluări
- SMP Kakaskasen English Exam QuestionsDocument5 paginiSMP Kakaskasen English Exam QuestionsadeÎncă nu există evaluări