Documente Academic
Documente Profesional
Documente Cultură
Iron-Carbon Phase Diagram (A Review) See Callister Chapter 9
Încărcat de
babiker mortada0 evaluări0% au considerat acest document util (0 voturi)
26 vizualizări13 paginiiron carbon diagram
Titlu original
FeC3
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentiron carbon diagram
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
26 vizualizări13 paginiIron-Carbon Phase Diagram (A Review) See Callister Chapter 9
Încărcat de
babiker mortadairon carbon diagram
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 13
MSE 300 Materials Laboratory Procedures
Iron-Carbon Phase
Diagram (a review) see
Callister Chapter 9
University of Tennessee, Dept. of Materials Science and Engineering 1
MSE 300 Materials Laboratory Procedures
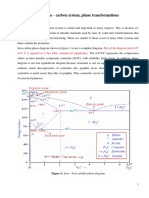
The Iron–Iron Carbide (Fe–Fe3C) Phase Diagram
In their simplest form, steels are alloys of Iron (Fe) and
Carbon (C). The Fe-C phase diagram is a fairly complex
one, but we will only consider the steel part of the diagram,
up to around 7% Carbon.
University of Tennessee, Dept. of Materials Science and Engineering 2
MSE 300 Materials Laboratory Procedures
Phases in Fe–Fe3C Phase Diagram
! !-ferrite - solid solution of C in BCC Fe
• Stable form of iron at room temperature.
• The maximum solubility of C is 0.022 wt%
• Transforms to FCC "-austenite at 912 #C
! "-austenite - solid solution of C in FCC Fe
• The maximum solubility of C is 2.14 wt %.
• Transforms to BCC $-ferrite at 1395 #C
• Is not stable below the eutectic temperature
(727 # C) unless cooled rapidly (Chapter 10)
! $-ferrite solid solution of C in BCC Fe
• The same structure as !-ferrite
• Stable only at high T, above 1394 #C
• Melts at 1538 #C
! Fe3C (iron carbide or cementite)
• This intermetallic compound is metastable, it
remains as a compound indefinitely at room T, but
decomposes (very slowly, within several years)
into !-Fe and C (graphite) at 650 - 700 #C
! Fe-C liquid solution
University of Tennessee, Dept. of Materials Science and Engineering 3
MSE 300 Materials Laboratory Procedures
A few comments on Fe–Fe3C system
C is an interstitial impurity in Fe. It forms a solid solution
with !%&"%&$&&phases of iron
Maximum solubility in BCC !-ferrite is limited (max.
0.022 wt% at 727 #C) - BCC has relatively small interstitial
positions
Maximum solubility in FCC austenite is 2.14 wt% at 1147
#C - FCC has larger interstitial positions
Mechanical properties: Cementite is very hard and brittle -
can strengthen steels. Mechanical properties also depend
on the microstructure, that is, how ferrite and cementite are
mixed.
Magnetic properties: ! -ferrite is magnetic below 768 #C,
austenite is non-magnetic
Classification. Three types of ferrous alloys:
• Iron: less than 0.008 wt % C in !'ferrite at room T
• Steels: 0.008 - 2.14 wt % C (usually < 1 wt % )
!-ferrite + Fe3C at room T (Chapter 12)
• Cast iron: 2.14 - 6.7 wt % (usually < 4.5 wt %)
University of Tennessee, Dept. of Materials Science and Engineering 4
MSE 300 Materials Laboratory Procedures
Eutectic and eutectoid reactions in Fe–Fe3C
Eutectic: 4.30 wt% C, 1147 #C
L ( " + Fe3C
Eutectoid: 0.76 wt%C, 727 #C
"(0.76 wt% C) ( ! (0.022 wt% C) + Fe3C
Eutectic and eutectoid reactions are very important in
heat treatment of steels
University of Tennessee, Dept. of Materials Science and Engineering 5
MSE 300 Materials Laboratory Procedures
Development of Microstructure in Iron - Carbon alloys
Microstructure depends on composition (carbon
content) and heat treatment. In the discussion below we
consider slow cooling in which equilibrium is maintained.
Microstructure of eutectoid steel (I)
University of Tennessee, Dept. of Materials Science and Engineering 6
MSE 300 Materials Laboratory Procedures
Microstructure of eutectoid steel (II)
When alloy of eutectoid composition (0.76 wt % C) is
cooled slowly it forms perlite, a lamellar or layered
structure of two phases: !-ferrite and cementite (Fe3C)
The layers of alternating phases in pearlite are formed for
the same reason as layered structure of eutectic structures:
redistribution C atoms between ferrite (0.022 wt%) and
cementite (6.7 wt%) by atomic diffusion.
Mechanically, pearlite has properties intermediate to soft,
ductile ferrite and hard, brittle cementite.
In the micrograph, the dark areas are
Fe3C layers, the light phase is !-
ferrite
University of Tennessee, Dept. of Materials Science and Engineering 7
MSE 300 Materials Laboratory Procedures
Microstructure of hypoeutectoid steel (I)
Compositions to the left of eutectoid (0.022 - 0.76 wt % C)
hypoeutectoid (less than eutectoid -Greek) alloys.
" ) ! + " ) ! + Fe3C
University of Tennessee, Dept. of Materials Science and Engineering 8
MSE 300 Materials Laboratory Procedures
Microstructure of hypoeutectoid steel (II)
Hypoeutectoid alloys contain proeutectoid ferrite (formed
above the eutectoid temperature) plus the eutectoid perlite
that contain eutectoid ferrite and cementite.
University of Tennessee, Dept. of Materials Science and Engineering 9
MSE 300 Materials Laboratory Procedures
Microstructure of hypereutectoid steel (I)
Compositions to the right of eutectoid (0.76 - 2.14 wt % C)
hypereutectoid (more than eutectoid -Greek) alloys.
" ) " + Fe3C ) ! + Fe3C
University of Tennessee, Dept. of Materials Science and Engineering 10
MSE 300 Materials Laboratory Procedures
Microstructure of hypereutectoid steel (II)
Hypereutectoid alloys contain proeutectoid cementite
(formed above the eutectoid temperature) plus perlite that
contain eutectoid ferrite and cementite.
University of Tennessee, Dept. of Materials Science and Engineering 11
MSE 300 Materials Laboratory Procedures
How to calculate the relative amounts of proeutectoid
phase (! or Fe3C) and pearlite?
Application of the lever rule with tie line that extends from
the eutectoid composition (0.76 wt% C) to ! – (! + Fe3C)
boundary (0.022 wt% C) for hypoeutectoid alloys and to (!
+ Fe3C) – Fe3C boundary (6.7 wt% C) for hypereutectoid
alloys.
Fraction of ! phase is determined by application of the
lever rule across
University the entire
of Tennessee, Dept.(! + Fe3C)
of Materials phase
Science field:
and Engineering 12
MSE 300 Materials Laboratory Procedures
Example for hypereutectoid alloy with composition C1
Fraction of pearlite:
WP = X / (V+X) = (6.7 – C1) / (6.7 – 0.76)
Fraction of proeutectoid cementite:
WFe3C = V / (V+X) = (C1 – 0.76) / (6.7 – 0.76)
University of Tennessee, Dept. of Materials Science and Engineering 13
S-ar putea să vă placă și
- Control and Analysis in Iron and SteelmakingDe la EverandControl and Analysis in Iron and SteelmakingEvaluare: 3 din 5 stele3/5 (2)
- Pages From FeCDocument13 paginiPages From FeCFuhad HasanÎncă nu există evaluări
- Materials Data for Cyclic Loading: Aluminium and Titanium AlloysDe la EverandMaterials Data for Cyclic Loading: Aluminium and Titanium AlloysEvaluare: 1 din 5 stele1/5 (1)
- Fe-C Phase DiagramDocument34 paginiFe-C Phase DiagramYoung-long Choi100% (1)
- Iron-Carbon Phase Diagram (A Review) See Callister Chapter 9Document34 paginiIron-Carbon Phase Diagram (A Review) See Callister Chapter 9Zefa Erliana YullahÎncă nu există evaluări
- Fe CDocument34 paginiFe CZaza ArifinÎncă nu există evaluări
- Material Science and MettalurgyDocument21 paginiMaterial Science and MettalurgySR-71 BLACKBIRDÎncă nu există evaluări
- Fec DiagramDocument15 paginiFec DiagramShankarÎncă nu există evaluări
- M. Tech. (FFT) Technology of Ferrous Casting Phase DiagramDocument7 paginiM. Tech. (FFT) Technology of Ferrous Casting Phase DiagramRajulapati Sunil KumarÎncă nu există evaluări
- Phase Diagrams - 040823Document23 paginiPhase Diagrams - 040823Anthony MubangaÎncă nu există evaluări
- 9 Engineering AlloysDocument17 pagini9 Engineering AlloysdavidtomyÎncă nu există evaluări
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 paginiUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongÎncă nu există evaluări
- Iron Carbon DiagramDocument10 paginiIron Carbon DiagramsivakumarÎncă nu există evaluări
- Fe-Fe3C Phase Diagram GuideDocument55 paginiFe-Fe3C Phase Diagram GuideMrDOTÎncă nu există evaluări
- Chapter Outline:: Heat Treatment (And Temperature)Document24 paginiChapter Outline:: Heat Treatment (And Temperature)DSGÎncă nu există evaluări
- Fe-Fe3C phase diagram analysisDocument10 paginiFe-Fe3C phase diagram analysisAlina OmerovicÎncă nu există evaluări
- 8.fe - Fe3C Phase DiagramDocument27 pagini8.fe - Fe3C Phase DiagramMhackSahuÎncă nu există evaluări
- Fracture Dependence On Heat Treatment: Table 1-Dependence of Absorbed Energy (For Crack Propagation) Over TemperatureDocument4 paginiFracture Dependence On Heat Treatment: Table 1-Dependence of Absorbed Energy (For Crack Propagation) Over Temperaturelokomoko1Încă nu există evaluări
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument39 paginiFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (14)
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument79 paginiFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgÎncă nu există evaluări
- 18bem0046 Materials Da 2Document20 pagini18bem0046 Materials Da 2QUANTUM SOLEOSÎncă nu există evaluări
- Chapter Outline: Phase Transformations. KineticsDocument23 paginiChapter Outline: Phase Transformations. Kineticsmpcd07Încă nu există evaluări
- Iron Carbon Equilibrium DiagramDocument11 paginiIron Carbon Equilibrium Diagramganesh82Încă nu există evaluări
- Fe-C Phase Diagram Lecture 33Document15 paginiFe-C Phase Diagram Lecture 33Manish GuptaÎncă nu există evaluări
- 3 Fe-Fe3C Phase DiagramDocument33 pagini3 Fe-Fe3C Phase DiagramRajat Mishra100% (1)
- FALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramDocument33 paginiFALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramFazal KhanÎncă nu există evaluări
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 paginiCh-27.5 Iron Carbon Equilibrium DiagramManojÎncă nu există evaluări
- Fe CdiagramDocument36 paginiFe CdiagramGeorge SingerÎncă nu există evaluări
- Ferrous Metals GuideDocument113 paginiFerrous Metals GuideAbhishek ChavanÎncă nu există evaluări
- Weldability of Metals - NPTELDocument18 paginiWeldability of Metals - NPTELKaushal Gandhi0% (1)
- 3 Iron Carbon DiaDocument21 pagini3 Iron Carbon DiaChhavi SharmaÎncă nu există evaluări
- What Is PearliteDocument4 paginiWhat Is Pearliteardy cornettoÎncă nu există evaluări
- Carbon Steels: ME 215/205:materials Science LaboratoryDocument9 paginiCarbon Steels: ME 215/205:materials Science Laboratoryfaizan997Încă nu există evaluări
- BG2802 Heat Treatment and Mechanical Properties of SteelsDocument11 paginiBG2802 Heat Treatment and Mechanical Properties of SteelsVenus LimÎncă nu există evaluări
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 paginiIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatÎncă nu există evaluări
- Research of Phase Transformation On Fe-8.7Al-28.3Mn-1C-5.5Cr AlloyDocument4 paginiResearch of Phase Transformation On Fe-8.7Al-28.3Mn-1C-5.5Cr AlloyMarina PiermannÎncă nu există evaluări
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument58 paginiCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfÎncă nu există evaluări
- Iron - Carbon Phase DiagramDocument19 paginiIron - Carbon Phase DiagramGaurav DograÎncă nu există evaluări
- Iron Carbon Phase DiagramDocument4 paginiIron Carbon Phase DiagramMizanur RahmanÎncă nu există evaluări
- Sudipta Nath: Materials EngineeringDocument19 paginiSudipta Nath: Materials EngineeringSudipta NathÎncă nu există evaluări
- Phase Diagram 3Document26 paginiPhase Diagram 3Tony StarkÎncă nu există evaluări
- Engineering Materials 27-29Document40 paginiEngineering Materials 27-29Sanu SouravÎncă nu există evaluări
- 8.fe - Fe3C Phase Diagram PDFDocument27 pagini8.fe - Fe3C Phase Diagram PDF13311A0341 S SHIVA SAI KIRANÎncă nu există evaluări
- Week 5 Phase DiagramDocument51 paginiWeek 5 Phase DiagramMohaiminul Islam TalhaÎncă nu există evaluări
- Chapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMDocument13 paginiChapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMPAUL NDIRITUÎncă nu există evaluări
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 paginiSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserÎncă nu există evaluări
- Industrial Materials (B)Document15 paginiIndustrial Materials (B)Muhammad Bilal SahiÎncă nu există evaluări
- Dokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dDocument16 paginiDokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dAfrizal Adithya PÎncă nu există evaluări
- TMP CBECDocument14 paginiTMP CBECFrontiersÎncă nu există evaluări
- Fe-Fe3C Phase Diagram and MicrostructuresDocument42 paginiFe-Fe3C Phase Diagram and MicrostructuresTalaat Ahmed Mohamed El-Benawy100% (2)
- Materials of Construction and Selection: Faculty of Chemical Engineering Universiti Teknologi MaraDocument80 paginiMaterials of Construction and Selection: Faculty of Chemical Engineering Universiti Teknologi MaraAisyah Addia AzizanÎncă nu există evaluări
- EMM 2312 - Fe-CDocument53 paginiEMM 2312 - Fe-CCalebÎncă nu există evaluări
- SteelMicrostructuresLab (4) (1)Document7 paginiSteelMicrostructuresLab (4) (1)muhammad.amir14333Încă nu există evaluări
- 2 Physical Metallurgy (Btcmtpcc403) Btech 2nd Year 2nd Semester Module 2Document39 pagini2 Physical Metallurgy (Btcmtpcc403) Btech 2nd Year 2nd Semester Module 2ranjan.metal98Încă nu există evaluări
- Chapter 10Document4 paginiChapter 10Izzat NasrullahÎncă nu există evaluări
- Microstructure of Steels and Cast IronDocument14 paginiMicrostructure of Steels and Cast IronPraicy Ann josephÎncă nu există evaluări
- Iron Carbon Diagram (ChE Handbook)Document21 paginiIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- ASSIGNMENT # 3 W KEYDocument16 paginiASSIGNMENT # 3 W KEYRashedul IslamÎncă nu există evaluări
- Engineering Material-II: Iron Carbide Phase DiagramDocument16 paginiEngineering Material-II: Iron Carbide Phase DiagramAla ZiÎncă nu există evaluări
- Ductile Iron: Rio Tinto Iron & TitaniumDocument18 paginiDuctile Iron: Rio Tinto Iron & TitaniumarirodriguezdiazÎncă nu există evaluări
- Foundry, LR PDFDocument8 paginiFoundry, LR PDFbabiker mortadaÎncă nu există evaluări
- Thin Wall Cast Iron: Phase II: Project TeamDocument10 paginiThin Wall Cast Iron: Phase II: Project Teambabiker mortadaÎncă nu există evaluări
- Grey Cast IronDocument97 paginiGrey Cast IronSiddharth GuptaÎncă nu există evaluări
- Vol8no3 Aujtechno07Document5 paginiVol8no3 Aujtechno07rakeshkaushikÎncă nu există evaluări
- Design Study PDFDocument28 paginiDesign Study PDFbabiker mortadaÎncă nu există evaluări
- Gating CalDocument1 paginăGating Calbabiker mortada100% (1)
- The Effect of Gating Design on Aluminum Casting QualityDocument20 paginiThe Effect of Gating Design on Aluminum Casting Qualitybabiker mortadaÎncă nu există evaluări
- MY4130 Mid Term Exam KeyDocument3 paginiMY4130 Mid Term Exam Keybabiker mortadaÎncă nu există evaluări
- SOLIDWORKS Advanced Part ModellingDocument3 paginiSOLIDWORKS Advanced Part Modellingbabiker mortadaÎncă nu există evaluări
- Web Only 0406123Document12 paginiWeb Only 0406123Rifki AuliaÎncă nu există evaluări
- 1 PDFDocument10 pagini1 PDFbabiker mortadaÎncă nu există evaluări
- 10 1 1 389 8478Document41 pagini10 1 1 389 8478Khalil LasferÎncă nu există evaluări
- اساسيات الاوتوكاد 2017Document3 paginiاساسيات الاوتوكاد 2017babiker mortadaÎncă nu există evaluări
- CASTING Design GuideDocument54 paginiCASTING Design GuideXin Yu75% (4)
- Design InfluenceDocument80 paginiDesign InfluenceSelva RamÎncă nu există evaluări
- Sheet Metal Design Solidworks CapabiltiesDocument59 paginiSheet Metal Design Solidworks Capabiltiesbabiker mortada100% (3)
- Foundry, LR PDFDocument8 paginiFoundry, LR PDFbabiker mortadaÎncă nu există evaluări
- Production 12Document33 paginiProduction 12babiker mortadaÎncă nu există evaluări
- 2 CastingDocument16 pagini2 Castinggubeguru100% (11)
- 2 CastingDocument16 pagini2 Castinggubeguru100% (11)
- Phase Equilibria in Materials (MME 330) : Laboratory ManualDocument33 paginiPhase Equilibria in Materials (MME 330) : Laboratory ManualAhmed Nawaz DanishÎncă nu există evaluări
- PD Binary PDFDocument81 paginiPD Binary PDFmanilrajkrr6302Încă nu există evaluări
- API CatalogueDocument20 paginiAPI CatalogueMadirley Pimenta100% (1)
- EHB en 7-Sizing PDFDocument83 paginiEHB en 7-Sizing PDFChristopher Kenneth ChoaÎncă nu există evaluări
- 4.122 MSC Chem Phy Sem III and IVDocument32 pagini4.122 MSC Chem Phy Sem III and IVAsma MerchantÎncă nu există evaluări
- Phono Par PDFDocument8 paginiPhono Par PDFvranceanu.ovidiu-1Încă nu există evaluări
- UV Vis Spectroscopy Reveals Electronic TransitionsDocument4 paginiUV Vis Spectroscopy Reveals Electronic Transitionsurwah naveed100% (1)
- Geotechnical Design Manual FinalDocument190 paginiGeotechnical Design Manual FinalBrhane W Ygzaw100% (4)
- Tem-290 Process Validation Protocol Template SampleDocument5 paginiTem-290 Process Validation Protocol Template SampleJonatan Dominguez Perez100% (2)
- Bhushan Arun Patil - Formulation Development (R and D)Document3 paginiBhushan Arun Patil - Formulation Development (R and D)Bhushan PatilÎncă nu există evaluări
- Squeezing Ground ConditionDocument16 paginiSqueezing Ground ConditionMourad HosniÎncă nu există evaluări
- Astm C 473-03 Standard Test Methods For Physical Testing ofDocument13 paginiAstm C 473-03 Standard Test Methods For Physical Testing ofCharwin Picao100% (1)
- JL-98-November-December Restraint Moments in Precast Prestressed Concrete Continuous BridgesDocument18 paginiJL-98-November-December Restraint Moments in Precast Prestressed Concrete Continuous Bridgesjrobert123321Încă nu există evaluări
- Grupo 5. LCA Chemical SolventDocument9 paginiGrupo 5. LCA Chemical SolventJuan Manuel FlorezÎncă nu există evaluări
- Class X Science Question PaperDocument24 paginiClass X Science Question PaperKalpna RaniÎncă nu există evaluări
- Ujian RemediDocument1 paginăUjian RemediJokoSuswonoÎncă nu există evaluări
- Visible Particles Regulatory and Compendial RequirementsDocument31 paginiVisible Particles Regulatory and Compendial Requirementsdavincicode888Încă nu există evaluări
- Study of Equipment Presses of Cocoa Powder (Theobroma Cacao, L) To Produce Quality Fat Cocoa and Analysis of The ResultingDocument8 paginiStudy of Equipment Presses of Cocoa Powder (Theobroma Cacao, L) To Produce Quality Fat Cocoa and Analysis of The ResultingAlex Ramadhan SabananyoÎncă nu există evaluări
- ALS Minerals Service Schedule USDDocument44 paginiALS Minerals Service Schedule USDGAUCHEX697355Încă nu există evaluări
- CaseStudy2 WindmillDocument8 paginiCaseStudy2 WindmillAnthony BergemannÎncă nu există evaluări
- Alloy 286Document6 paginiAlloy 286shivam.kumarÎncă nu există evaluări
- 1-2003-Laskowski Et Al-2003-The Canadian Journal of Chemical EngineeringDocument7 pagini1-2003-Laskowski Et Al-2003-The Canadian Journal of Chemical EngineeringjvchiqueÎncă nu există evaluări
- FormulaDocument6 paginiFormulaLars RembrandtÎncă nu există evaluări
- Worksheet 2 - TLC - Updated Summer 2021Document4 paginiWorksheet 2 - TLC - Updated Summer 2021Bria PopeÎncă nu există evaluări
- Rr322105-High Speed AerodynamicsDocument8 paginiRr322105-High Speed AerodynamicsSRINIVASA RAO GANTAÎncă nu există evaluări
- Assignment 1 CarboxylicDocument8 paginiAssignment 1 CarboxylicYu HuiÎncă nu există evaluări
- Metrology and MeasurementsDocument58 paginiMetrology and MeasurementsShishir Fawade75% (4)
- STANKIEWICZ, !!!! Process Intensification 2002 PDFDocument5 paginiSTANKIEWICZ, !!!! Process Intensification 2002 PDFFranco A. ZavaletaÎncă nu există evaluări
- Welcome To This Course Physics 1Document31 paginiWelcome To This Course Physics 1andrewsiby63Încă nu există evaluări
- Structure and Bonding (Chapter 3) Exam Questions: 141 Minutes 141 MarksDocument34 paginiStructure and Bonding (Chapter 3) Exam Questions: 141 Minutes 141 Marksrejymol100% (1)
- A Dictionary of ColoursDocument528 paginiA Dictionary of ColoursMohammed Mahfouz78% (9)
- Atomic StructureDocument2 paginiAtomic Structureapi-350245383Încă nu există evaluări