Documente Academic
Documente Profesional
Documente Cultură
1b Periodic Table Notes
Încărcat de
api-289539870Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
1b Periodic Table Notes
Încărcat de
api-289539870Drepturi de autor:
Formate disponibile
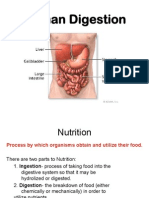
The Periodic Table of Elements
What is the Periodic Table?
It is a table of all of the chemical elements. An element is something that is made of
only one kind of atom. An atom is a very small particle, and every element is made
of a different kind of atom.
How are Atoms of Different Elements Different?
In 1800, the English chemist John Dalton came up with his theory that all elements
are made of different atoms, and that atoms of different elements all have different
mass. For example, a single atom of oxygen is heavier than a single atom of
hydrogen.
He also explained his experiments by saying that different atoms could join together
to make a particle with more than one atom. This is called a molecule. Molecules
can be made of atoms of the same element, or made of atoms of different
elements, called compounds.
Molecules
Atoms of one made of atoms
element of both
elements
Atoms of another element
Where does the Periodic Table fit in?
In 1869, the Russian scientist Dmitri Mendeleev noticed that some
elements behave similar to others. In other words, they have very similar
physical and chemical properties. For example, metals like Silver and
Aluminium are often Malleable (bendy) and Lustrous (shiny); Lithium
and Sodium are both very reactive; Silicon and Carbon both form
similar crystals.
Because of this, Mendeleev grouped all the elements that were similar into groups.
These groups are the columns in the periodic table. He also arranged the groups
horizontally to take into account Dalton’s ideas about mass. The atoms of an
element get heavier as you go across each row.
How do we Write Molecules?
The chemical formula of a molecule tells us how many of each atom are in each
molecule. It also tells us how many molecules we have. The diagram below explains
this for Water Molecules, which are made of two hydrogens and one oxygen (H20).
This shows there are 3 water
3H20
molecules
This shows there are 2
Hydrogen atoms per molecule
S-ar putea să vă placă și
- Particle Nature of MatterDocument6 paginiParticle Nature of MatterAlvin John Song ConcepcionÎncă nu există evaluări
- Composition of Matter: Atom, Molecule Chapter-1: Answer To The Short QuestionsDocument15 paginiComposition of Matter: Atom, Molecule Chapter-1: Answer To The Short QuestionsG M Ali KawsarÎncă nu există evaluări
- Chapter 01Document46 paginiChapter 01dostinÎncă nu există evaluări
- Presented by GROUP 5Document10 paginiPresented by GROUP 5cq8bvzccw9Încă nu există evaluări
- Elements & Compounds: Done By: Joe Sarkis. Zaid Abdo. Majed Ayman. Mohammed Abdel MalikDocument10 paginiElements & Compounds: Done By: Joe Sarkis. Zaid Abdo. Majed Ayman. Mohammed Abdel MalikazarÎncă nu există evaluări
- Chemistery Unit 1Document28 paginiChemistery Unit 1abdiabu701Încă nu există evaluări
- Atoms and MoleculesDocument113 paginiAtoms and MoleculesUdyat Vishesh - IX-BÎncă nu există evaluări
- M2 1Document58 paginiM2 1Abdullah MÎncă nu există evaluări
- Global Indian International School Uppal, Hyderabad Subject: Chemistry Chapter 3 .Atoms and MoleculesDocument13 paginiGlobal Indian International School Uppal, Hyderabad Subject: Chemistry Chapter 3 .Atoms and MoleculesKhatrasÎncă nu există evaluări
- General Chemistry Chapter 2Document8 paginiGeneral Chemistry Chapter 2Celive SiendaÎncă nu există evaluări
- ChemistryDocument17 paginiChemistryPhương Mai Nguyễn LêÎncă nu există evaluări
- C16 14a Pt3 Atomic TheoryDocument49 paginiC16 14a Pt3 Atomic TheoryJohn Michael AlcachupasÎncă nu există evaluări
- CH 3 Atoms and Molecules 2Document51 paginiCH 3 Atoms and Molecules 2SHIVAKUMAR H PÎncă nu există evaluări
- Unit 7 Elements and CompoundsDocument17 paginiUnit 7 Elements and CompoundsZonzaw HtooÎncă nu există evaluări
- 8F The Periodic TableDocument33 pagini8F The Periodic TableJohanna UrwinÎncă nu există evaluări
- Atoms and MoleculesDocument10 paginiAtoms and MoleculesUtsav Kumar MathurÎncă nu există evaluări
- Chemistry Module 3Document14 paginiChemistry Module 3MASHÎncă nu există evaluări
- ATOM Class 7Document23 paginiATOM Class 7Eureka MaterialÎncă nu există evaluări
- Conceptos y Definiciones Science G9Document5 paginiConceptos y Definiciones Science G9Gabriel LorenttyÎncă nu există evaluări
- Chemistry - Copy of Lesson 7.1 - Putting Atoms TogetherDocument21 paginiChemistry - Copy of Lesson 7.1 - Putting Atoms TogetherarielÎncă nu există evaluări
- 1.1 CHM3100 Basic Quantum Theory-1Document41 pagini1.1 CHM3100 Basic Quantum Theory-1NicholasYeohÎncă nu există evaluări
- Chapter 2 Chem IDocument16 paginiChapter 2 Chem IStudy LionÎncă nu există evaluări
- Class Ix Chapter 3Q QND Answer With NumericalsDocument17 paginiClass Ix Chapter 3Q QND Answer With NumericalsABHAY PRATAP SINGH TOMARÎncă nu există evaluări
- L1 Che101Document19 paginiL1 Che101Musa Ahammed MahinÎncă nu există evaluări
- Chemical Language 1Document1 paginăChemical Language 1TrnScÎncă nu există evaluări
- 2 - Atoms Molecules Moles - Lecture 2Document61 pagini2 - Atoms Molecules Moles - Lecture 2Abdulrahman El KhatibÎncă nu există evaluări
- General Science and Environment MKT-125 (3 Credits) : Prof. Dr. Mohammed Almujaddade AlfasaneDocument28 paginiGeneral Science and Environment MKT-125 (3 Credits) : Prof. Dr. Mohammed Almujaddade AlfasaneMuhibur Rahman AbirÎncă nu există evaluări
- Atom, Molecul and IonDocument62 paginiAtom, Molecul and IonChamilass YayaÎncă nu există evaluări
- Chap 1 ModDocument32 paginiChap 1 ModMuaawia B. ArshadÎncă nu există evaluări
- AsgvdhDocument6 paginiAsgvdhhe ytherÎncă nu există evaluări
- Notes 1 - Basic ConceptsDocument5 paginiNotes 1 - Basic Conceptsochiengjoseph122Încă nu există evaluări
- Bio 110 - Ch2Document30 paginiBio 110 - Ch2محسن الشاطريÎncă nu există evaluări
- Chemistry Lecture PowerpointDocument39 paginiChemistry Lecture Powerpointapi-308666520Încă nu există evaluări
- ENG Natural Sciences GR 8 Introduction To The Periodic TableDocument8 paginiENG Natural Sciences GR 8 Introduction To The Periodic TableCharlotte MitchellÎncă nu există evaluări
- 9th Atoms and Molecules Revision Notes-1Document10 pagini9th Atoms and Molecules Revision Notes-1ADITYA RAI100% (1)
- CHEM - Elements, Compounds and MoleculesDocument2 paginiCHEM - Elements, Compounds and MoleculesSiCi ZhuangÎncă nu există evaluări
- 2 1 OverviewDocument34 pagini2 1 Overviewapi-262378640Încă nu există evaluări
- Chapter 2 PowerpointDocument87 paginiChapter 2 PowerpointGladys BuslatanÎncă nu există evaluări
- Dalton'S Atomic TheoryDocument4 paginiDalton'S Atomic TheoryAkane StarNightÎncă nu există evaluări
- Dwnload Full Chemistry 9th Edition Zumdahl Solutions Manual PDFDocument35 paginiDwnload Full Chemistry 9th Edition Zumdahl Solutions Manual PDFelijah3oa4knight100% (13)
- Chemistry 9th Edition Zumdahl Solutions ManualDocument35 paginiChemistry 9th Edition Zumdahl Solutions Manualstrewmerils1ej3n100% (14)
- Lesson 5 - Corpuscles To Chemical Atomic TheoryDocument21 paginiLesson 5 - Corpuscles To Chemical Atomic TheoryEji AlcorezaÎncă nu există evaluări
- Ix L-3 Notes 1Document3 paginiIx L-3 Notes 1Zerah MaryamÎncă nu există evaluări
- The Summary of Biologi Text Book Campbell Reece (Benjamin Cummings)Document7 paginiThe Summary of Biologi Text Book Campbell Reece (Benjamin Cummings)LirofiatillahÎncă nu există evaluări
- Atoms and MoleculesDocument28 paginiAtoms and MoleculesthinkiitÎncă nu există evaluări
- The Nature of MatterDocument31 paginiThe Nature of MatterxspiirO100% (1)
- Structure of Substance - Lesson - 1Document14 paginiStructure of Substance - Lesson - 1samsonÎncă nu există evaluări
- Mod 1 - Properties and Structure of Matter NotesDocument114 paginiMod 1 - Properties and Structure of Matter NotesRadhika BakshiÎncă nu există evaluări
- Fundamentals of Inorganic Chemistry - SCH1101 - 2021Document84 paginiFundamentals of Inorganic Chemistry - SCH1101 - 2021amoskipngetich87Încă nu există evaluări
- Atomsmolecules and Ions Ppt. FinalDocument44 paginiAtomsmolecules and Ions Ppt. Finalmain.20002245Încă nu există evaluări
- Dwnload Full Chemistry 10th Edition Zumdahl Solutions Manual PDFDocument35 paginiDwnload Full Chemistry 10th Edition Zumdahl Solutions Manual PDFlifelike.anenstkq2h100% (11)
- Chemistry 10th Edition Zumdahl Solutions ManualDocument35 paginiChemistry 10th Edition Zumdahl Solutions Manualpouterhawebakefzc8eb100% (32)
- Atoms AndmoleculesDocument51 paginiAtoms Andmoleculesmirzamehdihassan6Încă nu există evaluări
- SSC Mts Ex: Studymaterialfor GenralawarenessDocument8 paginiSSC Mts Ex: Studymaterialfor GenralawarenessAmrit SinghÎncă nu există evaluări
- Unit 6Document4 paginiUnit 6psychomaniac1771Încă nu există evaluări
- General ScienceDocument14 paginiGeneral ScienceJodessa_Mae_To_1855Încă nu există evaluări
- Atoms, Molecules and IonsDocument47 paginiAtoms, Molecules and Ionszekarias wondafrashÎncă nu există evaluări
- Basic Chemistry NotesDocument50 paginiBasic Chemistry NotesJames ReiterÎncă nu există evaluări
- 1c Thermal Energy NotesDocument1 pagină1c Thermal Energy Notesapi-289539870Încă nu există evaluări
- Junior Periodic Table Y10Document1 paginăJunior Periodic Table Y10api-289539870Încă nu există evaluări
- As 91602Document2 paginiAs 91602api-289539870Încă nu există evaluări
- Sedimentary RockDocument4 paginiSedimentary Rockapi-289539870Încă nu există evaluări
- Y9 ScienceDocument3 paginiY9 Scienceapi-289539870Încă nu există evaluări
- Yr 12 Course Outline 2016Document1 paginăYr 12 Course Outline 2016api-289539870Încă nu există evaluări
- MetarocksDocument12 paginiMetarocksapi-289539870Încă nu există evaluări
- As 91606Document3 paginiAs 91606api-324594246Încă nu există evaluări
- IgneousrocksDocument20 paginiIgneousrocksapi-289539870Încă nu există evaluări
- Plate Boundaries 2Document42 paginiPlate Boundaries 2api-289539870Încă nu există evaluări
- Powerpoint Patterns EvolutionDocument22 paginiPowerpoint Patterns Evolutionapi-289539870Încă nu există evaluări
- ThedigestivesystemDocument48 paginiThedigestivesystemapi-289539870Încă nu există evaluări
- Y10cie Course Outline 2015Document1 paginăY10cie Course Outline 2015api-289539870Încă nu există evaluări
- Chemistry MattersDocument1 paginăChemistry MattersemadÎncă nu există evaluări
- Block 5: Atomic Physics: #Thenuclearatom #RadioactivityDocument70 paginiBlock 5: Atomic Physics: #Thenuclearatom #RadioactivityMac Justine JimenezÎncă nu există evaluări
- Chapter 2 Test BankDocument14 paginiChapter 2 Test Bank陳禹誌Încă nu există evaluări
- Week 6 and 7 ElsDocument146 paginiWeek 6 and 7 ElsJomar CarabotÎncă nu există evaluări
- Olevels Chemistry Notes - Pure ChemistryDocument82 paginiOlevels Chemistry Notes - Pure ChemistryMarcusNg100% (11)
- Grade 9 Chemistry Block PlanDocument9 paginiGrade 9 Chemistry Block Planapi-354019245Încă nu există evaluări
- Achievement TestDocument28 paginiAchievement Testmuhdattique26Încă nu există evaluări
- GenChem 1 Q1 M2Document36 paginiGenChem 1 Q1 M2Jabeguero Marvelyn JessicaÎncă nu există evaluări
- Class 7 ScienceDocument40 paginiClass 7 ScienceQulb e AbbasÎncă nu există evaluări
- Poem About Mixtures and Pure SubstancesDocument4 paginiPoem About Mixtures and Pure SubstancesjobenmÎncă nu există evaluări
- Modern Chemistry Chapter 3 Homework 3-1 AnswersDocument8 paginiModern Chemistry Chapter 3 Homework 3-1 Answersafmtjuhob100% (1)
- Shannon 02 Activity NoodliumDocument4 paginiShannon 02 Activity NoodliumShannon McMahonÎncă nu există evaluări
- CHEM 2101 Lecture 1 (Atomic Structure)Document4 paginiCHEM 2101 Lecture 1 (Atomic Structure)Asif UddinÎncă nu există evaluări
- Spearfishing Diving ManualDocument71 paginiSpearfishing Diving ManualLodewyk Spies100% (7)
- NCERT Solutions For Class 10 Science Chapter 5 Periodic Classification of Elements - AglaSem SchoolsDocument8 paginiNCERT Solutions For Class 10 Science Chapter 5 Periodic Classification of Elements - AglaSem SchoolsBalaji ashokkumarÎncă nu există evaluări
- Sample Question Paper: Class X SCIENCEDocument4 paginiSample Question Paper: Class X SCIENCEABHAY PRATAP SINGH TOMARÎncă nu există evaluări
- Chemical Formular of Compounds: Valency ElementDocument3 paginiChemical Formular of Compounds: Valency ElementNsereko AmilÎncă nu există evaluări
- TCR Engineering Services Profile 2010Document50 paginiTCR Engineering Services Profile 2010aayasirÎncă nu există evaluări
- British Airways Engineering Training Electrical Fundamentals - Book 1Document406 paginiBritish Airways Engineering Training Electrical Fundamentals - Book 1BenitoKameloÎncă nu există evaluări
- Motivation CompoundsDocument3 paginiMotivation CompoundsJohn Perseus LeeÎncă nu există evaluări
- PhET - Build An AtomDocument2 paginiPhET - Build An Atommlongo24100% (1)
- Chapter 5.1Document6 paginiChapter 5.1api-19624513Încă nu există evaluări
- Let ReviewerDocument137 paginiLet ReviewerHannah Katreena Joyce JuezanÎncă nu există evaluări
- Chemistry ChecklistDocument3 paginiChemistry ChecklistJackie van der HoefÎncă nu există evaluări
- Element Ball ProjectDocument3 paginiElement Ball ProjectCarolina CastroÎncă nu există evaluări
- Basic ChemistryDocument58 paginiBasic ChemistryFrancesco MauriÎncă nu există evaluări
- Page 1 of 26: Masterton, W.L., Et. Al. Principles and Reactions: Chemistry For Engineering Students, Philippine Ed. 2016Document26 paginiPage 1 of 26: Masterton, W.L., Et. Al. Principles and Reactions: Chemistry For Engineering Students, Philippine Ed. 2016The Hamster VoyageÎncă nu există evaluări
- Alondra Solomon - Physical Science Week 1Document5 paginiAlondra Solomon - Physical Science Week 1Emy SolomonÎncă nu există evaluări
- 2 Electronic Configuration WSDocument19 pagini2 Electronic Configuration WSAbdoÎncă nu există evaluări
- The Periodic Table of Elements: Daniel LundbergDocument2 paginiThe Periodic Table of Elements: Daniel LundbergAHNAF AJMAINÎncă nu există evaluări
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceDe la EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceEvaluare: 4 din 5 stele4/5 (51)
- A Brief History of Time: From the Big Bang to Black HolesDe la EverandA Brief History of Time: From the Big Bang to Black HolesEvaluare: 4 din 5 stele4/5 (2193)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseDe la EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseEvaluare: 3.5 din 5 stele3.5/5 (69)
- Lost in Math: How Beauty Leads Physics AstrayDe la EverandLost in Math: How Beauty Leads Physics AstrayEvaluare: 4.5 din 5 stele4.5/5 (125)
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyDe la EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyÎncă nu există evaluări
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincDe la EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincEvaluare: 3.5 din 5 stele3.5/5 (137)
- Summary and Interpretation of Reality TransurfingDe la EverandSummary and Interpretation of Reality TransurfingEvaluare: 5 din 5 stele5/5 (5)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessDe la EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessEvaluare: 4 din 5 stele4/5 (6)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldDe la EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldEvaluare: 3.5 din 5 stele3.5/5 (64)
- The End of Everything: (Astrophysically Speaking)De la EverandThe End of Everything: (Astrophysically Speaking)Evaluare: 4.5 din 5 stele4.5/5 (157)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldDe la EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldEvaluare: 4.5 din 5 stele4.5/5 (54)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterDe la EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterEvaluare: 4.5 din 5 stele4.5/5 (410)
- The Beauty of Falling: A Life in Pursuit of GravityDe la EverandThe Beauty of Falling: A Life in Pursuit of GravityÎncă nu există evaluări
- Bedeviled: A Shadow History of Demons in ScienceDe la EverandBedeviled: A Shadow History of Demons in ScienceEvaluare: 5 din 5 stele5/5 (5)
- The Beginning of Infinity: Explanations That Transform the WorldDe la EverandThe Beginning of Infinity: Explanations That Transform the WorldEvaluare: 5 din 5 stele5/5 (60)
- Black Holes: The Key to Understanding the UniverseDe la EverandBlack Holes: The Key to Understanding the UniverseEvaluare: 4.5 din 5 stele4.5/5 (13)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeDe la EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeEvaluare: 4.5 din 5 stele4.5/5 (3)
- Quantum Physics: What Everyone Needs to KnowDe la EverandQuantum Physics: What Everyone Needs to KnowEvaluare: 4.5 din 5 stele4.5/5 (49)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeDe la EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeÎncă nu există evaluări
- Packing for Mars: The Curious Science of Life in the VoidDe la EverandPacking for Mars: The Curious Science of Life in the VoidEvaluare: 4 din 5 stele4/5 (1396)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessDe la EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessEvaluare: 4.5 din 5 stele4.5/5 (57)