Documente Academic
Documente Profesional
Documente Cultură
Polyflux R Dialyzers Spec Sheet
Încărcat de
Shshank87Descriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Polyflux R Dialyzers Spec Sheet
Încărcat de
Shshank87Drepturi de autor:
Formate disponibile
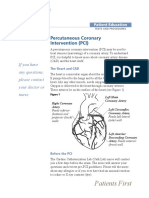
Designed for Reuse Steam Sterilized
• High Flux dialyzer with a synthetic membrane • The only reuse dialyzer sterilized by steam
capable of multiple reuses • Never E-Beam or EtO sterilized
• Available in three sizes, 1.7 m2, 2.1 m2 and 2.4 m2,
Sales Success
to address your patients’ needs
• POLYFLUX R has been sold in the US for over ten
• Non-detachable headers designed for secure line
years accounting for millions of treatments1
and safety.
POLYFLUX R is intended for use in hemodialysis for the treatment of chronic and acute renal failure. POLYFLUX R may be reprocessed for
reuse on the same patient.
Baxter authorizes reprocessing of POLYFLUX R only by using the Renatron Dialyzer Reprocessing System and Renalin as disinfectant. For
this reuse method, the safety and performance of the POLYFLUX R were validated both in vitro and in vivo for up to 15 reuse cycles. The
maximum number of reuse for each POLYFLUX R and the efficiency of cleaning may vary from patient to patient and from clinic to clinic.
POLYFLUX R Dialyzer
Established Reuse Performance
Making possible personal.
Performance data
POLYFLUX R Dialyzer IN VITRO Measured according to EN 1283
Clearances in vitro after first use
(mL/min) +/-10%: QD=500 mL/min and UF=0 mL/min POLYFLUX 17R POLYFLUX 21R POLYFLUX 24R
QB (mL/min) 200 300 400 300 400 500 300 400 500
Urea 191 254 295 267 315 346 274 326 360
Creatinine 179 229 260 245 282 308 255 296 322
Phosphate 176 223 253 240 275 299 249 288 314
Vitamin B12 136 159 174 178 197 209 192 214 229
UF-coefficient in vitro (mL/h, mmHg) +/-20% 71 83 77
Priming volume in vitro (mL) 121 152 165
Maximum TMP (mmHg) 600 600 600
Range of QB (mL/min) 200-400 200-500 300-500
Range of QD (mL/min) 500-800 500-800 500-800
Performance in vitro, after reuse 500-800
QD=500 mL/min; UF=0 mL/min, clearance in vitro (mL/min) +/- 10%, variation for Vitamin B12 +/-20% measured according to ISO 8637
Hemodialysis
1 reuse
QB (mL/min), UF=0 mL/min 200 300 400 300 400 500 300 400 500
Urea 190 253 294 264 310 341 253 315 385
Creatinine 179 228 258 241 277 302 244 290 358
Vitamin B12 135 157 168 174 191 203 177 213 229
UF-coefficient*(mL/h. mmHg) +/-20% 57 63 61
15 reuses
QB (mL/min), UF=0 mL/min 200 300 400 300 400 500 300 400 500
Urea 185 248 284 255 297 325 274 328 358
Creatinine 170 218 245 230 262 285 249 294 316
Vitamin B12 124 140 149 161 176 187 177 199 213
UF-coefficient (mL/h. mmHg) +/-20% 52 44 39
SPECIFICATIONS
Code Number 101785 100691 104324
Membrane
Material PAES/PVP PAES/PVP PAES/PVP
Effective surface area (m2) 1.7 2.1 2.4
Fiber dimensions
Sterilization agent Steam Steam Steam
Component
Membrane Polyflux**
Potting material Polyurethane (PUR)
Housing and caps Polycarbonate (PC)
Sterile plugs Polypropylene (PP)
* Measured with bovine blood, Hematocrit=32%, Protein 60 g/l at 37°C. ** Polyflux is a blend of Polyarylethersulfone, Polyvinylpyrrolidone, Polyamide.
For Customer Support call, 800-525-2623
Rx Only. For safe and proper use of these devices mentioned herein refer to the Instructions for Use.
Baxter, Gambro and Polyflux are trademarks of Baxter International Inc., or its subsidiaries.
USMP/MG160/14-0003(1) 250 12/15
S-ar putea să vă placă și
- The Latest in Peritoneal DialysisDocument114 paginiThe Latest in Peritoneal DialysisDavid Brown100% (1)
- Teaching ScheduleDocument2 paginiTeaching Schedulebpt2Încă nu există evaluări
- Genitourinary: Drafnan Abdirahman MohamedDocument163 paginiGenitourinary: Drafnan Abdirahman MohamedMuse MuseÎncă nu există evaluări
- Biomedical Instrumentation Syllabus of Pondicherry UniversityDocument1 paginăBiomedical Instrumentation Syllabus of Pondicherry UniversityRekhamtrÎncă nu există evaluări
- Catalogo Uro Nuevo PDFDocument294 paginiCatalogo Uro Nuevo PDFMei Córdova PinkasÎncă nu există evaluări
- History of Dialysis: DR Manjunath JDocument65 paginiHistory of Dialysis: DR Manjunath JDani Dany100% (1)
- 67 PDFDocument7 pagini67 PDFshihabÎncă nu există evaluări
- Dialysis Dose Prescription: Presented by Dr. UjjawalDocument54 paginiDialysis Dose Prescription: Presented by Dr. UjjawalZH. omg sarÎncă nu există evaluări
- Alternate Dialysis Platforms:: Sorbents SorbentsDocument17 paginiAlternate Dialysis Platforms:: Sorbents SorbentsJoe Single100% (2)
- A Brief Look at Renal AnatomyDocument15 paginiA Brief Look at Renal AnatomyKristina De Peralta Willy100% (1)
- Renal Replacement Therapy HD, PD, Renal TransplantationDocument65 paginiRenal Replacement Therapy HD, PD, Renal TransplantationparciÎncă nu există evaluări
- HD Guideline Cleaning Disinfecting HD MachinesDocument14 paginiHD Guideline Cleaning Disinfecting HD MachinesMendes NovatoÎncă nu există evaluări
- Dialysis: Notes MS Vol. 2 Krizza Myrrh Gomera Balcita, RNDocument52 paginiDialysis: Notes MS Vol. 2 Krizza Myrrh Gomera Balcita, RNWhimsey CipresÎncă nu există evaluări
- DIALYZERDocument2 paginiDIALYZERassilamorÎncă nu există evaluări
- AV Fistula Examination: Tarek ElerakyDocument37 paginiAV Fistula Examination: Tarek Elerakyadi amali100% (1)
- Dileesh.C Dialysis Technician Medical Allied Healthcare DHA Eligibility DHA/LS/1572017/731817 +971 54 302 2150Document4 paginiDileesh.C Dialysis Technician Medical Allied Healthcare DHA Eligibility DHA/LS/1572017/731817 +971 54 302 2150Gloria JaisonÎncă nu există evaluări
- Basics of HemodialysisDocument43 paginiBasics of HemodialysisDani DanyÎncă nu există evaluări
- Dialysis: Duaa Farooq Khan 11634 Amina Bashir 11283 Syed Uzair Iftikhar 13608 Qamar Ud Din 13071Document11 paginiDialysis: Duaa Farooq Khan 11634 Amina Bashir 11283 Syed Uzair Iftikhar 13608 Qamar Ud Din 13071Aimal SafdarÎncă nu există evaluări
- Dialysis Outcomes in India A Pilot Study PDFDocument22 paginiDialysis Outcomes in India A Pilot Study PDFVashistmohan A.P.Încă nu există evaluări
- Hemodialysis Machines Testing Your UnderstandingDocument30 paginiHemodialysis Machines Testing Your UnderstandingstarykÎncă nu există evaluări
- How Does Haemodialysis WorkDocument19 paginiHow Does Haemodialysis Workhello_khay100% (1)
- Hemodialysis and Artificial Kidneys ExplainedDocument48 paginiHemodialysis and Artificial Kidneys ExplainedRI NAÎncă nu există evaluări
- HemodialysisDocument48 paginiHemodialysisChandan HansiÎncă nu există evaluări
- Disorders of The Kidneys and Ureters3962Document110 paginiDisorders of The Kidneys and Ureters3962Nikki M. ArapolÎncă nu există evaluări
- AP Paramedical NotificationDocument36 paginiAP Paramedical NotificationGirish challa50% (2)
- Hemodialysis in ChildrenDocument28 paginiHemodialysis in ChildrenKarna Yuli sitanggangÎncă nu există evaluări
- Artis First Q PMDocument22 paginiArtis First Q PMAhmed HaiderÎncă nu există evaluări
- 6.principle of HDDocument24 pagini6.principle of HDCaerulus Fuad Abdul BaqiÎncă nu există evaluări
- Membranes for Life SciencesDe la EverandMembranes for Life SciencesKlaus-Viktor PeinemannÎncă nu există evaluări
- 1478765732niprohemodializsurdial X PDFDocument9 pagini1478765732niprohemodializsurdial X PDFZlatko JusupovicÎncă nu există evaluări
- Infosys - Aptitude-Model Papers - 1473177928759Document25 paginiInfosys - Aptitude-Model Papers - 1473177928759rahul khandelwalÎncă nu există evaluări
- Understanding HemodialysisDocument16 paginiUnderstanding HemodialysisIsha MohantyÎncă nu există evaluări
- TorayDocument8 paginiTorayWildan Novaldi IrawanÎncă nu există evaluări
- Pyelonephritis: Dr. Shraddha Koirala Department of Pathology NMCTHDocument26 paginiPyelonephritis: Dr. Shraddha Koirala Department of Pathology NMCTHUrusha MagarÎncă nu există evaluări
- Fluid, Electrolyte and Acid-Base BalanceDocument42 paginiFluid, Electrolyte and Acid-Base BalanceMiss Vina100% (1)
- Peritoneal DialysisDocument23 paginiPeritoneal DialysisNur Aida JoeÎncă nu există evaluări
- Dialysis Treatment Options and Techniques for Kidney FailureDocument11 paginiDialysis Treatment Options and Techniques for Kidney FailureGail Leslie HernandezÎncă nu există evaluări
- Uf & Sodium ProfilingDocument32 paginiUf & Sodium Profilinggramaseva Parishath100% (1)
- The AutoFlow Function For The 5008 Therapy SystemDocument9 paginiThe AutoFlow Function For The 5008 Therapy SystemtigercatÎncă nu există evaluări
- Solus Dan KonsentratDocument8 paginiSolus Dan KonsentratA.H. SudjadiÎncă nu există evaluări
- CatheterizationDocument32 paginiCatheterizationJohn Dave V. VillarmenteÎncă nu există evaluări
- Model 3150 Downflow Installation InstructionsDocument32 paginiModel 3150 Downflow Installation InstructionsWattsÎncă nu există evaluări
- Nutritional Management of Renal DiseasesDocument3 paginiNutritional Management of Renal Diseasescbac1990Încă nu există evaluări
- Blood Transfusion On Dialysis Guidelines Aug 2017 PDFDocument5 paginiBlood Transfusion On Dialysis Guidelines Aug 2017 PDFYolanda IrawatiÎncă nu există evaluări
- Peritoneal DialysisDocument29 paginiPeritoneal DialysisMicah Alexis CandelarioÎncă nu există evaluări
- Modes of VentilatorDocument17 paginiModes of VentilatorFahrizal MuhammadÎncă nu există evaluări
- Aami Standard For Reusehemodialysis2Document32 paginiAami Standard For Reusehemodialysis2Nexi anessaÎncă nu există evaluări
- Preoperative Vein Diameter Predicts AVF OutcomesDocument40 paginiPreoperative Vein Diameter Predicts AVF OutcomesMikael AngelooÎncă nu există evaluări
- ULTRAFILTRATIONDocument22 paginiULTRAFILTRATIONpragati dwivedi100% (2)
- DialyzerDocument10 paginiDialyzerfatima rasheedÎncă nu există evaluări
- Theranova 400 4p Datasheet 2018Document3 paginiTheranova 400 4p Datasheet 2018Henller UsecheÎncă nu există evaluări
- Acute Kidney Injury (AKI)Document68 paginiAcute Kidney Injury (AKI)Alex beharuÎncă nu există evaluări
- Innovation EDDocument32 paginiInnovation EDAETCM Emergency medicineÎncă nu există evaluări
- Guidelines for EU Nurses to Register in MaltaDocument9 paginiGuidelines for EU Nurses to Register in MaltaAndra ApostolÎncă nu există evaluări
- Percutaneous Coronary Intervention 10 05Document8 paginiPercutaneous Coronary Intervention 10 05benypermadiÎncă nu există evaluări
- 2012 Cath Lab Consensus DocumentDocument85 pagini2012 Cath Lab Consensus DocumentDorin DocÎncă nu există evaluări
- Renal Function Test (RFT) : Muhammad Asif Shaheen Lecturer Pathology Kemu, LahoreDocument14 paginiRenal Function Test (RFT) : Muhammad Asif Shaheen Lecturer Pathology Kemu, LahoreRimsha MustafaÎncă nu există evaluări
- AV Shunt-Brescia Cimino - EditDocument53 paginiAV Shunt-Brescia Cimino - EditYufriadi100% (1)
- Dialysis History English PDFDocument20 paginiDialysis History English PDFgimenÎncă nu există evaluări
- Dialyser Filter-Meditechsys RevaclearDocument4 paginiDialyser Filter-Meditechsys Revaclearhojjat ghaznaviÎncă nu există evaluări
- Annotated Bibliography Final DraftDocument3 paginiAnnotated Bibliography Final Draftcchurc13Încă nu există evaluări
- Regional Studies in Marine ScienceDocument11 paginiRegional Studies in Marine ScienceBOUCHNANÎncă nu există evaluări
- Birads PosterDocument1 paginăBirads PosterGopalarathnam BalachandranÎncă nu există evaluări
- Methodology Tapping Methodology of WaterlineDocument15 paginiMethodology Tapping Methodology of WaterlineBryÎncă nu există evaluări
- A Medical Outreach Elective CourseDocument11 paginiA Medical Outreach Elective CourseRobert SmithÎncă nu există evaluări
- E136Document4 paginiE136Subramanya RaoÎncă nu există evaluări
- Maintenance Scheduling For Electrical EquipmentDocument82 paginiMaintenance Scheduling For Electrical Equipmentduonza100% (6)
- Cardiovascular NotesDocument18 paginiCardiovascular NotesCathy SantosÎncă nu există evaluări
- Nursing Assignment SampleDocument12 paginiNursing Assignment Sampleswetha swethaÎncă nu există evaluări
- The Costly Business of DiscriminationDocument46 paginiThe Costly Business of DiscriminationCenter for American Progress100% (1)
- NCP Gastric CancerDocument7 paginiNCP Gastric CancerAnonymous XvwKtnSrMR100% (4)
- Slaked Lime MSDS Safety SummaryDocument7 paginiSlaked Lime MSDS Safety SummaryFurqan SiddiquiÎncă nu există evaluări
- Confidence Intervals For Ratio of Two Poisson Rates Using The Method of Variance Estimates RecoveryDocument23 paginiConfidence Intervals For Ratio of Two Poisson Rates Using The Method of Variance Estimates RecoveryJaneÎncă nu există evaluări
- Effects of Vitamin B-12 Supplementation On Neurologic and Cognitive Function in Older People: A Randomized Controlled TrialDocument9 paginiEffects of Vitamin B-12 Supplementation On Neurologic and Cognitive Function in Older People: A Randomized Controlled TrialzuzuoonÎncă nu există evaluări
- NCERT Solutions For Class 7 Science Chapter 2Document5 paginiNCERT Solutions For Class 7 Science Chapter 2SANJEEV KUMARÎncă nu există evaluări
- Class 7 PolityDocument10 paginiClass 7 PolityNakka nikithaÎncă nu există evaluări
- Risk Assessment For Balustrade Glass InstallationDocument3 paginiRisk Assessment For Balustrade Glass InstallationNicos PapadopoulosÎncă nu există evaluări
- Paul B. Bishop, DC, MD, PHD, Jeffrey A. Quon, DC, PHD, FCCSC, Charles G. Fisher, MD, MHSC, FRCSC, Marcel F.S. Dvorak, MD, FRCSCDocument10 paginiPaul B. Bishop, DC, MD, PHD, Jeffrey A. Quon, DC, PHD, FCCSC, Charles G. Fisher, MD, MHSC, FRCSC, Marcel F.S. Dvorak, MD, FRCSCorlando moraÎncă nu există evaluări
- MsdsDocument6 paginiMsdsJackyÎncă nu există evaluări
- Immunology Serology Blood BankingDocument5 paginiImmunology Serology Blood BankingEdsss Villar100% (3)
- DK50 Developer Parts ABDocument15 paginiDK50 Developer Parts ABedu3ipbÎncă nu există evaluări
- Oet Reading Part A Additional - GlucomaDocument8 paginiOet Reading Part A Additional - Glucomaafacean25% (8)
- Practical Research 2 Quarter 1 Activity SheetsDocument8 paginiPractical Research 2 Quarter 1 Activity SheetsJonnis Estillore100% (1)
- GoalSettingWorkbookFinal PDFDocument21 paginiGoalSettingWorkbookFinal PDFDato KhutsishviliÎncă nu există evaluări
- Postpartum Health TeachingDocument8 paginiPostpartum Health TeachingMsOrange96% (24)
- Hvis Msds PDFDocument6 paginiHvis Msds PDFsesbasar sitohangÎncă nu există evaluări
- 380 Final PaperDocument46 pagini380 Final Paperapi-538048965Încă nu există evaluări
- Tbf-531Bodyfat Monitor/Scale: Instruction ManualDocument13 paginiTbf-531Bodyfat Monitor/Scale: Instruction ManualJose JimenoÎncă nu există evaluări
- Wirmen Beautycare Cloth Pad SDN - BHDDocument9 paginiWirmen Beautycare Cloth Pad SDN - BHDadilahÎncă nu există evaluări
- VetcareDocument18 paginiVetcareMy binÎncă nu există evaluări