Documente Academic
Documente Profesional
Documente Cultură
Pinsky 2015 - Lung Cancer Risk by Years Since Quitting in 30+ Pack Year Smokers
Încărcat de
NavinJohnsonDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Pinsky 2015 - Lung Cancer Risk by Years Since Quitting in 30+ Pack Year Smokers
Încărcat de
NavinJohnsonDrepturi de autor:
Formate disponibile
XML Template (2015) [22.4.
2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
J Med Screen OnlineFirst, published on April 29, 2015 as doi:10.1177/0969141315579119
Original Article
J Med Screen
0(0) 1–7
Lung cancer risk by years since quitting ! The Author(s) 2015
Reprints and permissions:
in 30þ pack year smokers sagepub.co.uk/journalsPermissions.nav
DOI: 10.1177/0969141315579119
msc.sagepub.com
Paul F Pinsky, Claire S Zhu and Barnett S Kramer
Abstract
Objective: Current United States recommendations for low-dose computed tomography (LDCT) lung cancer screening limit
eligibility to ever-smokers with 30þ pack-years, with former smokers eligible only within 15 years of quitting. The 15 year limit
is partly based on perceived decreases in lung cancer risk as years since quitting (YSQ) increase. We examine the relationship
between lung cancer risk and YSQ among 30þ pack-year former smokers.
Methods: In the Prostate, Lung, Colorectal, and Ovarian trial, participants aged 55–74 were randomized to screening or usual
care; screened subjects received annual chest-radiographs for lung cancer screening. Subjects completed a baseline question-
naire; smoking history included average cigarettes per day and age at starting and stopping smoking. Subjects were followed
13 years. Cox proportional hazards models were utilized to estimate hazard ratios (HRs) associated with YSQ, with YSQ
treated as a time-varying covariate. The models adjusted for age and sex.
Results: Of 154899 subjects randomized, 27101 were former smokers with 30þ pack-years, and 69182 were never smokers.
HRs relative to never smokers ranged from 30.8 (95% CI:23.4–40.5) for YSQ 45 to 6.4 (95% CI:5.1–8.0) for YSQ > 30. For
YSQ of >10–15, >15–20, and >20–25, HRs were 14.8 (95% CI:11.9–18.2), 13.5 (95% CI:11.3–16.2), and 9.9 (95% CI: 8.1–12.0),
respectively.
Conclusions: Lung cancer risk decreases gradually with YSQ in 30þ pack year former smokers. A range of upper limits on
YSQ may be supportable for LDCT screening.
Keywords
lung cancer, low-dose CT, smoking, years since quit
Date received: 7 January 2015; accepted: 5 March 2015
Introduction None of the studies on the relationship between years
The United States Preventive Services Task Force 2014 since quitting smoking (YSQ) and lung cancer risk have
recommendations for low-dose computed tomography been tailored to the LDCT screening scenario, in terms of
(LDCT) screening for current and former smokers with focusing on those with 30þ pack years and examining the
at least 30 pack years of cigarette smoking limited eligible short term risk of incident lung cancer, which is relevant in
former smokers to those who had quit smoking within the deciding who should be screened at a given time point.
past 15 years.1 The 15 year cutoff, as well as the 30 pack These studies also vary in important aspects, eg: specific
year minimum, mirrored the eligibility requirements for intervals of YSQ for which relative risk estimates are pre-
the National Lung Screening Trial (NLST), which pro- sented, study design (case-control versus cohort), end-
vided much of the evidence base for the task force deci- points (incidence or mortality), and analytic approach
sion.2 Other LDCT screening guidelines, and the 2015 (smoothed estimates using modeling or non-parametric
Medicare coverage guidelines, also use the 15 year limit.3,4 estimates for each YSQ interval).5–8
The 15 year limit may have been chosen to cover The Prostate, Lung, Colorectal and Ovarian (PLCO)
screening in a population similar to that in the NLST Cancer Screening Trial randomized trial evaluated chest
(to date the only randomized trial showing LDCT bene- radiograph for lung cancer screening.9 Unlike the NLST,
fit), and to restrict screening to those at sufficiently high it had no smoking history eligibility requirement, so the
lung cancer risk, in whom the evidence that the benefits of
screening outweigh the harms is strongest. There may be a
perception that lung cancer risk decreases substantially Division of Cancer Prevention, National Cancer Institute
15þ years since quitting smoking, however, no direct evi-
Corresponding author:
dence or quantitative risk estimates have been cited to Paul F Pinsky, Div. of Cancer Prevention, National Cancer Institute, 9609
support the 15 year limit. Medical Center Dr, Rm 5E108, Bethesda, MD, 20892.
Email: pp4f@nih.gov
Downloaded from msc.sagepub.com by guest on July 26, 2015
XML Template (2015) [22.4.2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
2 Journal of Medical Screening 0(0)
range of YSQ values through 30þ years can be examined. should be based on the current risk of lung cancer and
We examine lung cancer risk by YSQ in the PLCO cohort, not, for example, the risk over the next decade. Because
among ever smokers meeting the 30þ pack year require- YSQ increases over study time in former smokers, to
ment of the various guidelines. accurately estimate the short term risk associated with a
given YSQ level, subjects’ assigned YSQ values should be
changing over study time so that lung cancer incidence in
Methods a given study year is related to YSQ levels at that same
In the PLCO trial10 participants aged 55–74 were rando- study year. We therefore employed Cox proportional haz-
mized from 1993 to 2001 at ten screening centers across ards modeling with YSQ included as a time varying cov-
the United States. Exclusion criteria included history of a ariate, increasing by one year as study time increased by
PLCO cancer, current cancer treatment, and removal of one year. Sex and age (time-varying) were included as
one lung. Intervention arm participants were offered covariates; YSQ was grouped into 5 year categories for
annual posterior-anterior chest-radiograph for four analysis.
years. Chest radiograph screens were considered positive The supplemental questionnaire data for 30þ pack-
if a nodule, mass, or other abnormality suspicious for lung year former smokers with YSQ 45 at baseline
cancer was noted. Diagnostic evaluation was decided by (N ¼ 3576) showed that a non-negligible proportion
the patients and their physicians, not by trial protocol. All (13%) relapsed to current smoking at the time of the ques-
diagnosed cancers were ascertained, primarily by means of tionnaire. For 30þ pack-year subjects with baseline
a mailed Annual Study Update (ASU) questionnaire, YSQ > 5 (N ¼ 12466), the relapse rate on the supplemental
which asked about type and date of any cancers diagnosed questionnaire was low, 1.8% overall and under 4% for
in the prior year. Participants not returning ASUs were each 5-year baseline YSQ category. Therefore, subjects
contacted by repeat mailing or telephone. Cancers were with baseline YSQ 4 5 were censored at 3 years from
confirmed by medical records and trained abstractors rec- baseline, to minimize contamination of that group with
orded cancer histology and stage. Deaths were ascertained (relapsed) current smokers. Subjects with baseline
through the ASUs and other means, with supplementation YSQ > 5 were followed for the full study period.
by National Death Index searches. Participants were fol- Never smokers were assumed to continue their never
lowed for up to 13 years, or until 31 December 2009, smoking status through all study years. Current smokers,
whichever came first. however, could have quit after enrollment, which would
At study entry, participants completed a baseline ques- change their status to former smoker. Of baseline current
tionnaire on demographics, medical history, smoking his- smokers with 30þ pack-years who responded to the sup-
tory, and past screenings. The smoking-related questions plemental questionnaire (N ¼ 5970), 35.4% reported on
asked if subjects had ever smoked cigarettes regularly for that questionnaire that they were currently former smo-
6 months or longer, if they smoke regularly now, the start kers. Therefore, to insure minimal contamination of the
and (last) stopping ages of regular smoking, and the current smoker category, this group was also censored at 3
number of cigarettes usually smoked per day during per- years from baseline.
iods when the subject smoked, with categories of 1–10, To examine possible interactions of the YSQ and lung
11–20, 21–30, 31–40, 41–60, 61–80, and 81 or more. cancer risk relationship by sex and study arm, we
A supplemental questionnaire was mailed to PLCO par- restricted the basic model to former smokers at baseline
ticipants at 6–12 (median 9.3) years from enrollment, and utilized a continuous measure of YSQ, along with an
asking similar questions about smoking, and additional interaction term of YSQ by sex or YSQ by study arm.
questions concerning smoking craving, passive smoking Among incident lung cancers we examined the relation-
exposure, and attempts to quit. ship of YSQ and histology. The Chi-squared test was used
to test for the statistical significance of the association of
YSQ (415 versus > 15) and histology (with categories of
Quantitative Methods adenocarcinoma, squamous cell carcinoma, other NSCLC
YSQ at baseline for former smokers was calculated by and small cell). The YSQ level at the time of cancer diag-
subtracting the age at which regular smoking was last nosis was used for this analysis.
stopped from current age. Pack-years were computed as
the product of years of smoking and cigarettes per day
Results
(CPD) divided by 20, with years of smoking calculated as
either current age (for current smokers) or age at which Of 154899 subjects randomized, 149930 (96.8%) com-
smoking was last stopped (for former smokers) minus age pleted the smoking section of the baseline questionnaire.
at which smoking started, and CPD was calculated using There were 69182 (46.1%) never smokers, 16057 (10.7%)
the high point of each CPD category range (and 100 CPD current smokers and 64691 (43.2%) former smokers. For
for the 81 or more category). current smokers, 15869 (98.8%) had calculable pack years
To assess lung cancer risk by YSQ in the context of (based on completing the age started smoking and cigar-
LDCT screening, we are interested in short term risk. The ettes per day questions), and of these, 12243 (77.2%) had
decision to undergo LDCT screening in a given year 30þ pack years. Among former smokers, 63402 (98.0%)
Downloaded from msc.sagepub.com by guest on July 26, 2015
XML Template (2015) [22.4.2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
Pinsky et al. 3
Table 1. Baseline demographics and smoking history of the analysis cohort.
Baseline Smoking Median (25/75th) Median Median Age % Non-Hispanic
History Category N Pack Years (25th/75th) CPD1 (25/75th) % Male White
Never Smoker 69182 N/A N/A 62 (58/67) 38.8 88.5
30þ Pack Years:
Current 12243 53 (43/72) 20 (20/30) 60 (57/65) 58.3 88.5
YSQ 4 5 6480 54 (42/74) 30 (20/40) 61 (57/65) 58.9 90.0
YSQ > 5–10 6013 51 (39/70) 30 (20/40) 62 (58/66) 61.0 91.0
YSQ > 10–15 5621 48 (37/66) 30 (20/40) 63 (59/67) 67.0 92.2
YSQ > 15–20 3924 44 (35/60) 30 (30/40) 63 (59/68) 71.8 92.2
YSQ > 20–25 2514 42 (34/56) 40 (30/40) 65 (61/69) 76.4 92.8
YSQ > 25–30 1649 40 (33/52) 40 (30/60) 66 (62/69) 81.1 94.0
YSQ > 30 900 38 (33/48) 40 (40/60) 68 (65/71) 87.7 95.2
All Former 27101 48 (38/66) 30 (20/40) 63 (59/67) 66.9 91.7
1
CPD (cigarettes per day) was assessed in categories of 1–10, 11–20, 21–30, 31–40, 41–60, 61–80, >80.
YSQ ¼ years since quitting. High value of range was used to compute CPD and pack-years.
Table 2. Cox Proportional Hazards Model.
Smoking History Rate per 10000 HR (95% CI) controlling
Category Person Years1 Lung Cancers Person Years for age, sex
Never Smoker 779504 253 3.2 Referent
Current Smoker 36312 271 74.6 35.9 (29.0–44.5)
Former Smoker
YSQ 4 5 12758 83 65.1 30.8 (23.4–40.5)
YSQ > 5–10 17615 90 51.1 22.1 (16.9–28.9)
YSQ > 10–15 39205 151 38.5 14.8 (11.9–18.2)
YSQ > 15–20 55422 236 42.6 13.5 (11.3–16.2)
YSQ > 20–25 47138 173 36.7 9.9 (8.1–12.0)
YSQ > 25–30 32163 101 31.4 8.1 (6.4–10.2)
YSQ > 30 36986 111 30.0 6.4 (5-.1–8.0)
All 241287 945 39.2 –
YSQ ¼ years since quitting. HR ¼ hazard ratio.
Only first 3 study years included for baseline current smokers and for former smokers with YSQ < ¼ 5 at baseline.
1
For former smokers, person years at risk, number of lung cancers and rate were computed based on time-varying YSQ.
completed the age at which smoking stopped question, former smokers, median pack-years decreased from 54 for
enabling calculation of YSQ. Of the 63402, 63,000 baseline YSQ 4 5 to 38 for baseline YSQ > 30. Median
(99.4%) had calculable pack years, with 27101 (42.7%) CPD was higher for former than current smokers, and
of these having 30þ pack years. Mean follow-up time increased with YSQ.
for the analysis cohort was 11.2 years. Over the analysis period, there were 271, 945, and 253
Table 1 gives baseline demographics and pack-year lung cancers diagnosed in baseline current, former, and
characteristics of the analysis cohort by smoking history never smokers, respectively (Table 2). Corresponding
category. The never smoker group had a substantially lung cancer rates (per 10,000 person years) were 74.6,
lower proportion of men (38.8%) than the current 39.3, and 3.2, respectively. The median (25th/75th) age at
smoker (58.3%) or former smoker groups (58.9% for lung cancer diagnosis was 70 (65/75). Table 2 also displays
baseline YSQ 45 years to 87.7% for baseline the results of the Cox proportional hazards models. For
YSQ > 30). Median age (at baseline) was 62 for never the multivariate model controlling for age and sex, the
and 60 for current smokers; median age range for hazard ratio (HR) (relative to never smokers) was 35.9
former smokers was 61 (YSQ 45 at baseline) to 68 (95% CI: 29.0–44.5) for current smokers and 30.8 (95%
(YSQ > 30 at baseline). Median pack-years was slightly CI: 23.4–40.5) for former smokers with YSQ 4 5. HRs for
lower for former (48) than current smokers (53); among former smokers decreased as YSQ increased, with HRs of
Downloaded from msc.sagepub.com by guest on July 26, 2015
XML Template (2015) [22.4.2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
4 Journal of Medical Screening 0(0)
Figure 1. Hazard ratios of lung cancer risk for various years since quitting (YSQ) levels. Solid and dotted line are for referent group never
smokers and current smokers, respectively.
Table 3. Lung cancer histology by smoking history.
Smoking History
Histology Never Current Former, YSQ 415 Former, YSQ 15þ
Adenocarcinoma 163 (64.4) 102 (37.6) 147 (38.7) 258 (45.7)
Squamous Cell 22 (8.7) 54 (19.9) 103 (27.1) 102 (18.1)
NSCLC Other 54 (21.3) 67 (24.7) 85 (22.4) 142 (25.1)
Small Cell 14 (5.5) 48 (17.7) 45 (11.8) 63 (11.2)
Total 253 271 380 565
YSQ ¼ years since quitting. Chi-squared - YSQ 4 15 vs YSQ > 15 (p ¼ 0. 0004). YSQ represents value at time of diagnosis
14.8 (95% CI: 11.9–18.2), 13.5 (95% CI: 11.3–16.2), 9.9 the highest proportion of adenocarcinoma (64%) and the
(95% CI: 8.1–12.0), 8.1 (95% CI: 6.4–10.2), and 6.4 (95% lowest proportion of squamous cell (8.7%).
CI: 5.1–8.0) for YSQ levels of >10–15, >15–20, >20–25,
>25–30, and >30, respectively.
Figure 1 displays HRs versus YSQ levels with never
Discussion
smokers as the referent group, as in Table 2, and also Our analysis of PLCO trial data in subjects with 30þ
with current smokers as the referent group. HRs versus pack-years of smoking showed that the decrease in lung
current smokers were 0.41 (95% CI: 0.33–0.51) for YSQ cancer risk with YSQ is gradual, with no dramatic drop-
>10–15, 0.38 (95% CI: 0.30–0.47) for YSQ >15–20, 0.27 off after 15 years since quitting. Even with YSQ > 30, lung
(95% CI: 0.22–0.35) for YSQ >20–25 and 0.22 (95% CI: cancer risk was still substantially elevated compared with
0.17–0.29) for YSQ >25–30. that of never smokers.
There were no significant interactions of YSQ by sex Although there is no literature directly addressing the
(p ¼ 0.84) or study arm (p ¼ 0.43). question of risk reduction with YSQ among 30þ pack-
Table 3 shows lung cancer histology by smoking his- year former smokers, several large studies have addressed
tory. There was a statistically significant association the more general question of YSQ and lung cancer risk. A
(p ¼ 0.007) of histology by YSQ category (4 15 ver- 1990 United Kingdom case-control study showed Relative
sus > 15); squamous cell carcinoma was more common for Risks (RRs) for lung cancer incidence compared with cur-
YSQ 4 15 (27.1 versus 18.1%), whereas adenocarcinoma rent smokers of 0.66, 0.44, and 0.20 for YSQ values of
was less common (38.7 versus 45.7%). Never smokers had <10, 10–19, and 20–29, respectively in men, and RRs of
Downloaded from msc.sagepub.com by guest on July 26, 2015
XML Template (2015) [22.4.2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
Pinsky et al. 5
Figure 2. Relative Risk (RR) or hazard ratio (HR) estimates of lung cancer risk compared with current smokers by YSQ for various studies.
Solid line is current (PLCO) study, long- dashed line is Knoke et al study, short-dashed line is Peto et al study, dotted line is Halpern et al. study.
For Halperin et al. and Peto et al., the means of the RRs for men and women are plotted. Sizes of dots are proportional to the total number of
lung cancer events among former smokers in the study.
0.69 (YSQ < 10) and 0.21 (YSQ 10–19) in women.5 >15–20, >20–25, and >25–30, respectively. This com-
Halperin et al. used statistical modeling to estimate lung pares with observed HRs of 14.8, 13.5, 9.9, and 8.1,
cancer mortality risk for different age and age at quitting respectively, for these YSQ categories. For YSQ > 15,
groups in the CPS-II cohort.6 Translating these into YSQ, the decrease in risk is close to that predicted by the
RRs (for those aged 65) relative to current smokers were model, but slightly less steep.
0.56, 0.29, and 0.18 (men) and 0.60, 0.33, and 0.22 In our analysis, limited to those with 30þ pack-years,
(women) for YSQ levels of >5–10, >10–15, and >15–25, median pack-years were similar for current (53) versus
respectively. Knoke et al. analyzed CPS-I data and dis- former (48) smokers, and the decrease in median pack-
played age-standardized lung cancer mortality rates by years from current smokers to YSQ > 30 was only 28%
YSQ among former smokers.7 Utilizing the group with (53 versus 38). Cigarettes per day were higher in former
YSQ < 3 as representing the risk level of current smokers, smokers (median ¼ 30) than in current smokers (med-
RRs (age standardized) compared with current smokers ian ¼ 20). In contrast, for all of PLCO, not limited by a
were 0.39 (YSQ > 5–10), 0.22 (YSQ > 10–15), 0.15 minimum pack-years, the differential was much greater,
(YSQ > 15–20), and 0.13 (YSQ > 20–25). To compare with medians of 46 for current smokers, 24 for former
these findings with each other, and with those of the cur- smokers overall, and 7 for former smokers with
rent study, RRs (or HRs) for lung cancer risk compared YSQ > 30 (85% decrease from current smokers); current
with current smokers are plotted against YSQ (Figure 2). and former smokers both had a median of 20 cigarettes
Despite differences in study design, analytic methods, and per day. Therefore, for many of the studies cited above,
eligible populations, the studies are generally consistent in pack-years were probably substantially lower for long-
how much risk falls off with YSQ. term former smokers than for current smokers, which
A recent meta-analysis examined 85 studies with would tend to augment the rate of decrease in risk
data on lung cancer risk in former smokers grouped by observed with YSQ.
YSQ category, as well as in current and never smokers.8 Two primary lines of reasoning underlie the YSQ limit
Using a model in which smoking-related excess lung recommendations. The first is that, below a certain level of
cancer risk decayed exponentially with YSQ, the authors lung cancer risk, the harms of LDCT screening may out-
estimated an average half-life of 9.9 years, which corres- weigh the benefits, and relatedly, that LDCT screening is
ponds to an exponential decay parameter of C ¼ 0.07 not cost effective below a certain level of risk. Implied in
years–1. Using this value of C, with the observed HR in this reasoning is that for YSQ > 15, lung cancer risk may
PLCO of 35.9 for current smokers, and the midpoints of drop below this required level. None of the analyses of the
each 5 year YSQ category, the HRs predicted by this benefits to harms tradeoff, and of the cost-effectiveness of
model are 15.6, 11.3, 8.2, and 6.1 for YSQ of >10–15, LDCT, has specifically examined these metrics with
Downloaded from msc.sagepub.com by guest on July 26, 2015
XML Template (2015) [22.4.2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
6 Journal of Medical Screening 0(0)
respect to the YSQ cutoff.1,11,12 Because these types of It is also important to consider how much of a differ-
analyses are imprecise, and may include subjective judg- ence removing the 15 year requirement might make. An
ments or rely on models that incorporate numerous analysis of population data showed that the recommended
untestable assumptions, it would be difficult to conclu- LDCT screening guidelines of 30þ pack-years, current
sively determine a specific optimal YSQ cutoff above smoking or having quit within 15 years, and age 55 to
which LDCT screening is reasonable and below which it 74 would cover 6.2% of the United States population
should not be recommended. Although the 15 year limit over age 40. Removing the YSQ limitation would increase
may be reasonable, other limits may also be valid. this by approximately 3 million persons, to 8.3%.15 Based
Further, within some range of YSQ, personal patient pref- on models of smoking history and lung cancer risk, the
erences may determine screening decisions. The choice of estimated proportion of subjects with incident lung cancer
the 15 year YSQ limit in the NLST could not be based on who would be screened would correspondingly increase
an analysis of the benefits to harms tradeoff of LDCT from 26.7% to 32.5%.
screening, because the magnitude of the LDCT benefit In conclusion, in former smokers with 30þ pack-years,
was unknown when planning the trial. The requirement lung cancer risk decreases gradually with YSQ. The
reflected a compromise on having an efficient trial (max- 15 year YSQ limit for screening eligibility appears reason-
imizing endpoint events per enrolled subject) that able, but other YSQ eligibility ranges may also be
would be representative of risk groups that might bene- supportable.
fit from LDCT screening. Within a reasonable set of par- The data set for this analysis can be requested via:
ameters, the decision was to some extent arbitrary. The https://biometry.nci.nih.gov/cdas.
pilot study for NLST used a 10 year cutoff for YSQ, as
does the ongoing European NELSON LDCT screening Funding
trial.13,14 This research received no specific grant from any funding agency
The other reasoning is that it is undesirable to extrapo- in the public, commercial, or not-for-profit sectors.
late beyond the population represented by the NLST cri-
teria, as this was the only study to directly show LDCT
Conflict of interest
benefit. This is a generally reasonable principle, but for a
cancer screening trial, an argument can be made for at None declared.
least limited extrapolation beyond some trial parameters.
The effectiveness of LDCT screening, assessed through References
percentage reduction in lung cancer mortality, will be 1. Moyer VA. Screening for lung cancer: U.S. Preventive
principally a function of the types of lung cancers (defined Services Task Force Recommendation Statement. Ann Int
by histology, molecular sub-types, etc) presenting in the Med 2014;160:330–338.
population, and the overall health status of the popula- 2. National Lung Screening Trial Research Team. Reduced
tion, including ability to undergo and withstand curative lung-cancer mortality with low-dose computed tomographic
lung resection. In the current study, although exploratory screening. N Engl J Med 2011;365:395–409.
analyses revealed some potential differences, the overall 3. Center for Medicare Services. National Coverage Analysis for
histologic pattern was generally similar across YSQ cate- screening for lung cancer with low dose computed tomog-
gories, and the histology distribution for those with raphy (LDCT). http://www.cms.gov/medicare-coverage-
YSQ > 15 was actually more similar to that of current database/details/nca-details.aspx?NCAId¼274 (accessed 7
February 2015).
than to that of never smokers. Data on molecular sub-
4. American Cancer Society. American Cancer Society guide-
types are not available, however, it is unlikely that small lines for the early detection of cancer. http://www.cancer.org/
changes in YSQ would have a large effect on molecular healthy/findcancerearly/cancerscreeningguidelines/american-
sub-types. Subjects with increased YSQ would be as, or cancer-society-guidelines-for-the-early-detection-of-cancer
more, likely to be able to withstand surgical resection. (accessed 23 December 2014).
Reduction in risk of heart disease and all-cause mortality 5. Peto R, Darby S, Deo H, et al. Smoking, smoking cessation,
after sustained smoking cessation is well-established. and lung cancer in the UK since 1950: combination of
There are arguments in support of the 15 year limit. As national statistics with two case control studies. Br Med J
seen in some other screening programmes (eg. prostate- 2000;321:323–329.
specific antigen screening for prostate cancer) and medical 6. Halperin MT, Gillespie BW, Warner KE. Patterns of abso-
interventions, there is a danger that factors such as the fee lute risk of lung cancer mortality in former smokers.
J National Cancer Inst 1993;85:457–464.
for services medical landscape prevalent in the United
7. Knoke JD, Burns DM, Thun MJ. The change in excess risk of
States, and pressures from patient interest groups, pro- lung cancer attributable to smoking following smoking cessa-
mote use of an intervention among groups clearly unlikely tion: an examination of different analytic approaches to using
to receive net benefit from it. If the 15 year limit were CPS-I data. Cancer Causes Control 2008;19:207–219.
relaxed, then there could also be pressures for other 8. Fry JS, Lee PN, Forey BA, et al. How rapidly does the excess
limits, such as the 30þ pack-year requirement, to be risk of lung cancer decline following quitting smoking? A
relaxed, with an end result of LDCT screening occurring quantitative review using the negative exponential model.
in relatively low-risk populations. Regul Toxicol Pharmacol 2013;67:13–26.
Downloaded from msc.sagepub.com by guest on July 26, 2015
XML Template (2015) [22.4.2015–2:34pm] [1–7]
//blrnas3.glyph.com/cenpro/ApplicationFiles/Journals/SAGE/3B2/MSCJ/Vol00000/150010/APPFile/SG-MSCJ150010.3d (MSC) [PREPRINTER stage]
Pinsky et al. 7
9. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the 13. Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings
Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer of a randomized feasibility trial of lung cancer screening
Screening Trial. Controlled Clinical Trials 2000;21(6 with spiral CT scan vs chest radiograph. Chest
Suppl): 273S–309S. 2004;126:114–121.
10. Oken MM, Hocking WG, Kvale PA, et al. Screening by 14. Horeweg N, van der Aalst CM, Vliegenthart R, et al.
chest radiograph and lung cancer mortality: The Prostate, Volumetric computed tomography screening for lung
Lung, Colorectal, and Ovarian (PLCO) randomized trial. cancer: three rounds of the NELSON trial. Eur Respir J
JAMA 2011;306(17):1865–1873. 2013;42:1659–1667.
11. Villanti AC, Jiang Y, Abrams DB, et al. A cost-utility ana- 15. Pinsky PF, Berg C. Applying the National Lung Screening
lysis of lung cancer screening and the additional benefits of Trial eligibility criteria to the US. population: what percent
incorporating smoking cessation interventions. PLOS ONE of the population and of incident cancers would be covered?
2013;8(8):e71379. J Med Screen 2012;19:154–156.
12. Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of
CT screening in the National Lung Screening Trial.
New Engl J Med 2014;371:1793–1802.
Downloaded from msc.sagepub.com by guest on July 26, 2015
S-ar putea să vă placă și
- CPG Lung CaDocument15 paginiCPG Lung CaTina de JesusÎncă nu există evaluări
- Lung Cancer Screening. Patient Selection and Implementation. 2020Document11 paginiLung Cancer Screening. Patient Selection and Implementation. 2020Mary CogolloÎncă nu există evaluări
- Lung Cancer Screening Final RecommendationDocument9 paginiLung Cancer Screening Final RecommendationChon ChiÎncă nu există evaluări
- Nihms 659315Document13 paginiNihms 659315Pratiwi Dewi Indah PakartiÎncă nu există evaluări
- Lungcancerscreening: Why, When, and How?Document19 paginiLungcancerscreening: Why, When, and How?Nelson William UsnayoÎncă nu există evaluări
- Tamizaje Ca de PulmonDocument11 paginiTamizaje Ca de Pulmonjuan CARLOS vaRGASÎncă nu există evaluări
- Screening For Lung Cancer JAMADocument9 paginiScreening For Lung Cancer JAMACecille ZacateÎncă nu există evaluări
- Impact of Lung Cancer Screening Results On Smoking CessationDocument8 paginiImpact of Lung Cancer Screening Results On Smoking CessationAndreea TanaseÎncă nu există evaluări
- Lung Cancer Screening: Clinical PracticeDocument7 paginiLung Cancer Screening: Clinical PracticefuadizzuddinÎncă nu există evaluări
- Estudio Nelson - NejmDocument11 paginiEstudio Nelson - NejmCarlos LuqueÎncă nu există evaluări
- 10 11648 J Ijmi 20170505 12Document5 pagini10 11648 J Ijmi 20170505 12Anchit AggarwalÎncă nu există evaluări
- Integrating Artificial Intelligence Into Lung Cancer Screening: A Randomised Controlled Trial ProtocolDocument6 paginiIntegrating Artificial Intelligence Into Lung Cancer Screening: A Randomised Controlled Trial Protocolstardust.m002Încă nu există evaluări
- Lung Cancer ScreeningDocument17 paginiLung Cancer ScreeningNguyen Minh DucÎncă nu există evaluări
- Screening For Lung Cancer: What Have We Learned?: Jeffrey P. KanneDocument6 paginiScreening For Lung Cancer: What Have We Learned?: Jeffrey P. KanneBitaÎncă nu există evaluări
- Referensi 1Document9 paginiReferensi 1Indah Syaidatul MÎncă nu există evaluări
- 408 2020 Article 407Document11 pagini408 2020 Article 407CuchuluuÎncă nu există evaluări
- CT Lung Cancer Screening in The UK: CommentaryDocument3 paginiCT Lung Cancer Screening in The UK: CommentaryBitaÎncă nu există evaluări
- Clinical Efficacy of Stereotactic Ablative RadiothDocument8 paginiClinical Efficacy of Stereotactic Ablative RadiothuswahsnddinÎncă nu există evaluări
- Lung Cancer Screening With Low Dose CT SimulatingDocument7 paginiLung Cancer Screening With Low Dose CT SimulatingeugeniaÎncă nu există evaluări
- Predicting Lung Nodules MalignancyDocument7 paginiPredicting Lung Nodules MalignancyhajajÎncă nu există evaluări
- Radiotherapy and Oncology: Original ArticleDocument8 paginiRadiotherapy and Oncology: Original ArticleJuancarlos Pari SalasÎncă nu există evaluări
- Management of The Solitary Pulmonary NoduleDocument6 paginiManagement of The Solitary Pulmonary Nodulevictor ibarra romeroÎncă nu există evaluări
- Cancer Network - Small-Cell Lung Cancer Mesothelioma and Thymoma - 2016-01-27Document14 paginiCancer Network - Small-Cell Lung Cancer Mesothelioma and Thymoma - 2016-01-27Rio YansenÎncă nu există evaluări
- Colorectal Cancer ESGARDocument8 paginiColorectal Cancer ESGARscepanÎncă nu există evaluări
- 1 SMDocument10 pagini1 SMAnindita GaluhÎncă nu există evaluări
- WJR 7 189Document6 paginiWJR 7 189GarryÎncă nu există evaluări
- Good 1Document11 paginiGood 11eenahaÎncă nu există evaluări
- Lung Cancer Research Paper PDFDocument6 paginiLung Cancer Research Paper PDFhyavngvnd100% (1)
- Seminar: Fred R Hirsch, Giorgio V Scagliotti, James L Mulshine, Regina Kwon, Walter J Curran, Yi-Long Wu, Luis Paz-AresDocument13 paginiSeminar: Fred R Hirsch, Giorgio V Scagliotti, James L Mulshine, Regina Kwon, Walter J Curran, Yi-Long Wu, Luis Paz-AresseruniallisaaslimÎncă nu există evaluări
- 1 s2.0 S001236922200575XDocument9 pagini1 s2.0 S001236922200575XYulia Niswatul FauziyahÎncă nu există evaluări
- Manejo Nodulo PulmonarDocument8 paginiManejo Nodulo PulmonarLuis Maximiliano Tinte SegoviaÎncă nu există evaluări
- Dissertation On Prostate CancerDocument24 paginiDissertation On Prostate CancerZainab ShahidÎncă nu există evaluări
- ESR/ERS Statement Paper On Lung Cancer Screening: European Radiology (2020) 30:3277 - 3294Document18 paginiESR/ERS Statement Paper On Lung Cancer Screening: European Radiology (2020) 30:3277 - 3294MeeqatÎncă nu există evaluări
- Review Article: Screening For Lung Cancer With Low-Dose Computed Tomography: A Review of Current StatusDocument16 paginiReview Article: Screening For Lung Cancer With Low-Dose Computed Tomography: A Review of Current StatusAnnizada Intan PratiwiÎncă nu există evaluări
- Lung Nodules Assessment - Analysis of Four GuidelinesDocument9 paginiLung Nodules Assessment - Analysis of Four GuidelinesdonaskÎncă nu există evaluări
- Screening For Lung CancerDocument7 paginiScreening For Lung CancerdtmÎncă nu există evaluări
- RetrieveDocument13 paginiRetrieves1138084Încă nu există evaluări
- Nej Mo A 1201637Document11 paginiNej Mo A 1201637GianÎncă nu există evaluări
- 2022 48 3 3675 EnglishDocument10 pagini2022 48 3 3675 EnglishRaul Matute MartinÎncă nu există evaluări
- Prognostic Value of F-FDG Pet/Ct in Surgical Non-Small Cell Lung Cancer: A Meta-AnalysisDocument19 paginiPrognostic Value of F-FDG Pet/Ct in Surgical Non-Small Cell Lung Cancer: A Meta-AnalysisAmina GoharyÎncă nu există evaluări
- Review of Radiation Issues For Computed Tomography: Donald P. FrushDocument8 paginiReview of Radiation Issues For Computed Tomography: Donald P. Frushradiologi rsudÎncă nu există evaluări
- Lung Cancer Current Therapies and New Targeted TreatmentsDocument13 paginiLung Cancer Current Therapies and New Targeted TreatmentsWlad PaCaÎncă nu există evaluări
- MicronodulosDocument9 paginiMicronodulosWildcane SalmeronÎncă nu există evaluări
- Research Paper Lung CancerDocument7 paginiResearch Paper Lung Cancerjvfmdjrif100% (1)
- Abordaje de Los Nodulos Pulmonares Indeterminados en El Tamizaje de Cancer de Pulmon Estudio Con PET CTDocument6 paginiAbordaje de Los Nodulos Pulmonares Indeterminados en El Tamizaje de Cancer de Pulmon Estudio Con PET CTAnaÎncă nu există evaluări
- SNR and CNRDocument12 paginiSNR and CNRp110054Încă nu există evaluări
- Bronchogenic CarcinomaDocument13 paginiBronchogenic CarcinomakvishwanathÎncă nu există evaluări
- Reliability of The Brazilian Version of The Functional Assessment of Cancer Therapy-Lung (FACT-L) and The FACT-Lung Symptom Index (FLSI)Document5 paginiReliability of The Brazilian Version of The Functional Assessment of Cancer Therapy-Lung (FACT-L) and The FACT-Lung Symptom Index (FLSI)Jairo BermúdezÎncă nu există evaluări
- Minimally Important Differences For InterpretingDocument7 paginiMinimally Important Differences For InterpretingAndrés Felipe Hurtado MejíaÎncă nu există evaluări
- Interval Colorectal Cancers in Inflammatory Bowel Disease: The Grim Statistics and True StoriesDocument12 paginiInterval Colorectal Cancers in Inflammatory Bowel Disease: The Grim Statistics and True StoriespancholarpancholarÎncă nu există evaluări
- A Review Article On Lung Cancer Diagnosis & Treatment: Research and Reviews Journal of Medical and Health SciencesDocument9 paginiA Review Article On Lung Cancer Diagnosis & Treatment: Research and Reviews Journal of Medical and Health Sciencesmeilvi19Încă nu există evaluări
- ACCP Evidence-Based Clinical Practice: Management of Small Cell Lung CancerDocument18 paginiACCP Evidence-Based Clinical Practice: Management of Small Cell Lung CancerCharles Xavier KimÎncă nu există evaluări
- Lepechoux 2020Document7 paginiLepechoux 2020Aniketh BaghoriyaÎncă nu există evaluări
- 40-60 Chemoprevention of Lung CancerDocument21 pagini40-60 Chemoprevention of Lung CancerCARDIOTORAX HCMÎncă nu există evaluări
- Risk Adapted Starting Age For Personalized Colorectal Cancer Screening Validated Evidence From National Population Based StudiesDocument21 paginiRisk Adapted Starting Age For Personalized Colorectal Cancer Screening Validated Evidence From National Population Based Studieszhe zhÎncă nu există evaluări
- Editorial Surveillance in Testicular Cancer: Who, When, What and How?Document3 paginiEditorial Surveillance in Testicular Cancer: Who, When, What and How?Jocho NeavesÎncă nu există evaluări
- s12876 022 02492 7Document13 paginis12876 022 02492 7ppdsipd2019Încă nu există evaluări
- Alcohol Drinking Cessation and The Risk of Laryngeal and Pharyngeal Cancers: A Systematic Review and Meta-AnalysisDocument11 paginiAlcohol Drinking Cessation and The Risk of Laryngeal and Pharyngeal Cancers: A Systematic Review and Meta-Analysisjustin_saneÎncă nu există evaluări
- Clinical Practice GuidelinesDocument7 paginiClinical Practice GuidelinesRoppeÎncă nu există evaluări
- 2019E 11 - Adjuvant Cytotoxic and Targeted TherapyDocument15 pagini2019E 11 - Adjuvant Cytotoxic and Targeted Therapyrusgal8992Încă nu există evaluări
- (Comprehensive Gynecology and Obstetrics) Mikio Mikami - Surgery For Gynecologic Cancer-Springer Singapore (2019)Document428 pagini(Comprehensive Gynecology and Obstetrics) Mikio Mikami - Surgery For Gynecologic Cancer-Springer Singapore (2019)Bakar Benk100% (2)
- Lung CancerDocument15 paginiLung CancerO.r. CadzÎncă nu există evaluări
- Endo FinalDocument52 paginiEndo Finalspamboy6464Încă nu există evaluări
- DR Ajay Kumar - Time, Dose & FractionationDocument31 paginiDR Ajay Kumar - Time, Dose & Fractionationoncology KMC-KnlÎncă nu există evaluări
- DashBoard Health SampleDocument1 paginăDashBoard Health SampleAliChana1Încă nu există evaluări
- Conjunctival Pigmented LesionsDocument0 paginiConjunctival Pigmented LesionsBhartendu Agarwal0% (1)
- PECOMADocument25 paginiPECOMAAnan JaiswalÎncă nu există evaluări
- Marine Corps Institute: Semper Fit Advanced Fitness CourseDocument222 paginiMarine Corps Institute: Semper Fit Advanced Fitness CoursesrollinsÎncă nu există evaluări
- The Brain in Love by Daniel G. Amen, M.D. - ExcerptDocument31 paginiThe Brain in Love by Daniel G. Amen, M.D. - ExcerptCrown Publishing Group81% (16)
- Optic Nerve Tumours: Presenter-Dr Adheela Abdulla Moderator - DR Shikha BassiDocument54 paginiOptic Nerve Tumours: Presenter-Dr Adheela Abdulla Moderator - DR Shikha BassiMohammed Jazeel 2549Încă nu există evaluări
- Pediatric LeukemiasDocument42 paginiPediatric LeukemiasFilbertaÎncă nu există evaluări
- DR Eric and Sabrina Zielinski Non Toxic Body Care Diy RecipeDocument141 paginiDR Eric and Sabrina Zielinski Non Toxic Body Care Diy Recipefizz100% (1)
- LABSTAND Azucena Case DigestsDocument19 paginiLABSTAND Azucena Case Digestslleiryc7100% (1)
- IHFG Part B Oncology Medical ChemotherapyDocument20 paginiIHFG Part B Oncology Medical ChemotherapyFransiska OktavianiÎncă nu există evaluări
- June 2009 Nle HirapDocument35 paginiJune 2009 Nle HirapDavid BrilloÎncă nu există evaluări
- Template Continued On Page 2: Indication: References: NCCN Supportive CareDocument2 paginiTemplate Continued On Page 2: Indication: References: NCCN Supportive CareJaneÎncă nu există evaluări
- Medstar ObGyn 2nd EditionDocument570 paginiMedstar ObGyn 2nd EditionMerahit Abera100% (2)
- Health of Women in Pakistan SOGP Annual Report 2009Document8 paginiHealth of Women in Pakistan SOGP Annual Report 2009WINSOMETABÎncă nu există evaluări
- Msds PlutoniumDocument12 paginiMsds PlutoniumarizÎncă nu există evaluări
- Abnoba ViscumDocument44 paginiAbnoba ViscumAlexandre Funcia100% (1)
- Robbins Pathology NotesDocument48 paginiRobbins Pathology NotesRajesh Kumar Asunala91% (93)
- Lecture 15 PDFDocument20 paginiLecture 15 PDFSrramÎncă nu există evaluări
- National Geographic USA - 2022-AprilDocument126 paginiNational Geographic USA - 2022-Aprildayne81s100% (2)
- Ijcri 201312ab Full IssueDocument88 paginiIjcri 201312ab Full IssueDedi SuciptaÎncă nu există evaluări
- IMRT & Stereotactic Radiotherapy: Christopher GolbyDocument41 paginiIMRT & Stereotactic Radiotherapy: Christopher GolbyAmr MuhammedÎncă nu există evaluări
- Cancer - 2011 - Hajdu - A Note From History Landmarks in History of Cancer Part 2Document10 paginiCancer - 2011 - Hajdu - A Note From History Landmarks in History of Cancer Part 2Pilar AufrastoÎncă nu există evaluări
- Gall Bladder CarcinomaDocument29 paginiGall Bladder CarcinomaUsman FarooqÎncă nu există evaluări
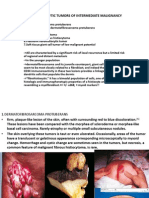
- Tumori Fibrohistiocitare IntermediareDocument39 paginiTumori Fibrohistiocitare IntermediareOana BarbuÎncă nu există evaluări
- USMLE Step 2CK Notes With AdditionsDocument103 paginiUSMLE Step 2CK Notes With AdditionsLuong Anh100% (1)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDe la EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsÎncă nu există evaluări
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDDe la EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDEvaluare: 5 din 5 stele5/5 (3)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDe la EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionEvaluare: 4 din 5 stele4/5 (404)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDe la EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityEvaluare: 4 din 5 stele4/5 (32)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDe la EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeEvaluare: 2 din 5 stele2/5 (1)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDe la EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)De la EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Evaluare: 3 din 5 stele3/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDe la EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedEvaluare: 4.5 din 5 stele4.5/5 (82)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDe la EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsEvaluare: 4 din 5 stele4/5 (4)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisDe la EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisEvaluare: 4.5 din 5 stele4.5/5 (42)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDe la EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsEvaluare: 5 din 5 stele5/5 (1)
- The Obesity Code: Unlocking the Secrets of Weight LossDe la EverandThe Obesity Code: Unlocking the Secrets of Weight LossEvaluare: 4 din 5 stele4/5 (6)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesDe la EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesEvaluare: 4.5 din 5 stele4.5/5 (1412)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDe la EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeEvaluare: 4.5 din 5 stele4.5/5 (254)
- The Marshmallow Test: Mastering Self-ControlDe la EverandThe Marshmallow Test: Mastering Self-ControlEvaluare: 4.5 din 5 stele4.5/5 (60)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDe la EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisEvaluare: 5 din 5 stele5/5 (8)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.De la EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Evaluare: 4.5 din 5 stele4.5/5 (110)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDe la EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityEvaluare: 4 din 5 stele4/5 (5)
- To Explain the World: The Discovery of Modern ScienceDe la EverandTo Explain the World: The Discovery of Modern ScienceEvaluare: 3.5 din 5 stele3.5/5 (51)
- Summary: Thinking, Fast and Slow: by Daniel Kahneman: Key Takeaways, Summary & Analysis IncludedDe la EverandSummary: Thinking, Fast and Slow: by Daniel Kahneman: Key Takeaways, Summary & Analysis IncludedEvaluare: 4 din 5 stele4/5 (61)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisDe la EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisEvaluare: 3.5 din 5 stele3.5/5 (2)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryDe la EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryEvaluare: 4 din 5 stele4/5 (46)