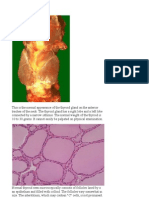
Documente Academic
Documente Profesional
Documente Cultură
Chapter 7 Neoplasia 1 2 Robbins and Cotran Pathologic Basis of Disease PDF
Încărcat de
ChethranTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Chapter 7 Neoplasia 1 2 Robbins and Cotran Pathologic Basis of Disease PDF
Încărcat de
ChethranDrepturi de autor:
Formate disponibile
6. CHORISTOMA – have normal tissue, but not in normal place (ie.
NEOPLASIA I endometriosis)
Nomenclature – For Benign • heterotopic rest of normal tissue
Introduction Characteristics BENIGN MALIGNANT
•
nd st
2 leading cause of death in the US (1 = CVD) 1. MESENCHYMAL – organs lined with mesothelium
• Decreased mortality in the last decade of the 20th century and • Attach the suffix “oma” to the cell of origin
– Osteoma - bone Differentiation and Well differentiated Well to poorly/
in a downward course anaplasia undifferentiated/
– Chondroma - cartilagenous
– Fibroma – fibrous tissue anaplastic
Nomenclature Rate of growth Indolent/slow Rapid, erratic pace
*Neoplasma = tumor – Lipoma lipid tissue (not fat/ adipose)
growing/expansile-
• Neoplasia - new growth capsule
• Tumor - swelling 2. EPITHELIAL
• Oncology – study of tumors or neoplasms • Attach the suffix “oma” to the cell of origin, microscopic
pattern or macroscopic architecture Local invasion Absent Present-progressive
NEOPLASM infiltration/destruction
– Adenoma – glandular; forming glands
• Abnormal mass of tissue of
• Growth exceeds and uncoordinated with that of the normal – Papillomas – finger-like projections
– Polyp – protrudes from the surface of the organs surrounding/adjacent
tissue tissue
• Persists in the same manner after the cessation of stimuli – Cystadenomas, papillary cystadenomas
• Exception: Hepatoma, Lymphoma, Seminoma, Melanoma,
which evoked the change Metastasis Absent Present
Mesothelioma-malignant (found in pleura)
• Autonomous
• Clonal-arise from single cell that incurred genetic damage *all “-oma” are malignant
BENIGN Nomenclature – Malignant
1. CARCINOMA-from epithelial Differentiation & Anaplasia
• Relatively innocent
• Adenocarcinoma DIFFERENTIATION
• Remain localized
• Squamous cell carcinoma, basal cell carcinoma • Extent to which neoplastic parenchymal cells resemble the
• Cannot spread to other sites
corresponding normal parenchymal cells both
• Amenable to surgical removal
2. SARCOMA-from mesenchymal morphologically and functionally
• Patient generally survives
• liposarcoma, leiomyosarcoma, osteosarcoma ANAPLASIA – poorly differentiated
MALIGNANT
• fibrosarcoma, chondrosarcoma, angiosarcoma, • lack/loss of differentiation, associated with other morphologic
• Cancer
rhabdomyosarcoma changes
• Invade and destroy adjacent structures
• Spread to distant sites
• Cause death 3. MIXED TUMOR Changes in Anaplasia
• Divergent differentiation of a single neoplastic clone 1. PLEOMORPHISM – hallmark ok neoplasia
2 BASIC COMPONENTS • Pleomorphic adenoma of salivary gland (parotid) • Variation in size and shape – ex.big/small cells
2. ABNORMAL NUCLEAR MORPHOLOGY
1. parenchyma-clonal neoplastic cells
4. TERATOMA – from testis, embryo, ovarian • increased N:C ratio (1:6è1:1)
• determines tumor’s behavior and pathologic
• totipotential cell that differentiate along 3 germ cell layer – • hyperchromatic, large nucleoli
consequences
totipotent means it can make a whole body/anything 3. MITOSES – mitosis is normale in labile cells
2. stroma supporting tissues; tells how fast will grow
• benign (mature) or malignant (immature) • increased, atypical, bizarre
• growth & evolution-blood supply
4. LOSS OF POLARITY
• connective tissue-framework
5. HAMARTOMAS – not necessary a neoplasia, it does not grow bigger • disturbed orientation
– Scant-soft and fleshy ex. Lymphoma,
Fibrosarcoma • disorganized but benign appearing masses composed of 5. TUMOR GIANT CELLS – NOT macrophages (ie. Langhan cells in
mature specialized cells or tissue indigenous to the particular TB)
– Abundant-desmoplasia (excessive stroma),
site • Not macrophages
stony hard ex. Scirrhous CA of breast
• pulmonary chondroid hamartoma, hemangioma 6. DYSPLASIA – if severe dysplasia, it is already equal in
carcinoma in situ (lesions confined in basement/noninvasive)
• loss in uniformity & architectural orientation of the cells
Prepared by: EGBautistaII
•disordered growth, often occurs in metaplastic – More aggressive, more rapidly growing, larger size- – Women: significant decline in death rates from
epithelium greater likelihood of metastasis cancer of breast & colorectal CA & most notably
7. METASTASIS – Approximately 30% of newly diagnosed individuals cervix-earlier diagnosis –Pap smear
• tumor implants discontinuous with the primary tumor with solid tumors present with metastasis – Men: nearly 80% of the total decrease in lung,
Rate of Growth – something to do w/stroma, needs blood supply prostate & colorectal CA
1. Doubling time of tumor cells – multiplication time remains the
same (3) Pathways of Spread
– Cell cycle time not shortened – time it takes to • Seeding of body cavities & surfaces
divide – Carcinomas arising from the ovaries Pseudomyxoma Geographic & Environmental Factors
2. Fraction of tumor cell in the replicative pool peritonei, appendiceal CA • Environmental > hereditary
– Growth fraction – affects the G0 (ie. Stable cells) • Lymphatics – carcinoma • Cultural > racial
• low in breast & colon, high in some – Skip metastasis – coz’ the passageway is obstructed • Environmental factors
leukemia, lymphoma & small cell CA – Sentinal nodes – Home - Outdoors
• Correlate with level of differentiation • Hematogenous – sarcoma – most common route – Work - Medication
• Hormonal stimulation, adequacy of blood – Veins>arteries *Look at table 7-3
supply, etc – Organ area for metastasis (liver & lung) = need to • Most overweight individuals have a higher death rate from CA
3. Rate at which cells are shed or die check fist • Alcohol abuse-increase the risk for oropharynx, larynx,
• Transplantation – rare esophagus, liver
CANCER STEM CELLS & CANCER CELL LINEAGES – If undergo surgery = it will spread because same • Smoking-mouth, pharynx, larynx, esophagus, pancreas,
• Cancer could arise from scalpel is used in making cuts bladder, kidney
– normal tissue stem cells or • Risk of cervical CA: age of first intercourse, number of sex
– More differentiated cells that acquire the property Epidemiology partners-HPV
of self-renewal • Study of cancer patterns
• CML- from stem cells • Contributes substantially to knowledge about origins of Age
• AML- differentiated myeloid precursors cancer • Most CA occur in later years of life >55yrs
• Tumor initiating cells (T-ICs) • Provides major insights on the cause of cancer • Main cause of death among women aged 40-79 and men
– Allow cells to grow indefinitely – environmental, racial (hereditary) & cultural aged 60-79 ….carcinomas
– May represent 25% of the total cellularity influences • Acute leukemia & neoplasm of the CNS-approx 60% of deaths
• Cancer incidence in children
Local Invasion Male Female • Common neoplasms of infancy & childhood-small round blue
• Most benign tumors develop a rim of compressed connective – 1. Prostate 1. Breast cell tumors, which includes:
tissue called a capsule – this are normal tissue that are – 2. Lung 2. Lung – Neuroblastoma, Wilms tumor, retinoblastoma,
pushed outside the lining forming capsule – 3. Colon 3. Colon acute leukemias, & rhabdomyosarcomas
• Cancers show infiltration, invasion and destruction of – 4. Bladder 4. Uterus
• Death - most people die w/lung ca
surrounding tissue Genetic Predisposition
– Invasiveness Male Female
1. Autosomal Dominant Inherited Cancer Syndromes
• most reliable feature that differentiates – 1. Lung (31%) 1. Lung (26%)
2. Defective DNA Repair Syndromes
benign from malignant tumor next to – 2. Prostate (10%) 2. Breast (15%)
3. Familial Cancers
development of metastasis – 3. Colon (8%) 3. Colon (9%)
– 4. Pancreas (5%) 4. Pancreas & Ovary (6%)
1. Autosomal Dominant Cancer Syndromes
Metastasis • Inheritance of single autosomal dominant mutant gene
• Unequivocally marks a tumor malignant Cancer Incidence greatly increases the risk of developing a tumor
• With few exceptions, all malignant tumors can metastasize • Increased death rate • Usually a point mutation in single allele of tumor suppressor
– Gliomas of CNS, basal cell carinoma of skin-locally – HCC (hepatocellular ca)-caused by HCV (hepa C) gene (RB)
invasive but rarely metastasize • Decreased death rate • Defect in the second allele occurs in somatic cells as a
consequence of chromosome deletion or recombination
Prepared by: EGBautistaII
• Inherited variations (polymorphisms) of enzymes that – Genes involved in DNA repair
1. Carriers of mutant RB tumor suppressor gene have 10,000 metabolize procarcinogens to their active carcinogenic forms 4. Haploinsufficiency-loss of gene function caused by damage to
folds increased risk-bilateral retinoblastoma can influence the susceptibility to cancer a single allele
– Increased risk of developing second cancer, osteogenic – cytochrome P-450 enzymes – Single cell is palpak but the other one is normal. A
sarcoma total of 2 alelles
2. Familial adenomatous polyposis-inherit AD mutation of APC 5. Carcinogenesis is a multistep process at both the phenotypic
tumor suppressor gene (APC0adenomatous polycystic & genetic levels, resulting from the accumulation of multiple
carcinoma) mutations
– innumerable adenomas in colon, virtually 100% fated to – Tumor progression
develop CA
3. Li-Fraumeni syndrome-germ line mutation in p53 gene
4. Multiple Endocrine Neoplasia (MEN) 1 & 2 mutation in menin Nonhereditary Predisposing Conditions
and RET tyrosine kinase CHRONIC INFLAMMATION –can lead to neoplastic
5. Hereditary Nonpolyposis colon cancer (HNPCC)-inactivation of • ex. Ulcerative colitis, Crohn disease, H. pylori gastritis, viral
mismatch repair gene (HNPCC is both AD&AR) hepatitis, chronic pancreatitis
• it is d/t Cytokines stimulate growth of transformed cells – that
Inherited Cancer Syndromes stimulate jak stat receptors
GENERAL CHARACTERISTICS • Increase pool of tissue stem cells
• Tumors tend to arise in specific sites and tissues • Production of Reactive Oxygen Species
– Except Li Fraumeni • Evidence: COX-2 increased in colon CA
• Associated with a specific marker phenotype
– Lisch nodule & café-au-lait spots PRECANCEROUS CONDITIONS
7 FUNDAMENTAL CHANGES
• non-neoplastic disorders
2. Defective DNA Repair Syndromes – chronic atrophic gastritis of pernicious anemia, solar 1. Self-sufficiency in growth factors
• Generally autosomal recessive keratosis, skin chronic ulcerative colitis, & 2. Insensitivity to growth inhibitory signals
• Defects in DNA repair can cause DNA instability leukoplakia of oral vulva, penis & oral cavity 3. Evasion of apoptosis
• Xeroderma pigmentosum, Ataxia telagiectasia, Bloom • Neoplasic 4. Defects in DNA repair
syndrome – Villous adenoma-increase in size, develops CA in 5. Limitless replicative potential
• ★HNPCC – AD w/DNA repair defect 50% of cases 6. Sustained angiogenesis
7. Ability to invade and metastasize
– colon CA, small int., endometrium, ovary Molecular Basis of Cancer
– most common cancer predisposition syndrome FUNDAMENTAL PRINCIPLES
1. Nonlethal genetic damage lies in the heart of carcinogenesis
3. Familial Cancers – mutation may be acquired-action of environmental
• Breast, ovary, pancreas, …..colon, brain, melanomas, agents: chemicals, radiation, viruses
lymphomas – inherited in the germ line
• Characteristic features – if cells died, there will be no replication & thus, it
– early age at onset - AR must not be lethal
– tumors arising in 2 or more close relatives 2. Tumor is formed by the clonal expansion of single precursor
– Multiple or bilateral tumors cell that has incurred the genetic damage
• Transmission pattern of familial cancers is not clear – Tumors are monoclonal
– Cells that gone crazy
Interactions Genetic & Nongenetic Factors 3. Four classes of normal regulatory genes are the principal
• Complex target of genetic damage
• Genotype can significantly influence the likelihood of – Protooncogenes-growth promoting
developing environmentally induced cancers – Tumor suppressor gene-growth inhibiting
– Genes that regulate apoptosis
Prepared by: EGBautistaII
SELF SUFFICIENCY IN GROWTH SIGNALS – kinase is transiently activated by binding of the • Can act in concert to reprogram somatic cells into
• ONCOGENES - Genes that promote autonomous growth specific GF, followed rapidly by receptor pluripotent stem cells
• PROTONCOGENES - Normal cellular counterpart dimerization & tyrosine phosphorylation of several • May also enhance self-renewal, block differentiation or
• ONCOPROTEINS - Products of genes without regulatory substrate that are part of signaling cascade both
function • Oncogenic/mutant receptors • Amplified in breast, colon, lung and many other
*Proto-oncogenes oncogene secretes oncoproteins – associated with constitutive dimerization & carcinomas
activation without binding to the GF, deliver • N-MYC & L-MYC in neuroblastomas & small cell CA of
continuous mitogenic signals to the cell even in the lungs respectively
absence of GF in the environment • Translocation-Burkitts lymphoma
• Can be constitutively activated in tumors by different
STEPS IN CELL PROLIFERATION
mechanisms CYCLINS
• Binding of growth factor to cell membrane receptor – Mutations
• Activation of signal transducing proteins – gene rearrangements
• Transmission of signal to nucleus – overexpression
• Activation of nuclear factors Ex. RET proto-oncogene : mutation with gene rearrangement
• Entry into cell cycle – MEN-2A & MEN-2B
• Over expression of normal forms of GF receptors
– By gene amplification and other unknown
mechanisms
Ex. EGFR
• ERBB1-overexpressed in SCCA of the lung,
glioblastomas, head & neck tumors Control orderly progression of cells thru the phases of the cell
cycle
Signal Transducing Proteins
CDK Inhibitors
• Oncoprotein can mimic signal transducing proteins
• CIP/WAF
• ex. RAS family of guanine triphosphate (GTP)-binding proteins
– p21,p27,p57
(G proteins)-RAS genes
– Inhibit all stages
• INK4
RAS Oncogene
– p15, p16, p18, p19
• Point mutation of RAS family genes-single most common
– Inhibit cyclin D
abnormality of protooncogene in human tumors-approx.
• CDK4 & CDK6
15-20% of all human tumors
• Check points at G1/S and G2/M mediated
• 90% in pancreatic adenoCA & cholangioCA
by p53, p21
• 50% in colon, endometrial, thyroid
Growth Factors • Major cause of genetic instability
• 30% in lung adenoCA & myeloid leukemia
• Normal cells • KRAS-colon & pancreas, HRAS-bladder, NRAS-
– need GF to stimulate proliferation SELF SUFFICIENCY OF GROWTH SIGNALS
hematopoeitic tumors
– paracrine action • Overexpression of growth factor genes
• Many cancer cells – PDGF & TGF
• Activated RAS stimulates downstream regulators of
– acquire the ability to synthesize the same GF to • Altered growth factor receptors
proliferation such as the mitogen-activated protein (MAP)
which they are responsive – RET
kinase cascade-floods the nucleus with signals for cell
– autocrine loop • Minicry of signal transducing proteins
proliferation
Ex. Glioblastoma-express PDGF &PDGFR – RAS
• Mutated RAs is trapped in its activated GTP-bound form, and
Sarcomas-TGFα • Oncoproteins as transcription factors
the cell is forced into continuously proliferating state
– MYC
Growth Factor Receptors • Dysregulation of cyclins
MYC Oncogene
• Normal
Prepared by: EGBautistaII
INSENSITIVITY TO GROWTH INHIBITORY SIGNALS PTCH- “Gorlin syndrome”, basal cell carcinoma
• TUMOR SUPPRESSORS
– Stop cell proliferation
• RB gene NEOPLASIA PART II
• P53 protein
• APC and NF-1
EVASION OF APOPTOSIS
• Reduced levels of CD95/Fas – inhibit extrinsic pathway
Knudson’s Hypothesis
• Inactivation of caspase 8 by FLIP – inhibit extrinsic path.
• “two hits” required
• Protection of tumor cells by BCL2
– First hit - inherited, all somatic cells
– Second hit- inherited or acquired, particular cell
• “loss of heterozygosity” LIMITLESS REPLICATIVE POTENTIAL
– Heterozygotes-no expression of cancer • Replicative senescence lies in telomere shortening
– Homozygotes-cancer • Short telomere activation of p53 checkpoint apoptosis
• Telomerase-prevents shortening of telomere
RB GENE • About 90% of tumors have telomerase activity
• RB protein regulates cell growth at G1/S • Some have alternative lengthening of telomeres
• Cyclin E- CDK2 and Cyclin D CDK 4,6 required
• Mutation associated with retinoblastoma and osteosarcoma SUSTAINED ANGIOGENESIS
• Loss of normal cell cycle control is central to malignant • Tumors >2mm require new blood supply
transformation and that at least 1 of 4 key regulators of the • Angiogenic switch- change in phenotype that induces
cell cycle is dysregulated in the vast majority of human APC/ CATENIN angiogenesis mostly controlled by hypoxia
cancers • APC protein down regulates catenin • Neovascularization (adult) or vasculogenesis – this 2 both
• Loss of APC causes continuous WNT signaling and -catenin occur in neoplasia
p53 (p63,p73) enters nucleus cell proliferation • Vasculature is abnormal- leaky, haphazard
• Most common target for genetic alteration • Vasculogenic mimicry – tumor cells resemble capillaries
• Located on chromosome 17p13.1 • The only difference is that there is no blood inside
• Stops neoplastic transformation by Notes:
– Cell cycle arrest (quiescence and senescence) • Decreased angiogenesis inhibitors – angiostatin, endostatin,
– initiation of apoptosis vasculostatin
• One mutant p53 = Li Fraumeni syndrome • Loss of p53 provides more permissive environment for
– 25 % increased risk of neoplasia angiogenesis
– Sarcomas, breast, leukemia • Vegf expressed on tumor cells – culprit in angiogenesis
• Vasculogenic mimiccry no longer in book!
METASTATIC CASCADE
• Two phases
1. Invasion of the extracellular matrix
2. Vascular dissemination, homing of tumor cells and
colonization
TUMOR SUPPRESSORS
NF1-Neurofibroma, glioma 1. INVASION
NF2- Schwannoma • Detachment of tumor cells from one another
VHL – renal cell ca, pheochromocytoma – Down regulation of E cadherins – becomes loose
PTEN- “Cowden Syndrome” epithelial cancers • Degradation of ECM
WTI- Wilms
Prepared by: EGBautistaII
– Secrete proteolytic enzymes: MMPs, cathepsin D, – Snail and twist promote EMT epithelial to mesenchymal – Loss of tumor suppressor genes
urokinase, plasminogen transition.which favors migration • Non hematopoietic solid tumors
• Attachment to matrix components • RB in retinoblastoma
– Intergrins attach to fibronectin, laminin Genomic Instability
• Migration of tumor cells or locomotion • Where DNA become vulnerable for mutations (irreversible) Gene Amplification
– Directed by cell derived cytokines/ amoeboid mov’t • Inherited defects in DNA repair genes allow mutations in • Two patterns are seen
other genes – double minutes (dms)
Notes: • DNA repair systems – Homogeneous Staining Regions (HSR)
• Down regulation of E catherins or mutation in catenin needed – Mismatch - can cause: – Ex. N-MYC – neuroblastoma, both patterns seen
for linkage • HHPCC – microsatellite instability – Ex. ERBB2 – breast ca
• Matrix metalloproteinases – remodelng insoluble components – Nucleotide excision repair
and release of growth factors • Xeroderma pigmentosa (AR) – sensitive to Epigenetic Changes
• Ameoboid migration- a second method of migration using uv light – Reversible, heritable changes in gene expression
collagen fibers – Recombination repair – Post translational modification
• Bloom, etc – hypersensitivity to DNA – Genome imprinting / loss of imprinting d/t
damaging agents – Methylation causes inactivation
• BRCA 1 &BRCA 2 breast cancer Ex BRCA 1 in breast cancer
Notes:
• Genonic instability occurs when both DNA repair genes are MiRNAs and Cancer
lost • Small non-coding single stranded RNA
• Microsatellite instability- tandems are shorter or longer • Mediate post-transcriptional gene silence
• UV radiation causes cross-linkage of pyrimidine residues that
now cannot be excised
Multistep Carcinogenesis
• Cancer must result from the accumulation of multiple
Warburg Effect – a metabolic alterations mutations
• Does not want yo breakdown glucose; inhibit kreb’s cycle
• Tumors shift to aerobic glycolysis
• Not on Oxygen-hunger more onn Glucose hunger
• Possible explanation
– Increased need for energy of dividing cell
2. VASCULAR DISSEMINATION – Abnormal vasculature
• Tumor cells -clump, form emboli, adhere to target (emboli is – Reduction in autophagy
filled with platelet & metabolite) Notes:
• At site -proliferate, develop a vascular supply, evade host • Used to visualize tumors in PET
defenses • Gives 2 ATP rather than 20
• Organ trophism –
– Adhesion molecules on the endothelial cells of the
target organ
Chromosomal Abnormalities
– Chemokine receptors
– Translocation
– Some tissues are unfavorable to metastasis ( where
– Overexpression of protooncogenes
we do not see metastasis, ex.skeletal muscles)
• MYC of Burkitt Lymphoma
Notes: Carcinogenesis
– Hybrid “chimaric” genes
– Some tissues, like skeletal muscles have an unfavorable Initiation-promotion sequence
– Constitutive tyrosine kinase activity
environment for growth 1. Initiation results from exposure of cells to a sufficient dose of
• BCR-ABL (Philadelphia chromosome 9) of CML
– CD44 – are adhesion molecule expressed on T cells; carcinogen
• BCR( 22) to ABL (9)
overexpression favors metatasis – Permanent DNA damage is rapid and irreversible
– Deletions
Prepared by: EGBautistaII
2. Promoters induce tumors in initiated cell without affecting • Bile salts • HPV also activate cyclins, inhibit apoptosis and combat cell
DNA directly and are reversible • High levels of dietary fat aging
– Not tumorigenic themselves; only cause cell • phenols • EBV uses CD 21 receptor to attach to B cells
proliferation
2. RADIATION CARCINOGENS Tumor Immunity
CARCINOGENS • Ultra Violet rays • Immune surveillance
1. Chemical – UV B in sunlight linked to all forms of skin cancer – – Guards against tumor development
2. Radiant energy can cause squamous cell Ca - melanoma • Cancer immunoediting
3. Microbial – Formation of pyrimidine dimers – Sculpting the immunogenic properties of tumors to
• Ionizing Electromagnetic Forces select tumor cells that escape immune elimination
1. CHEMICAL CARCINOGENS – X rays and gamma rays
• Initiation – and particles Tumor Antigens
– Exposure and mutation – linked to leukemias, thyroid ca etc 1. Products of mutated genes
• Promotion – Mutated tumor suppressor genes
– Reversible enhancement of proliferation Notes: – Mutated self protein
• Accumulated even exposure (SCC), short bursts of Notes:
INITIATORS – cause damage; direct (alkalyting agent); or indirect exposure (melanoma) – Tumor specific or tumor associated antigen no
• Direct acting • UVC more carcinogenic but filtered by ozone longer used!!
– Highly reactive electrophils – Antigens must be recognized by CTLs
– Weak carcinogens 3. MICROBIAL CARCINOGENS – Products of mutated genes become tumor antigens
• Indirect acting 1. RNA viruses - HTLV-1
– Require metabolic activation – T –cell leukemia, lymphoma 2. Overexpressed or aberrantly expressed cellular proteins
– Vary among individuals – tax genone causes proliferation and instabiity – Ex – tyrosinase and cancer testis antigen
2. Bacteria - Helicobacter pylori – Normal cellular proteins that are abnormally
DNA is primary target – Gastric adenocarcinomas and MALTomas expressed!
Altered cells must be able to undergo at least one cycle so – CagA cytotoxin gene and cytokines (Chronic 3. Produced by Oncogenic viruses
change can be made permanent inflammation) 4. Oncofetal antigens found on fetal and cancer cells
CARCINOGENIC CHEMICALS 5. Altered cell surface glycolipids and glycoproteins
* Liver cancer caused by Aflatoxin B has a signature mutation in (p53) – Higher concentration
Alkylating agents Naturally occurring Notes: – Abnormal forms
Weak - direct acting Aflatoxin B • Tax has transforming activity 6. Cell type specific differentiation antigens
Therapeutic agents Stored corn, rice, peanuts • H pylori – 1st bacteria called a carcinogen – Do not induce immune response
– target for immunotherapy
Polycyclic hydrocarbons Nitrosamines and amides 3. DNA viruses
Most potent Preservatives – Human Papilloma Virus (16 & 18) Anti Tumor Mechanisms
Tobacco, smoked meat/fish • Squamous cell ca of cervix d/t
• Cytotoxic T lymphocytes – cell mediated
• Overexpression of E6 & E7 oncogenes
• Natural killer cells
Aromatic amines/Azo dyes Others: – Epstein – Barr Virus
• Macrophages
Mainly affect liver asbestos, vinyl chloride, arsenic • Burkitt lymphoma, B cell (coz’ CD21)
• Antibodies - humoral
Food coloring Industrial exposure, insecticides lymphoma in HIV, Hodgkin,
Notes:
Nasopharyngeal Ca, gastric Ca,NK
• Mostly cell mediated
lymphoma
• Antibody against CD20 for B cell lymphoma
PROMOTERS • LMP-1 is oncogene
• Phorbol esters – Hepatitis B & C Viruses
Tumor Evasion of the Immune System
• Viral infection • Liver Cancer
• Cytokines and reactive oxygen species • Selective outgrowth of antigen-negative variants
• Hormones
• Loss or reduced expression of MHC molecules
Notes:
Prepared by: EGBautistaII
• Lack of costimulation • Tumor antigen cross reaction "The stage is T-3, N-1, M-0" which means "the cancer has spread
• Immunosuppression – ex. HIV 7. Acanthosis Nigricans – hyperpigmentation of the skin through the stomach wall, there is some spread to local lymph nodes,
• Antigen masking but no metastases in other parts of the body".
• Apoptosis of cytotoxic T cells 8. Hypertrophic osteoarthropathy (from bronchogenic)
• *Cancers more common in immunocompromised – New bone formation Laboratory Diagnosis
– Arthritis 1. Histologic & cytologic methods
Clinical Features of Tumors – Clubbing – Biopsy, needle aspiration (FNAB) & cytologic smears
Effects of Tumors on the Host 9. Vascular and hematologic (PAP) – stain not only for cervix
• Location & impingement on adjacent structures • Migratory thrombophlebitis (pancreas, lung) 2. Immunohistochemistry
• Functional activity such as hormone synthesis • Thrombotic endocarditis (adenocarcinoma) – Categorization of undifferentaiated malignancies
• Bleeding & secondary infection-ulcerate to surface • DIC (leukemia, prostate) – Determination of site of origin (PSA)
• Initiation of acute symptoms-rupture or infarction – Detection of molecules that have prognostic or
• Cachexia – 10% body weight loss Grading and Staging therapeutic value (ERBB2/ HER2-neu)
• Grading-degree of differentiation of the tumor cells and the * Cannot see loss of polarity, invasion, in FNAB and PAP
Local and Hormonal Effects number of mitosis within the tumor
• Destruction of remaining tissue-pituitary adenoma- – Usually 4 grades 3. Flow cytometry – give 3D view
endocrinopathies ie hypofunction • Staging is based on size of primary lesion, extent of regional – Individual cell characteristics
• Obstruction & intussuception lymph nodes, & presence or absence of blood borne 4. Molecular diagnosis – FISH & PCR, to look for cancer genes
• Fatal hypoglycemia-Beta cell adenoma metastasis. – Diagnosis
• Hematuria-renal – 2 staging system: UICC (TNM) & AJCC – Prognosis
• Melena-GIT – Detection of residual disease
* Stage more clinically significant than grade – Hereditary disposition
Cancer Cachexia *spread & size is important
• Progressive loss of body fat & lean body mass
• Weakness, anorexia and anemia T stands for tumour - how far the primary tumour has grown
• Increased basal metabolic rate locally.
• Action of soluble factors-cytokines-TNF, IL-1, IFN-gamma, N stands for nodes - if the cancer has spread to the local
Leukemia Inhibitory Factor (LIF) lymph nodes.
M stands for metastases - if the cancer has spread to other
parts of the body.
Paraneoplastic Syndromes
s/sx no connection with neoplastic T0 – carcinoma in situ; do not invade yet
1. May present the earliest manifestation of an occult neoplasm N0 – no lymph node involved Tumor Markers*
2. May represent significant clinical problems and may even be M0 – no metastasis • HCG
lethal – choriocarcinoma
3. May mimic metastatic disease and therefore confound Example: stomach cancer • AFP
treatment T-1 means the primary tumour is still in the stomach wall. T-3 – HCC
means the primary tumour has grown right through the • CEA
4. Endocrinopathies stomach wall and T-4 means it is invading nearby structures – colon, pancreas
• Cushing syndrome (most common endocrine), such as the pancreas. • PSA, PAP
ectopic hormone production in lung – prostate
N-0 means there is no spread to lymph nodes. N-1 means that
5. Hypercalcemia (most common paraneoplastic) • NSE
some local lymph nodes are affected. N-2 means more
• Osteolysis – SCCL and neuroblastoma
extensive spread to local lymph nodes.
• Calcemic humoral substances (PTHRP) • CA 125
• NOT due to skeletal metastasis M-0 means there are no metastases. M-1 means that there – ovarian
6. Neuromyopathic syndromes are metastases to some other area of the body such as the • CA-19-9
liver or brain.
Prepared by: EGBautistaII
– colon, pancreas
• CA-15-3
– breast
Prepared by: EGBautistaII
S-ar putea să vă placă și
- L6-PATHO-Neoplasia (Sept2821)Document12 paginiL6-PATHO-Neoplasia (Sept2821)Erald PaderangaÎncă nu există evaluări
- Table of Genetic Disorders: Download A Copy of This Study GuideDocument11 paginiTable of Genetic Disorders: Download A Copy of This Study Guideerica perezÎncă nu există evaluări
- CH 7 Genetic and Pediatric Diseases (P. 243-272, Nature of Genetic Abnormalities Contributing To Human DiseaseDocument16 paginiCH 7 Genetic and Pediatric Diseases (P. 243-272, Nature of Genetic Abnormalities Contributing To Human DiseaseJustine HungÎncă nu există evaluări
- Patho A 1. 5 Hemodynamic Disorders (Bongat, 2015)Document12 paginiPatho A 1. 5 Hemodynamic Disorders (Bongat, 2015)Grant GarcesÎncă nu există evaluări
- Week 7. Renal Pathology Continued.Document9 paginiWeek 7. Renal Pathology Continued.Amber LeJeuneÎncă nu există evaluări
- WBC Lymph Node SpleenDocument12 paginiWBC Lymph Node Spleendr brijesh TiwariÎncă nu există evaluări
- ENDOCRINE PATHOLOGY WebpathDocument35 paginiENDOCRINE PATHOLOGY Webpathapi-3766657Încă nu există evaluări
- Patho Common Stuff - RobbinsDocument7 paginiPatho Common Stuff - RobbinsMaf BÎncă nu există evaluări
- Chapter 10 - Diseases of Infancy and ChildhoodDocument17 paginiChapter 10 - Diseases of Infancy and ChildhoodAgnieszka WisniewskaÎncă nu există evaluări
- Malassezia Furfur An-An Ap-Ap Naturally Found On The SkinDocument48 paginiMalassezia Furfur An-An Ap-Ap Naturally Found On The SkinNikki ValerioÎncă nu există evaluări
- Trans 1 - Cells As A Unit of Health and DiseaseDocument12 paginiTrans 1 - Cells As A Unit of Health and DiseaseCedrick BunaganÎncă nu există evaluări
- Systemic Response To InjuryDocument5 paginiSystemic Response To InjuryJohn Christopher LucesÎncă nu există evaluări
- 4.1d - Pathology of The Pituitary - Nov.10 - Dr. GalangDocument4 pagini4.1d - Pathology of The Pituitary - Nov.10 - Dr. GalangMiel Raphael AranillaÎncă nu există evaluări
- Lymphomas and Leukemias ChartDocument2 paginiLymphomas and Leukemias ChartPA2014Încă nu există evaluări
- Systemic Effects of Inflammation The Acute Phase ResponseDocument3 paginiSystemic Effects of Inflammation The Acute Phase ResponseJenward Hostallero100% (1)
- 3 Surgery - Mediastinum and PleuraDocument6 pagini3 Surgery - Mediastinum and PleuraCassey Koi FarmÎncă nu există evaluări
- Neoplasia I - RecordingDocument6 paginiNeoplasia I - RecordingIS99057Încă nu există evaluări
- Clinical Medicine - Lecture: - Topic: - DateDocument3 paginiClinical Medicine - Lecture: - Topic: - DateqselmmÎncă nu există evaluări
- Pathology Description/Buzz Words DiseaseDocument5 paginiPathology Description/Buzz Words Diseasebea manzanoÎncă nu există evaluări
- Herpes, Pox, Rhabdo, Arena VIRUSDocument7 paginiHerpes, Pox, Rhabdo, Arena VIRUSErnie G. Bautista II, RN, MDÎncă nu există evaluări
- Cell Injury & AdaptationDocument22 paginiCell Injury & AdaptationUmam LoyalÎncă nu există evaluări
- Female Genital TractDocument5 paginiFemale Genital Tractsarguss14100% (1)
- Robbins Ch. 18 Liver and Biliary Tract Review QuestionsDocument12 paginiRobbins Ch. 18 Liver and Biliary Tract Review QuestionsPA2014Încă nu există evaluări
- Pathology - Lab: Pathology of The HeartDocument8 paginiPathology - Lab: Pathology of The HeartRazel PerezÎncă nu există evaluări
- Pathology B - Gastrointestinal Tract (Esguerra, 2015)Document18 paginiPathology B - Gastrointestinal Tract (Esguerra, 2015)Ars MoriendiÎncă nu există evaluări
- Boards' Fodder: Histologic Bodies: Benjamin A. Solky, M.D., Jennifer L. Jones, M.D., and Clare Pipkin, M.DDocument2 paginiBoards' Fodder: Histologic Bodies: Benjamin A. Solky, M.D., Jennifer L. Jones, M.D., and Clare Pipkin, M.DAreg JosephsÎncă nu există evaluări
- Chapter 7 - NeoplasiaDocument23 paginiChapter 7 - NeoplasiaAgnieszka WisniewskaÎncă nu există evaluări
- Pancreatic Hormones by KatzungDocument3 paginiPancreatic Hormones by KatzungChristian DeeÎncă nu există evaluări
- Exam 1 DiseasesDocument1 paginăExam 1 DiseasesSolomon Seth SallforsÎncă nu există evaluări
- Cellular Injury, Adaptation and Cell DeathDocument8 paginiCellular Injury, Adaptation and Cell DeathJessica Febrina Wuisan100% (1)
- Cocci Rod 4 Main Classifications: Gram Staph, Strep Bacillus Clostridium Neisseria Pleiomorphic Enterobact-EriceaeDocument2 paginiCocci Rod 4 Main Classifications: Gram Staph, Strep Bacillus Clostridium Neisseria Pleiomorphic Enterobact-EriceaeKimberly KanemitsuÎncă nu există evaluări
- Chapter 11 Blood Vessels 8th Ed NotesDocument7 paginiChapter 11 Blood Vessels 8th Ed NotesKyle Christopher SiaÎncă nu există evaluări
- Diseases - BiochemDocument4 paginiDiseases - BiochemJay FeldmanÎncă nu există evaluări
- Robbins - 12 The HeartDocument5 paginiRobbins - 12 The HeartCM NajitoÎncă nu există evaluări
- Anti FungalsDocument5 paginiAnti FungalskakuÎncă nu există evaluări
- Mnemonics For Medical PG EntranceDocument146 paginiMnemonics For Medical PG EntranceAlejandroPelaezÎncă nu există evaluări
- Table of Genetic DisordersDocument9 paginiTable of Genetic DisordersjeslymailÎncă nu există evaluări
- Coins Pathology PDFDocument7 paginiCoins Pathology PDFAthena BorjaÎncă nu există evaluări
- Divergent Differentiation, Creating So-Called Mixed Tumors: Seminoma Are Used For Malignant Neoplasms. TheseDocument6 paginiDivergent Differentiation, Creating So-Called Mixed Tumors: Seminoma Are Used For Malignant Neoplasms. TheseSherwin Kenneth Madayag100% (1)
- Flash Notes SyndromesDocument8 paginiFlash Notes SyndromesschxzerrydawnÎncă nu există evaluări
- Heart - PathologyDocument22 paginiHeart - Pathologyjmosser100% (1)
- Adequacy Criteria: ExceptionsDocument3 paginiAdequacy Criteria: ExceptionsPranayÎncă nu există evaluări
- 01 StudyGuide CellAdaptandNec Latham 0820-22Document8 pagini01 StudyGuide CellAdaptandNec Latham 0820-22ivankcurryÎncă nu există evaluări
- Small Intestine 01 PDFDocument9 paginiSmall Intestine 01 PDFfadoÎncă nu există evaluări
- I. Cellular Adaptations:: Cellular Injury, Cell Adaptation & Cell Death 1. Hyperplasia 3. AtrophyDocument4 paginiI. Cellular Adaptations:: Cellular Injury, Cell Adaptation & Cell Death 1. Hyperplasia 3. AtrophyShuaib SiddiquiÎncă nu există evaluări
- Robbins Ch. 26 Bones Joints and Soft-Tissue Tumors Review QuestionsDocument7 paginiRobbins Ch. 26 Bones Joints and Soft-Tissue Tumors Review QuestionsPA2014100% (1)
- Gastrointestinal PathologyDocument14 paginiGastrointestinal PathologyRahul ShuklaÎncă nu există evaluări
- 2011-07-PATHO-Basic Pathology of SkinDocument17 pagini2011-07-PATHO-Basic Pathology of SkindtimtimanÎncă nu există evaluări
- Anemia Type Pathogenesis Clinical Manifestations Diagnosis Peripheral Blood Lab FindingsDocument15 paginiAnemia Type Pathogenesis Clinical Manifestations Diagnosis Peripheral Blood Lab FindingsDanielle FosterÎncă nu există evaluări
- Anemia and Hematologic Drugs - KatzungDocument4 paginiAnemia and Hematologic Drugs - Katzungsarguss14100% (1)
- Skin Summary ChartDocument7 paginiSkin Summary ChartMarco HernandezÎncă nu există evaluări
- Toxicology USMLE NotesDocument15 paginiToxicology USMLE NotesDuncan JacksonÎncă nu există evaluări
- 2021 Systemic Pathology S4T1 - RBC and Bleeding Disorders PDFDocument27 pagini2021 Systemic Pathology S4T1 - RBC and Bleeding Disorders PDFAlexis Bondad100% (1)
- Acute Myeloproliferative Acute Lymphoproliferative Chronic Myeloproliferative Chronic Lymphoproliferative Plasma Cell NeoplasmDocument1 paginăAcute Myeloproliferative Acute Lymphoproliferative Chronic Myeloproliferative Chronic Lymphoproliferative Plasma Cell NeoplasmAudreySlitÎncă nu există evaluări
- Pediatric Pathology: Disease Cause/Risk Factors SymptomsDocument12 paginiPediatric Pathology: Disease Cause/Risk Factors SymptomsherethemindÎncă nu există evaluări
- TOPNOTCH Patho Supplement Handout For Sept 2018 UPDATED May 2018Document25 paginiTOPNOTCH Patho Supplement Handout For Sept 2018 UPDATED May 2018Waiwit KritayakiranaÎncă nu există evaluări
- Oncologic Nursing: Naming of CancerDocument7 paginiOncologic Nursing: Naming of CancerDianne MaeÎncă nu există evaluări
- Module 5. NEOPLASIADocument19 paginiModule 5. NEOPLASIAKryss Renato Engel BartolomeÎncă nu există evaluări
- Temporary - BbbhbRegistration - Non Schedule 7A FTPDocument8 paginiTemporary - BbbhbRegistration - Non Schedule 7A FTPChethranÎncă nu există evaluări
- New Doc 2019-10-23 15.16.57 - 20191023151720Document1 paginăNew Doc 2019-10-23 15.16.57 - 20191023151720ChethranÎncă nu există evaluări
- AllergicDocument18 paginiAllergicChethranÎncă nu există evaluări
- 01 Integration of Primary Care and Oh ServicesDocument5 pagini01 Integration of Primary Care and Oh ServicesChethranÎncă nu există evaluări
- Radiology Finals Pointers As Noted by Samira VDocument3 paginiRadiology Finals Pointers As Noted by Samira VChethranÎncă nu există evaluări
- New Doc 2019-10-23 15.15.15 - 20191023151531Document1 paginăNew Doc 2019-10-23 15.15.15 - 20191023151531ChethranÎncă nu există evaluări
- Definitions:: Impotence Here Means Incapable of Having Sexual RelationDocument2 paginiDefinitions:: Impotence Here Means Incapable of Having Sexual RelationChethranÎncă nu există evaluări
- Rvrtv5tgt5Abnormal Uterine BleedingDocument4 paginiRvrtv5tgt5Abnormal Uterine BleedingChethranÎncă nu există evaluări
- SS Plastic - General Principles (2014)Document11 paginiSS Plastic - General Principles (2014)ChethranÎncă nu există evaluări
- Rvrtv5tgt5Abnormal Uterine BleedingDocument4 paginiRvrtv5tgt5Abnormal Uterine BleedingChethranÎncă nu există evaluări
- A GYNE PrelimsuyguvuybDocument3 paginiA GYNE PrelimsuyguvuybChethranÎncă nu există evaluări
- 255067, Hibuinoubounojnounoun852 Cvs Clinical NotesDocument38 pagini255067, Hibuinoubounojnounoun852 Cvs Clinical NotesChethranÎncă nu există evaluări
- Optha Midterm: Saturday, May 16, 2015 10:59 PMDocument7 paginiOptha Midterm: Saturday, May 16, 2015 10:59 PMChethranÎncă nu există evaluări
- BREECH 2nijnjinijnuihiuhDocument7 paginiBREECH 2nijnjinijnuihiuhChethranÎncă nu există evaluări
- Traumassee 2 Notes - 20171202083816850Document14 paginiTraumassee 2 Notes - 20171202083816850ChethranÎncă nu există evaluări
- Answer: FDocument8 paginiAnswer: FChethranÎncă nu există evaluări
- Differential Diagnosis: Adenocarcinoma of Colon FOBT Positive Black Stool May Be Metastasis Has Gone To Form Liver AbcessDocument3 paginiDifferential Diagnosis: Adenocarcinoma of Colon FOBT Positive Black Stool May Be Metastasis Has Gone To Form Liver AbcessChethranÎncă nu există evaluări
- My Note (2) BB Gubtuvuvt7Document19 paginiMy Note (2) BB Gubtuvuvt7ChethranÎncă nu există evaluări
- Sunuohouhouhafari - 28 Feb 2019 at 11:19 Ouh0uhhDocument1 paginăSunuohouhouhafari - 28 Feb 2019 at 11:19 Ouh0uhhChethranÎncă nu există evaluări
- Bakdjdjedjnk Receipt - 20180910223833350Document2 paginiBakdjdjedjnk Receipt - 20180910223833350ChethranÎncă nu există evaluări
- Is A Traditional Colonoscopy or A Virtual Colonoscopy BetterDocument1 paginăIs A Traditional Colonoscopy or A Virtual Colonoscopy BetterPaul JensenÎncă nu există evaluări
- Preboard Exam Np3 Medical Surgical NursingDocument19 paginiPreboard Exam Np3 Medical Surgical NursingBhie Bhie100% (1)
- The Comparison of Surgery and Chemo-Radio Therapy in Locally Recurrent Colorectal CancerDocument4 paginiThe Comparison of Surgery and Chemo-Radio Therapy in Locally Recurrent Colorectal CancerInternational Journal of Innovative Science and Research TechnologyÎncă nu există evaluări
- Colonoscopy For DummiesDocument79 paginiColonoscopy For Dummiesmike.krawulski9224100% (3)
- Colonoscopy GameDocument1 paginăColonoscopy Gamenefft13Încă nu există evaluări
- Wang 2020Document9 paginiWang 2020cifha rajesh saldanhaÎncă nu există evaluări
- Duodenal Epithelial Polyps: A Clinicopathologic ReviewDocument16 paginiDuodenal Epithelial Polyps: A Clinicopathologic ReviewsilvanaÎncă nu există evaluări
- CA PretestDocument37 paginiCA PretestJoanna TaylanÎncă nu există evaluări
- Technical SeminarDocument11 paginiTechnical SeminarJeevika KsÎncă nu există evaluări
- Colorectal Cancer 1Document71 paginiColorectal Cancer 1Anupam SisodiaÎncă nu există evaluări
- makpress Η ψεύτικη έρευνα για τον καρκίνο και την πρόποληDocument4 paginimakpress Η ψεύτικη έρευνα για τον καρκίνο και την πρόποληfilesandimagesÎncă nu există evaluări
- Virtual Colonoscopy Vs Conventional Colonoscopy in Patients at High Risk of Colorectal Cancer - A Prospective Trial of 150 PatientsDocument8 paginiVirtual Colonoscopy Vs Conventional Colonoscopy in Patients at High Risk of Colorectal Cancer - A Prospective Trial of 150 PatientsPari Pengda BaliÎncă nu există evaluări
- Colon Surgical Pathology - Clinical CaseDocument2 paginiColon Surgical Pathology - Clinical CaseMariana UngurÎncă nu există evaluări
- Colorectal TraumaDocument44 paginiColorectal TraumaicalÎncă nu există evaluări
- General Surgery Board RevieDocument69 paginiGeneral Surgery Board RevieFadi Alkhassawneh88% (8)
- American Cancer SocietyDocument41 paginiAmerican Cancer SocietyEvi UtariÎncă nu există evaluări
- FCM3-3.02 Philippine Cancer Control ProgramDocument11 paginiFCM3-3.02 Philippine Cancer Control ProgramJoher MendezÎncă nu există evaluări
- Rubin - Ovarian Cancer 2ed PDFDocument207 paginiRubin - Ovarian Cancer 2ed PDFfdroooÎncă nu există evaluări
- Colitis, Ulcerative: Whitney D. Lynch Ronald HsuDocument8 paginiColitis, Ulcerative: Whitney D. Lynch Ronald HsuAlexis DF SanchezÎncă nu există evaluări
- Problem: DOB ASSESSMENT S: "Hindi Siya MakahingaDocument13 paginiProblem: DOB ASSESSMENT S: "Hindi Siya MakahingauzumakiruleÎncă nu există evaluări
- Worldwide Burden of Colorectal Cancer: A ReviewDocument5 paginiWorldwide Burden of Colorectal Cancer: A ReviewRomário CerqueiraÎncă nu există evaluări
- Thesis Book SampleDocument7 paginiThesis Book Sampleelizabethsnyderdesmoines100% (1)
- Contrast Enema ExaminationDocument40 paginiContrast Enema ExaminationOscar NogueraÎncă nu există evaluări
- 1 s2.0 S235230421930100X Main PDFDocument14 pagini1 s2.0 S235230421930100X Main PDFLorena RamosÎncă nu există evaluări
- A Sigmoidoscopy Only Examines Up To The Sigmoid. The Most Distal Part of The Colon, While Colonoscopy Examines The Whole Large BowelDocument5 paginiA Sigmoidoscopy Only Examines Up To The Sigmoid. The Most Distal Part of The Colon, While Colonoscopy Examines The Whole Large BowelMa. Ydela MeradoresÎncă nu există evaluări
- Colonic Inertia With Tortuous Colon Is Surgery MyDocument18 paginiColonic Inertia With Tortuous Colon Is Surgery MyMisa AjversonÎncă nu există evaluări
- Sleisenger IndiceDocument4 paginiSleisenger IndiceMario SchettinoÎncă nu există evaluări
- AGA Clinical Practice Update On Management of Inflammatory Bowel Disease in Elderly Patients Expert ReviewDocument22 paginiAGA Clinical Practice Update On Management of Inflammatory Bowel Disease in Elderly Patients Expert Reviewinfinity TangÎncă nu există evaluări
- Colon, Rectum and Anus-Dr. SigitDocument121 paginiColon, Rectum and Anus-Dr. SigitYuliana Latif100% (1)
- Dr. Ali's Uworld Notes For Step 2 CKDocument30 paginiDr. Ali's Uworld Notes For Step 2 CKuyesÎncă nu există evaluări