Documente Academic
Documente Profesional
Documente Cultură
Chapter 147:: Vascular Malformations:: Laurence M. Boon, Fanny Ballieux
Încărcat de
Jhauharina RfTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Chapter 147:: Vascular Malformations:: Laurence M. Boon, Fanny Ballieux
Încărcat de
Jhauharina RfDrepturi de autor:
Formate disponibile
22 Chapter 147 :: Vascular Malformations
:: Laurence M. Boon, Fanny Ballieux,
& Miikka Vikkula
VASCULAR common; see Chap. 118), and (2) vascular malforma-
tions (structural anomalies of blood vessels) that are
MALFORMATIONS subsequently subdivided, depending on the affected
vessel type, into arterial, capillary, lymphatic, or
venous malformations (VMs). In 1996, this classifica-
tion was adopted and further developed by the Inter-
Part 22
AT-A-GLANCE
national Society for the Study of Vascular Anomalies
■
(ISSVA; Table 147-1).4
The worldwide prevalence is roughly 0.3%, mostly
Vascular malformations mostly affect a single ves-
accounted for by capillary malformations.
::
sel type (Fig. 147-1), yet combined malformations
■ Congenital, localized (although sometimes
Vascular Diseases
also exist. They are named according to the affected
extensive or multifocal), and well-demarcated
vessel types, capillary-venous or venolymphatic
lesions of malformed vessels of various types:
malformation, for instance. In addition to isolated
capillary, venous, lymphatic, arteriovenous, and
forms, vascular malformations occur in syndromes
combined
such as Klippel-Trenaunay syndrome (KTS, capillary-
■ Histologically consist of enlarged, tortuous vessels lymphatic-VM with limb hypertrophy), Maffucci
of various types. syndrome (multiple enchondromas associated with
■ Caused by inherited or somatic mutations in multiple venous anomalies and high incidence of
various gene malignancy), CLOVES (congenital lipomatous over-
■ Can be isolated, combined, or part of a syndrome growth with vascular malformations, epidermal nevi,
■ Management: multidisciplinary approach and spinal or skeletal anomalies or scoliosis) syn-
drome, or Parkes Weber syndrome (high-flow vas-
cular malformation of the extremity with soft tissue
hypertrophy).4
INTRODUCTION
DEFINITION EPIDEMIOLOGY
Vascular malformations are believed to arise because Vascular malformations affect about 0.3% of the
of errors in the development of vessels that occur dur- population with most of these being capillary malfor-
ing the 4th to 10th weeks of intrauterine life. Most mations (CMs). Vascular malformations are mostly
vascular malformations are sporadic, although several congenital, even though they may be diagnosed later
families with inherited forms have been identified. in life.5
They are very heterogeneous.1
CLASSIFICATION
For many years, vascular anomalies were grouped CLINICAL FEATURES
under the term angioma, hampering precise classifica-
tion and leading to incorrect diagnosis and improper Vascular malformations grow proportionately with
management. For example, the term hemangioma has the patient. Usually they do not regress. Most fre-
been used both for vascular malformations, often quently, they are well-demarcated and localized.
venous (cavernous hemangioma), as well as for vas- They can affect any part of the body, including the
cular tumors (strawberry hemangioma). This nomen- viscera. In rare instances, they can be the stigmata
clature changed in 1982 with the development of a of deep lesions or the first sign of a syndrome. Vas-
biologic classification by Mulliken and Glowacki.2,3 cular malformations are rheologically divided into
This classification system organized vascular anoma- slow flow (capillary, lymphatic, venous, and com-
lies based on clinical, hemodynamic, radiologic, and bined) and fast flow (arterial, arteriovenous, and
histologic features. It divided vascular anomalies combined) (Table 147-2).4 They can lead to esthetic
into two major categories: (1) vascular tumors (with or functional impairment or even threaten life in
cellular proliferation, hemangioma being the most rare instances.6
Kang_CH147_p2636-2668.indd 2636 08/12/18 3:08 pm
TABLE 147-1 DIAGNOSIS
22
Classification of Vascular Anomalies
The diagnosis is usually based on clinical features in
VASCULAR TUMORS VASCULAR MALFORMATIONS 90% of superficial malformations.
Hemangiomas Capillary
Infantile hemangioma Capillary malformation (CM, also called
port-wine stain)
SUPPORTIVE STUDIES
Congenital hemangioma Telangiectasia (hereditary benign telan- Pathology: Histologically, vascular malforma-
giectasia, essential telangiectasia) tions consist of enlarged, tortuous vessels with quies-
Rapidly-involuting Hereditary hemorrhagic telangiectasia cent endothelium. In contrast to hemangioma, there
congenital (HHT) is neither a parenchymal mass nor overt cellular
hemangioma (RICH) proliferation.
Noninvoluting congenital Capillary malformation–arteriovenous
Imaging: Radiologic investigation is needed to delin-
Chapter 147 :: Vascular Malformations
hemangioma (NICH) malformation (CM-AVM)
eate the extent of a malformation but rarely for diagno-
Sturge-Weber syndrome
sis unless the malformation is deeply located. Doppler
Hemangioendothe- Venous ultrasonography is a very useful noninvasive radiologic
liomas examination that provides clues for differentiating the
Kaposiform hemangioen- Venous malformation (VM) various types. Magnetic resonance imaging (MRI) details
dothelioma the extension and precise location of the lesion.16
Tufted angioma Familial form: cutaneo-mucosal venous
malformation (VMCM)
Genetic Testing: Because a germline or somatic
genetic cause is known for a large number of vascular
Glomuvenous malformation (GVM)
anomalies, genetic testing can be used to confirm or
Blue rubber bleb nevus or Bean help make the precise diagnosis. For somatic mutations,
syndrome (BRBN)
a biopsy of the affected tissue is needed. For inherited
Angiosarcoma Lymphatic mutations, a blood test is sufficient to make the diagnosis.
Lymphatic malformation (LM)
Primary Lymphedemas
Arterial
MANAGEMENT
Arteriovenous malformation (AVM)
Capillary malformation–arteriovenous Treatment of vascular malformations depends on the
malformation (CM-AVM) affected vessel type, the location of the lesion, the
Arteriovenous fistula (AVF) age of the patient, and the symptoms. Because many
Syndromic Malformations lesions are extensive, patients should be aware that a
complete cure is often not possible and that recurrence
Slow flow
occurs. Treatment can be difficult and with severe com-
Klippel-Trenaunay syndrome (capillary– plications. Extensive or complex lesions should always
lymphatic–venous malformation with
be managed by a multidisciplinary team.
limb hypertrophy)
Maffucci syndrome
CLOVES (congenital lipomatous over-
growth with vascular malformations, CAPILLARY
epidermal nevi, and spinal or skeletal
anomalies or scoliosis) syndrome MALFORMATIONS
Fast flow
Parkes Weber syndrome AT-A-GLANCE
■ Worldwide occurrence. Prevalence is roughly 0.3%.
■ Congenital, slow-flow malformations of the capil-
lary bed
ETIOLOGY AND ■ Pinkish-red to purple in color; tend to darken and
thicken with time
PATHOGENESIS ■ Can be part of a sporadic syndrome, such as
Sturge-Weber syndrome or KTS
Several genes have been identified to be mutated ■ Can be part of the inherited capillary malformation-
in the inherited forms of vascular malformations arteriovenous malformation phenotype
(Table 147-3). Somatic genetic mutations have also
■ Pathology: capillaries that are increased in size and
been unraveled as the cause of many common spo- 2637
number with abnormal innervation.
radic lesions.7-15
Kang_CH147_p2636-2668.indd 2637 08/12/18 3:08 pm
22
A
B
Part 22
::
Vascular Diseases
C E
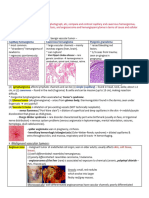
Figure 147-1 Vascular malformations of various vessel types. A, Capillary malformation of left lower extremity and genita-
lia. B, Subcutaneous lymphatic malformation invading the cubital nerve. C, Extensive venous malformation of right lower
extremity, causing pain, swelling, and coagulopathy. D, Arteriovenous malformation of the sole. E, Nidus of the lesion on
arteriography.
Capillary malformations mainly occur sporadically, Although most often an isolated finding, in rare
although there are well-documented pedigrees show- instances, CM can be the cutaneous hallmark of occult
ing autosomal dominant inheritance.17 When inherited, spinal dysraphism, especially if located in the lumbo-
they are usually multiple and part of the capillary mal- sacral area.24 Other blanchable, pink patches, known
formation–arteriovenous malformation (CM-AVM) as stork bite, angel’s kiss, salmon patch, nevus simplex, or
phenotype, which associates atypical CMs with arte- nevus flammeus neonatorum, are often confused with
riovenous malformation (AVM, see Arteriovenous CM. They are located on the nape of the neck (81%),
Malformations later).18-23 the eyelids (45%), or the glabella (33%).25 When located
on the occiput, the moniker Unna nevus may be used
(Fig. 147-2). These patches have a much higher inci-
dence (42%) in white infants than in black infants
EPIDEMIOLOGY (31%).26 They are also present in various syndromes,
such as Beckwith-Wiedemann and Rubinstein-Taybi
CMs, commonly called port-wine stain, is a slow-flow syndromes. These lesions (with the exception of the
2638 vascular malformation with a prevalence of 0.3%.5 Unna nevus) disappear spontaneously around the age
There is no sex preponderance. of 1 to 4 years. In contrast, true CMs persist lifelong.
Kang_CH147_p2636-2668.indd 2638 08/12/18 3:08 pm
TABLE 147-2
22
Clinical Characteristics of Vascular Malformations
CAPILLARY VENOUS LYMPHATIC ARTERIOVENOUS
Skin color Red Normal-bluish-purple Normal to yellowish-purple Normal to red
Aspect Flat Flat to raised Raised to vesicular Flat to raised
Temperature Normal Normal Normal Warm
Palpation Normal Compressible Firm, noncompressible Thrill, bruit
Associations — Phleboliths, deformation — Ulceration, deformation
Radiology No flow Slow flow Slow flow, cyst Fast flow
Histology Dilated capillaries Thin-walled venous channels Cystic lymphatic channels Arterialized veins
D2-40 negative D2-40 negative D2-40 positive D2-40 negative
Chapter 147 :: Vascular Malformations
Etiology See Table 147-3
Treatment Pulsed-dye laser Sclerotherapy, surgery, Surgery, sclerotherapy, Embolization followed by surgical resection
sirolimus medication sirolimus medication of nidus
SYNDROMIC DISORDERS DIFFUSE CAPILLARY
MALFORMATION WITH
CMs can be part of a syndrome, such as phako- OVERGROWTH
matosis pigmentovascularis (PPV), Sturge-Weber
syndrome (SWS), KTS, Parkes Weber syndrome, Patients with DCMO have hemihypertrophy, which
CLOVES syndrome, PTEN (phosphatase and ten- can be total, regional, or contralateral. CM can be dif-
sin homolog) Hamartoma tumor syndrome, diffuse fuse over the entire body. This entity is characterized
capillary malformation with overgrowth (DCMO), by a reticulated, ill-defined CM.30 The lesions do not
and macrocephaly–capillary malformation (M-CM, follow the lines of Blaschko (Fig. 147-5A).
also called megalencephaly–capillary malformation–
polymicrogyria syndrome). None of these syndromes
is inherited. MACROCEPHALY–CAPILLARY
MALFORMATION
PHAKOMATOSIS A well-delineated, dark CM of the vermillion bor-
der, the tip of the nose, or both associated with mac-
PIGMENTOVASCULARIS rocephaly is often pathognomonic of M-CM. These
PPV is thought to be an embryogenic anomaly affect- patients have megalencephaly and are at risk of mental
ing the vasomotor nerves and the melanocytes, both retardation.31 There are two reports of M-CM associ-
derived from neural crest. It manifests as a large, meta- ated with Wilms tumor in the literature.32
meric CMs, usually located on the trunk or the extrem-
ities, in association with pigmented cutaneous lesions,
such as a pigmented nevus, a nevus spilus, a café-
au-lait patch, or an atypical Mongolian spot (see CLINICAL FEATURES
Chap. 77) that is not located on the sacrum (Fig. 147-3).27
Nevus anemicus can also be seen in the vicinity as a
twin spot.28 CUTANEOUS FINDINGS
CM is a red, homogenous, congenital lesion that is
STURGE-WEBER SYNDROME often unilateral, sometimes bilateral, but usually not
median. CMs involve skin and subcutis and sometimes
When located in the frontopalpebral area, CM can be mucosa (see Fig. 147-5). Their color varies from pink-
part of SWS (Fig. 147-4A). This neuro-oculo-cutaneous ish-red to deep purple with a geographic contour or a
syndrome associates a cutaneous CM of the ophthal- dermatomal distribution. Lesions are flat and painless,
mic branch of the trigeminal nerve (V1) with a homo- do not bleed spontaneously, and are never warm on
lateral leptomeningeal capillary-venous malformation palpation in contrast to AVM (see later). Acquired CM
(CVM) and a choroid CVM. Glaucoma is often pres- can be seen after trauma.33
ent (Fig. 147-4B). SWS is associated with a high risk of Fifty percent of CMs are located on the face, where
epilepsy and mental retardation because of anomalies they follow the distribution of the trigeminal nerve:
of the venous drainage of the encephalon, as well as ophthalmic branch V1 (front and upper eyelid), max-
with glaucoma, buphthalmos, and sometimes retinal illary branch V2 (lower eyelid, cheek, and upper 2639
detachment.29 lip) (see Figs. 147-4A and 147-5B), or mandibular V3
Kang_CH147_p2636-2668.indd 2639 08/12/18 3:08 pm
22 TABLE 147-3
The Genetic Causes of Vascular Anomalies
SPORADIC MALFORMATIONS INHERITED MALFORMATIONS
DIAGNOSIS MUTATED GENE DIAGNOSIS MUTATED GENE
Capillary Malformations (CMs)
CM GNAQ CM-AVM1 RASA1
Sturge-Weber syndrome CM-AVM2 EPHB4
Phakomatosis pigmentovascularis (PPV) GNAQ or GNA11
Macrocephaly–capillary malformation (M-CM) AKT3, PIK3CA, PIK3R2
Venous Malformations (VMs)
VM TIE2 (60%) Mucocutaneous venous malformations TIE2
Part 22
PIK3CA (20%) (VMCM)
Blue rubber bleb nevus (BRBN) TIE2 Glomuvenous malformation (GVM) GLOMULIN
Multifocal venous malformation (MVM)
::
Verrucous venous malformation (VVM) MAP3K3
Vascular Diseases
Maffucci syndrome IDH1, IDH2
Lymphatic Malformations (LMs)
LM PIK3CA Nonne-Milroy lymphedema VEGFR3
CLOVES (congenital lipomatous overgrowth with Nonne-Milroy–like lymphedema VEGF-C
vascular malformations, epidermal nevi, and spinal or
skeletal anomalies or scoliosis)
Klippel-Trenaunay syndrome (KTS) Lymphedema distichiasis FOXC2
Microcephaly, chorioretinopathy, lymph- KIF11
edema, mental retardation
Hypotrichosis–lymphedema–telangiectasia SOX18
Hennekam lymphedema–lymphangiectasia 1 CCBE1
Hennekam lymphedema–lymphangiectasia 2 FAT4
Hennekam lymphedema–lymphangiectasia 3 ADAMTS3
Emberger syndrome GATA2
Fetal chylothorax ITGA9
Lymphedema–choanal atresia PTPN14
Four-limb lymphedema GJC2 (CX47)
Arteriovenous Malformations (AVMs)
Extracranial AVM MAP2K1 Hereditary hemorrhagic telangiectasia (HHT)1 ENG
Brain AVM KRAS HHT2 ACVRL1 (ALK1)
HHT3 SMAD4, GDF2
Phosphatase and tensin (PTEN) homolog PTEN
(protein encoded by PTEN gene)
(lower lip, chin, and mandible). CM can be diffuse with the child and are asymptomatic. Bier spots,
over the entire body, often with associated hemi- white cutaneous spots surrounded by a pale halo of
hypertrophy (see Fig. 147-5A). This entity is called redness, are seen in both entities. In contract to CM-
diffuse capillary malformation with overgrowth (see ear- AVM1, CMs in CM-AVM2 can be more telangiectatic
lier discussion). in appearance, especially periorally and on the upper
When inherited, CMs are usually small and multifo- thorax.23 CM-AVM1 is characterized by the presence
cal, such as in CM-AVM1 and 2. These small CMs vary of CMs in 97%, AVMs or arteriovenous fistulas (AVFs)
in size from a couple mm to several centimeters in in 24% (10% intra–central nervous system [CNS], 13%
size. They are often round-to-oval, pink, red, or brown extra-CNS), and Parkes Weber phenotype in 8% of
in color and are often surrounded with a pale halo patients.21,35 In CM-AVM2, the risk of a fast-flow lesion
(Fig. 147-6).18,34 The lesions are well-circumscribed and seems to be less frequent because the respective pen-
2640 blanch on pressure with a diascopy. Some of them are etrances are 93% (CM), 19% (AVM), and 7% (Parkes
present at birth, but others appear later. They grow Weber).
Kang_CH147_p2636-2668.indd 2640 08/12/18 3:08 pm
22
Chapter 147 :: Vascular Malformations
A B
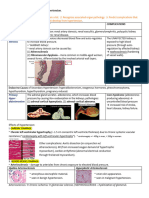
Figure 147-2 Nevus flammeus neonatorum (also called nevus simplex) on the glabella, upper eyelids, and upper lip (A)
of a 6-week-old neonate who also has an Unna nevus on the occiput (B).
NONCUTANEOUS FINDINGS SWS is associated with a high risk of epilepsy and
mental retardation because of anomalies of the venous
In rare instances, CM can be the stigmata of an under- drainage of the encephalon, as well as with glaucoma,
lying anomaly such as lumbar dysraphism underneath buphthalmos, and sometimes retinal detachment.29
a sacral CM. Cutaneous lesions of PPV can be associ- Patients with DCMO have hemihypertrophy, which
ated with systemic, visceral (hypoplasia of the larynx, can be total, regional, or contralateral. They have also
intestinal polyposis), muscular (scoliosis), or neuro- associated toe anomalies (in 30%) and prominent
logic (mental retardation, epilepsy, intracranial) signs veins.30 In CM-AVM1 or 2, there is a 20% to 30% risk
and symptoms in 60% of cases.36 of an associated AVM located on the skin, the brain,
or the spine.21,35 A well-delineated dark CM of the ver-
million border or the tip of the nose associated with
macrocephaly is often pathognomonic of M-CM, and
these patients have megalencephaly and are at risk of
mental retardation.31
COMPLICATIONS
The major concern for a patient with a CM is cosmetic
because of the visible discoloration. Hypertrophy of
soft (often lips and gums) and underlying hard tis-
sues can occur with time, especially when the CM
affects the V2 and V3 dermatomes or the extremities
(Fig. 147-7D and 147-7). Overgrowth of the maxilla or
mandible leads to skeletal asymmetry, occlusal tilt, and
open-bite deformity. When the child has atopic derma-
titis, psoriasis, or acne, lesions are worse in the area of
CM, a finding known as the Meyerson phenomenon.37
Evenly thickened skin, purple nodules, and pyogenic
granulomas can develop by adolescence (Fig. 147-7B
and 147-7C).38,39
ETIOLOGY AND
Figure 147-3 Clinical characteristics of phakomatosis pig- PATHOGENESIS
mentovascularis. A large metameric capillary malforma-
tion with atypical extensive dermal melanocytosis located Facial CM is thought to be caused by clonal expansion 2641
on the back. of abnormal cells originating from the neural crest.40
Kang_CH147_p2636-2668.indd 2641 08/12/18 3:08 pm
22 Part 22
A B
Figure 147-4 Extensive right facial capillary malformation. A, Involving the ophthalmic and maxillary branches of the
::
trigeminal nerve in a 6-month-old girl with Sturge-Weber syndrome. B, Choroid capillary–venous malformation causing
Vascular Diseases
vision loss in a 50-year-old man with Sturge-Weber syndrome.
Somatic activating mutations in GNAQ have been
identified as the cause of facial CM with hypertrophy DIAGNOSIS
as well as of SWS.13 GNAG is a q class of G-protein
α subunits that mediates signals between G protein–
coupled receptors and their downstream effectors.
SUPPORTIVE STUDIES
Mutations in GNAQ and GNA11 have also been identi- Pathology: Histologically, CM is characterized by
fied in pigmented lesions such as blue nevi, nevus of dilated capillaries of the papillary and upper reticular
Ota, uveal melanoma, and PPV.41,42 dermis combined with areas of increased number of
The inherited CM-AVM1 is caused by inactivating normal-looking capillaries (Fig. 147-8).44 Endothelial
mutations in RASA1.21 This gene encodes a GTPase- cells are flat. Factor VIII, fibronectin, and basement
activating protein, which negatively regulates Ras membrane protein are normal, but S100 staining shows
activity. CM-AVM2 is caused by inactivating muta- abnormal innervation.45,46
tions in EPHB4.23 EPHB4 is usually expressed in
venous endothelial cells and its ligand EPHINB2 on
arterial endothelial cells. They regulate arteriovenous Imaging: No imaging studies are mandatory for
identity. EPHB4 interacts with RASA1 to regulate RAS- a CM except in rare situations. If a so-called “CM”
MAPK signaling, and loss of EPHB4 or RASA1 in CM- is painful, warm, or spontaneously bleeds, Doppler
AVM likely activates this signaling pathway. M-CM is ultrasound is indicated to exclude the diagnosis of a
caused by activating mutations in AKT3, PIK3CA, and fast-flow malformation, such as an AVM, Parkes Weber
PIK3R2.43 syndrome, or a proliferating hemangioma.
A B C
2642 Figure 147-5 Several aspects of capillary malformations. A, Light red lesions involving half of the body. B, Dark red lesions
on the face with mucosal involvement and soft tissue hypertrophy. C, More localized on the shoulder and arm.
Kang_CH147_p2636-2668.indd 2642 08/12/18 3:08 pm
22
A B
Chapter 147 :: Vascular Malformations
C D
Figure 147-6 A–D, Examples of small atypical capillary malformations which belong to CM-AVM (capillary malformation-
arteriovenous malformation). D, Note the pale halo surrounding the lesion.
CM located in the frontopalpebral area, especially RASA1 and EPHB4. Pale, uncharacteristic, and incon-
if the inner part of the upper eyelid is involved, can spicuous CMs can be sign of PHTS, especially if these
be part of SWS. Therefore, for these CMs, an ophthal- vascular lesions are associated with macrocephaly (see
mologic and neurologic examination is mandatory. AVM later). Because of increased cancer risk, such
They need to be done during the first months of life patients should be screened for PTEN mutations. Iden-
and repeated once a year until puberty, even if normal tification of a germline mutation would enable precise
at younger age.47 A brain MRI should also be done counseling and surveillance.
to evaluate the occurrence of associated leptomen-
ingeal CVM. In patients with PPV, ophthalmologic,
neurologic, and orthopedic follow-up is mandatory
because of the common association of CM with sys- DIFFERENTIAL DIAGNOSIS
temic lesions. In patients with extensive CMs of the
lower extremity, such as in DCMO, a scaniometry See Table 147-4.
study (leg length films) is needed to evaluate pos-
sible progressive growth discrepancy around the age
of 4 to 6 years and needs to be repeated until the end
of growth to allow the orthopedist to correct the CLINICAL COURSE
discrepancy at the adequate time. Serial renal ultra-
sound examinations are also indicated to rule out
AND PROGNOSIS
the appearance of a Wilms tumor. MRI of the spinal
cord is indicated in the presence of a lumbosacral CM is present at birth and never regresses spon-
CM. Brain and spinal MRI should be considered in taneously. During the first weeks of life, they can
patients with CM-AVM. slightly fade, as the hemoglobin level of the newborn
decreases. Subsequently, the red hue stabilizes, and
Genetic Testing: Genetic testing is indicated, the lesion grows in proportion to the rest of the body.
especially in patients with multifocal lesions (CM- Around puberty, as well as later in life, CM slowly
AVM1 and 2) because of increased risk for intracerebral thickens and darkens with time. It often becomes 2643
fast-flow lesions. These patients should be screened for raised and nodular (see Fig. 147-7).38 Pyogenic
Kang_CH147_p2636-2668.indd 2643 08/12/18 3:08 pm
22 Part 22
A B C
::
Vascular Diseases
D E
Figure 147-7 Clinical evolution of capillary malformation: darkening and thickening with time. A, At age 6 months. B, At
age 33 years. C, Development of pyogenic granuloma. D, Soft tissue hypertrophy. E, Mucosal hypertrophy.
granuloma can occur with time, as well as soft tissue Procedures: Laser treatment is the gold standard
or bony hypertrophy.39 therapy for most CM. Pulsed-dye laser with its specific
wavelength (585 or 595 nm) and a short pulse duration
(400–1500 ms) currently gives the best results in infants
and children by lightening the red color of the CM.
MANAGEMENT There are few complications. Multiple sessions (6–12) are
needed, and general anesthesia may be necessary as the
The goal of management is to monitor for associated procedure is painful. Laser treatment is more efficient on
complications and minimize the impact of the CM the cervicofacial and trunk area than on the extremities.
on self-esteem and quality of life. Camouflage using Early treatment during childhood does not reduce the
a foundation such as Covermark is still an effectively number of laser sessions.48 The use of a dynamic cool-
approach (Fig. 147-9) ing system to avoid heating the epidermis allows higher
laser fluencies, resulting in optimal lightening of the
lesion. Recurrence can occur after cessation of therapy.49
Laser has no impact on associated hypertrophy.6,50
INTERVENTIONS Contour resection is considered to treat complications
such as pyogenic granuloma or lip hypertrophy.6
Orthopedic Considerations: In the presence
of leg-length discrepancy, an early-adapted shoe lift Counseling: Genetic and psychological counsel-
is needed if leg-length discrepancy is over 1.5 cm to ing for the patients and their families are part of mul-
prevent compensatory tilting of the pelvis. Epiphysio- tidisciplinary management. Patient organizations
2644 desis is sometimes necessary when the child is approx- dedicated to vascular anomalies also have an impor-
imately 11 to 13 years old. tant role in supporting patients and their families.
Kang_CH147_p2636-2668.indd 2644 08/12/18 3:08 pm
22
Chapter 147 :: Vascular Malformations
A B
Figure 147-8 A, Extensive capillary malformation of upper extremity. B, Histologically, capillary malformation is charac-
terized by dilated capillaries of normal number in the papillary and upper reticular dermis in combination with areas of
increased number of normal-looking capillaries (hematoxylin and eosin staining).
reducing visual impairment will depend on the
PREVENTION promptness of treatment. Prophylactic antiepileptic
medication to prevent neural cell death has also been
In patients with SWS, ophthalmologic follow-up, advocated, although randomized prospective studies
started immediately after birth is essential because to support efficacy are lacking at this time.51,52
A B
2645
Figure 147-9 Efficient noninvasive management of facial capillary malformation using cosmetic makeup.
Kang_CH147_p2636-2668.indd 2645 08/12/18 3:08 pm
22 TABLE 147-4 ■ Congenital slow-flow malformation of the venous
Differential Diagnosis of Capillary bed
Malformation ■ Bluish in color, localized or extensive, solitary or
multifocal
Based on Morphology
■ Compressible on palpation; presence of phleboliths
Most Likely
Faint pink median patch ■ Associated with consumptive coagulation abnor-
■ Unna nevus malities in about 40% of cases
■ Nevus flammeus neonatorum (nevus simplex) ■ Histologically consists of ectatic venous-like chan-
Multiple, inherited
nels with anomalies in mural cells
■ CM of CM-AVM
Telangiectatic patch ■ Mainly sporadic but can be inherited as an autoso-
■ CMTC mal dominant trait
■ HHT ■ Inherited venous anomalies: cutaneomucosal
■ CM-AVM2
Part 22
venous malformation (1%), glomuvenous malfor-
■ Essential telangiectasia mation (>5%)
■ Unilateral nevoid telangiectasia
■ Syndromic venous malformation: KTS, blue rubber
Warm on palpation
■ Proliferating hemangioma bleb nevus, Maffucci syndrome.
::
■ AVM
Vascular Diseases
■ AVF
Ulcerated or painful
■ AVM
■ AVF INTRODUCTION
Rule Out
Small, multiple
A VM is a congenital lesion made up of venous-type
■ Mastocytosis
vessels of the skin or mucosa but can involve any
■ CM of CM-AVM
structure (subcutis, muscles, bones, and nerves) and
Based on Location
any organ (CNS, gastrointestinal [GI] tract). Fifty per-
Most Likely
cent of VMs are located in the cervicofacial area, and
Occiput
37% are on the extremities. VMs are mainly isolated
■ Unna nevus
Glabella, upper eyelid, nape of neck but can be part of complex vascular disorders, such as
■ Stork bite, angel’s kiss, salmon patch the KTS, Maffucci, and blue rubber bleb nevus (BRBN)
Consider syndromes.53,54
Extremity
■ CMTC
■ Klippel-Trenaunay syndrome
■ Parkes Weber syndrome
Faint on trunk EPIDEMIOLOGY
■ M-CM
Rule Out Venous anomalies are the most common VMs
Scalp referred to specialized center with an overall inci-
■ Adams-Oliver syndrome dence of 1 in 10,000 in the population.54 They mainly
■ Multiple telangiectasia near canthus occur sporadically, although 1% are inherited muco-
■ Ataxia telangiectasia
cutaneous venous malformations (VMCMs) and 5%
AVF, arteriovenous fistula; AVM, arteriovenous malformation; CM, cap- are inherited glomuvenous malformations (GVMs).
illary malformation; CM-AVM, capillary malformation-arteriovenous No sex preponderance is reported.55 Both VMCM
malformation; CMTC, cutis marmorata telangiectatica congenita; HHT, and GVM have an age-dependant variation in pen-
hereditary hemorrhagic telangiectasia; M-CM, macrocephaly capillary
etrance, which reaches its maximum by 20 years of
malformation.
age (87% for VMCM and 92.7% for GVM).56 Large
VMCMs and GVMs are present at birth. However,
17% of affected individuals develop new small
lesions over time.55 BRBN syndrome is rare and
VENOUS ANOMALIES
occurs sporadically with about 200 cases reported in
the literature.
AT-A-GLANCE
■ Most common referral to specialized centers for
SYNDROMIC DISORDERS
vascular anomalies; incidence unknown but is
substantially lower than for CM and estimated at Syndromes with a venous anomaly include BRBN syn-
2646 1 in 10,000 drome, KTS (see Lymphatic Malformations later), and
Maffucci syndrome.
Kang_CH147_p2636-2668.indd 2646 08/12/18 3:08 pm
CLINICAL FEATURES
22
The features of venous anomalies are summarized in
Table 147-5.
CUTANEOUS FINDINGS
VMs are usually solitary but can be multifocal, the
latter suggesting an inheritable disorder in most
instances.54 They may be relatively localized or
extensive within an anatomic region. The most com-
Figure 147-10 Blue rubber bleb nevus syndrome. Pathog- mon location is the head and neck area (Fig. 147-12A
nomonic multiple small, dark blue, nipple-like lesions on and 147-12D–F). VMs can affect any tissue or organ
the toe.
Chapter 147 :: Vascular Malformations
(Figs. 147-12 to 147-14).
VMs are congenital, light to dark bluish lesions. Deep
VM has a normal overlying skin color and is often diag-
BLUE RUBBER BLEB NEVUS nosed only at puberty or later in life with the onset of
pain. Size varies from small spongy blebs to large lesions
SYNDROME of several centimeters in diameter. Skin temperature is
BRBN is characterized by numerous malformations normal. There is no thrill or bruit. VMs are larger when
of the venous system that involve the skin and vis- in a dependent position and can easily be emptied by
ceral organs. It is commonly characterized by one compression or facilitating venous drainage. Palpation
large “dominant” VM lesion associated with multiple is not painful unless thrombosis occurs.54,59
small, dark blue, nipple-like lesions, the latter being VM lesions in VMCM are small and multifocal. They
typically located on the palm and soles (Fig. 147-10).15 are more reminiscent of small sporadically occurring
These cutaneous lesions are associated with mul- VMs than BRBNs. They can enlarge with time and
tiple small GI VMs, often responsible for chronic are often asymptomatic. The VMs are generally small
anemia.15,57 (<5 cm), and more than 80% of patients have more than
two lesions. Some are present at birth; most lesions
appear by puberty. Mucosal lesions involve the lips,
MAFFUCCI SYNDROME tongue, and buccal mucosa.60,61
In rare instances, VM can be sporadic yet multiple.
Maffucci syndrome is a rare disorder characterized These multifocal venous malformations (MVMs) are
by multiple enchondromas associated with subcu- very similar to the VMCM lesions, yet there is no fam-
taneous VMs of the distal extremities (Fig. 147-11). ily history of the same findings.15
The disease starts during childhood with the devel- GVM is a bluish to purple, raised venous anomaly
opment of enchondromas of the bones of hands and characterized by multifocality, hyperkeratosis, and nod-
feet, as well as of the long bones. Deformities and ularity with a cobblestone surface (see Fig. 147-13C). In
shortening of extremities often occur. Subcutane- rare cases (especially in newborns), the lesions may be
ous vascular nodules appear later, around puberty, flat and purple in color, mimicking CM62; this plaque-
on the fingers and the toes. Phleboliths may become like GVM usually darkens with time. GVMs are usually
present.58 present at birth and slowly expand during childhood.
A B
Figure 147-11 Clinical (A) and radiologic (B) aspects of Maffucci syndrome. Multiple venous malformations and enchon- 2647
dromas deforming the hand and fingers.
Kang_CH147_p2636-2668.indd 2647 08/12/18 3:09 pm
22 TABLE 147-5
Characteristics of Venous Anomalies
VENOUS MALFORMATION GLOMUVENOUS
VENOUS MALFORMATION CUTANEOMUCOSAL MALFORMATION BLUE RUBBER BLEB NEVUS SYNDROME
Location 50% head and neck Extremities, mucosa 70% extremities Especially palms and soles
Skin color Normal to bluish-purple Bluish Bluish-red-purple Bluish-dark-blue
Aspect Flat to raised Raised, dome shaped Raised, cobblestone Dome shaped, nipple-like
Plaquelike, hyperkeratotic
Single, large Multiple, small Multiple Multiple, small, often + 1 large lesion
Palpation Phleboliths Compressible Noncompressible, painful Firm, rubbery, spongy
Associated Coagulopathy — — Visceral venous malformation,
Part 22
coagulopathy
Radiologic Phleboliths Venous channels No phlebolith Venous channels
Tissue involved Often deep location Cutis, subcutis Cutis, subcutis Often deep component
::
Histology Thin-walled venous Thin-walled venous Glomus cells Thin-walled venous channels
channels channels
Vascular Diseases
Inheritance Sporadic Autosomal dominant Autosomal dominant Sporadic
New small lesions appear with time. In contrast to VM, and intestinal hemorrhage is not present. Patients with
GVM is often painful on palpation and cannot be com- GVMs have normal mental and physical development.
pletely emptied by compression. GVMs are usually mul- Diagnosis of GVM is based on clinical and histologic
tifocal and located on the extremities, involving the skin evaluation of the cutaneous lesions. Genetic testing can
and subcutis. They are rarely encountered in mucosae, differentiate small multifocal GVMs from VMCMs.55
A B C
D E F
Figure 147-12 Various venous malformations. A. Of the soft palate, causing sleeping apnea. B and C, Of the knee, causing
2648 hemarthroses; confirmed by T2-weighted images. D. Of the mucosal eyelid. E and F, Of the cheek, causing facial asym-
metry and pain.
Kang_CH147_p2636-2668.indd 2648 08/12/18 3:09 pm
22
Chapter 147 :: Vascular Malformations
A B
C D
Figure 147-13 Venous malformations (VMs). A, Extensive hemifacial VM causing soft tissue hypertrophy and lip defor-
mation in a 6-month-old boy. B, Histologically, VM consists of ectatic venous-like channels with anomalies in mural cells
(hematoxylin and eosin). C, Superficial glomuvenous malformation of the inner thigh. Note the presence of mural glomus
cells on histology (hematoxylin and eosin). D, Mucosal VM involving the lower lip and floor of the mouth.
Verrucous Venous Malformation: vascular anomaly. Although VVM expresses an
Verrucous venous malformation (VVM), previously immunohistochemical profile similar to vascular
known as verrucous hemangioma, is a rare congenital neoplasms (Wilms tumor 1 and GLUT-1 positive), it
is distinct from infantile hemangioma because it is
usually noted at birth and never spontaneously invo-
lutes. Mainly located on the legs, VVM can also be
found on the head and the trunk. It initially appears
as a bluish macule that later becomes erythematous-
violaceous in color and after trauma and secondary
infections often evolves into a verrucous nodule
(see Fig. 147-14). It never involves muscles or deep
tissues.63
Figure 147-14 Verrucous venous malformation (previ- NONCUTANEOUS FINDINGS
ously known as verrucous hemangioma) on the lower
extremity, causing oozing. It is purple-red in color and Patients with VMCM, like those with sporadically 2649
slightly raised, with hyperkeratosis. occurring MVM, seldom have VMs located in internal
Kang_CH147_p2636-2668.indd 2649 08/12/18 3:09 pm
22 organs, such as the lungs, kidneys, brain, or GI tract.15
Extracutaneous manifestations of BRBN include GI tract
which encodes the angiopoietin receptor, TIE2.68,69 The
inherited mutations cause hyperphosphorylation of
involvement. The small bowel is the predominant region; the receptor even in the absence of a ligand. Somatic
however, VMs can occur in any site from the oral cavity to second hits have been found in VMCM tissues of two
the anal mucosa. In rare instances, VMs can also be seen patients: one caused loss of membranous expression
in brain, lungs, and kidneys, as well as other organs.57 of the second, wild-type, allele; two others occurred
on the allele with the inherited mutation, causing an
increase in hyperphosphorylation.15,70 These mutations
COMPLICATIONS activate the PI3K/AKT signaling pathway in endothe-
VM is usually unilateral, causing asymmetry and pro- lial cells.71,72
gressive, slowly worsening distortion (see Figs. 147-12 GVM is caused by dominant, loss-of-function muta-
and 147-13). Depending on their size and location, VMs tions in the glomulin gene.73 In addition, a somatic
can cause pain, particularly in the morning on awaken- second hit is required for lesions to form, leading
ing, and anatomic distortion and can even threaten life to complete loss of function of glomulin. The most
Part 22
because of bleeding, expansion, or obstruction of vital common second hit is acquired uniparental isodi-
structures. Migraine is a common feature when VM is somy, which leads to homozygosity of the inherited
located in the temporal muscle. Whereas oropharyngeal mutation.73,74 This explains the variable expressivity
VM can impair speech and cause difficulties in swallow- regarding penetrance, the extent of the lesions, and the
::
ing, pharyngeal or laryngeal lesions can compromise number of lesions within family members.75 So far, a
Vascular Diseases
the airway and cause snoring and sleeping apnea.54 mutation in glomulin has been found in almost all GVM
On the extremities, VMs often involve muscles and families tested, demonstrating locus homogeneity.75-77
joints. They cause muscle weakness, hypotrophy, and Glomulin seems to be expressed in vascular smooth
sometimes hypertrophy, resulting in leg-length dis- muscle cells, but its exact function remains unknown.
crepancy, which is less severe than in KTS. Intraarticu- Sporadic VMs are also caused by genetic mutations.
lar bleeding, if the malformation is located in the knee Sixty percent are caused by activating somatic muta-
joint, leads to early-onset arthrosis.64 Genital VM often tions in TIE2.70 The mutations differ from the inher-
occurs with limb VM and can cause dyspareunia. GI ited ones seen in VMCM. The most common mutation
VM may lead to chronic anemia. in VMs (L914F) has not been identified in VMCM,
Local thrombosis is usually responsible for acute and the most common inherited mutation in VMCM
pain that lasts for several days and resolves as phlebo- (R849W) has not been identified in single somatic
liths, which can be identified by palpation or by radi- VM. This suggests that whereas the recurrent somatic
ography. VM rarely causes pulmonary embolism, mutations have effects too detrimental to be supported
although this complication can occur when large in the germline and probably cause lethality, the inher-
draining veins exist.65,66 ited ones have weaker effects that require additional
Chronic localized intravascular coagulopathy changes (somatic second hits).70-72
(LIC) is associated with solitary or syndromic spongy Another 20% of sporadically occurring VMs are
VMs. It is characterized by elevated D-dimer level caused by somatic mutations in the PIK3CA gene.10
(>500 ng/mL) in 40% of patients. Severe LIC with The same mutations are seen in cancers. They activate
low fibrinogen levels is common in large VMs of the the PI3K/AKT signaling pathway, as do the mutations
extremities and rare in the cervicofacial area. A num- in TIE2, underscoring perturbations in this signaling
ber of events, such as surgery, sclerotherapy, and hor- activity to underlie pathogenesis of VMs.10,11
monal influences, can trigger the conversion of LIC to MVM is also caused by TIE2 mutations. Similar
disseminated intravascular coagulopathy (DIC).67 to VMCM, these patients often have two TIE2 muta-
In contrast to skin lesions, GI lesions, as seen in tions. Most frequently, a R915C mutation is present
patients with BRBN, have a tendency to bleed. They as a mosaic change, and a somatic Y897C mutation
may spontaneously rupture, causing acute hemorrhage occurs on top of the R915C change on the same allele.
and even death. However, most of the bleeding tends to This explains the lack of family history (the first muta-
progress slowly, resulting in chronic and occult blood tion has appeared de novo in a mosaic fashion in the
loss that can lead to iron-deficiency anemia. Other com- patient), multifocality (the mosaic mutation is a pre-
plications include intussusception, volvulus, and bowel disposing change, like the inherited mutations are
infarction, which are to be considered in patients with in VMCM), and the clinical similarity with VMCM
BRBN and abdominal pain. Morbidity depends on the lesions (both have similar, albeit not the same, double
extent of GI involvement.57 VVM often ulcerates.63 cis mutations).15 VVM is caused by somatic MAP3K3
mutations.78 BRBN syndrome, like VMCM, MVM,
and 60% of VMs, is also caused by mutations in TIE2.
BRBN mutations are not detectable in the blood; they
ETIOLOGY AND are somatic.15 Yet in distinct, distally located lesions of
the same patient with BRBN, the same double muta-
PATHOGENESIS tions can be identified with equal allele frequencies.
The most common is a T1105N–T1106P double muta-
2650 VMCM is inherited as an autosomal dominant disor- tion. This suggests that the separate and even newly
der caused by germline mutations in the TEK gene, appearing lesions are formed by endothelial cells
Kang_CH147_p2636-2668.indd 2650 08/12/18 3:09 pm
that originate form a single common site, such as the
dominant lesion or bone marrow. The BRBN-causing
location of the lesion (see Fig. 147-12).89 This will
highlight the more cellular component, as well as the
22
TIE2 mutations cause strong hyperphosphoryla- more superficial location of GVM compared with VM.
tion of the receptor, and the endothelial cells acquire Atypical MRI findings require a biopsy to rule out a
increased colony-forming capacity, survival, and faster sarcoma or neurofibroma.
migration.15 In the presence of palmar-plantar multifocal lesions
Maffucci syndrome is caused by somatic mutations typical of BRBN (see Fig. 147-10), an endoscopy, colo-
in IDH1 and 2.79-81 noscopy, or wireless capsule endoscopy should be per-
formed to detect GI lesions.
These radiologic examinations are mandatory for
pretherapeutic evaluation of any venous anomaly.
DIAGNOSIS
Genetic Testing: Consider genetic testing for
TIE2 and glomulin mutations in patients with multi-
SUPPORTIVE STUDIES focal lesions. This differentiates the conditions and
Chapter 147 :: Vascular Malformations
Laboratory Testing: A coagulation profile with enables genetic counseling.
platelet count, fibrinogen, and D-dimer level should
be done in any patients with a pure VM or complex
malformation with a venous component because the
D-dimer level is elevated in about 40% of VMs. This DIFFERENTIAL DIAGNOSIS
blood test, done at the first consultation, should be
repeated around puberty and whenever the malfor- See Table 147-6.
mation becomes painful. This is never the case in a
patient with GVM or other simple vascular anomalies.
Associated low fibrinogen is pathognomonic of severe
LIC. Severe LIC can easily decompensate to DIC with
TABLE 147-6
severe bleeding during interventional or surgical Differential Diagnosis of Venous Malformation
procedures.67,82-84 Based on Morphology
Most Likely
Pathology: VMs are histologically composed Bluish subcutaneous mass, noncompressible
of ectatic vascular channels of venous type with flat ■ Infantile hemangioma (deep)
endothelium. The vascular walls are thin and lined ■ Lymphatic malformation
by a discontinuous layer of mural smooth muscle With multiple enchondromas
cells positive for smooth muscle-α-actin.85 Basement ■ Maffucci syndrome
membranes are also thin. In contrast, GVM is histo- Consider
logically characterized by distended venous channels Hyperkeratotic
surrounded by mural “glomus cells,” which are aber- ■ GVM, if on extremity
■ Cutaneous lesions of CCM
rantly differentiated smooth muscle cells. Glomus cells
■ VVM
are round or polygonal, instead of being elongated, Rule Out
like the normal vascular smooth muscle cells.86 By Superficial, collateral normal veins
immunohistochemistry, glomus cells stain positively ■ Deep venous insufficiency, iatrogenic stenosis, or congenital
for smooth muscle-α-actin and vimentin, but they are agenesis
negative for desmin, von Willebrand factor, and S100.87 Bluish nonvascular lesion
By in situ hybridization, they are also negative for Based on Location
glomulin.76 Consider
Consider
VVM is characterized by hyperkeratotic epidermis Base of the nose
on top of small blood vessels with a multilaminated ■ Normal prominent veins
membrane located in the dermis and the subcutis. Scalp or forehead
Focal positive staining for GLUT-1 is seen.63 Histopath- ■ Sinus pericranii
ologic examination of patients with Maffucci syndrome ■ Underlying Galen malformation
Subungual
shows features of spindle cell hemangioendothelioma.
■ Solitary glomus tumor
Cheek
Imaging: Plain radiography can identify the calci- ■ Blue nevus
fication of phleboliths, which are pathognomonic of Brain
LIC in a VM. It can also show the presence of multiple ■ Developmental venous anomaly
enchondromas in patients with Maffucci syndrome ■ CCM
(see Fig. 147-11). Doppler ultrasonography is nonin- Rule Out
vasive and can confirm the slow-flow nature of the Neck; deep mass
lesion. In contrast to lymphatic malformations (LMs), ■ Thyroglossal duct
the venous channels are compressible with the probe.88 ■ Bronchogenic cyst
MRI imaging with spin-echo T1- and T2-weighted and CCM, cerebral cavernous malformation; GVM, glomuvenous malforma- 2651
fat-saturation sequences depicts the exact anatomic tion; VVM, verrucous venous malformation
Kang_CH147_p2636-2668.indd 2651 08/12/18 3:09 pm
22 An unusual pathway of cerebral drainage, a cere-
bral developmental venous anomaly that consists of
a preclinical VM-mouse model trial.90 A phase II clini-
cal trial for difficult-to-treat extensive VMs or complex
dilated intramedullary veins converging into a larger slow-flow vascular anomalies not amenable to conven-
draining vein, can be seen in 0.5% of the population. In tional management also gave encouraging results.90,91
contrast, it is present in 20% of patients with extensive Patients experienced almost complete relief of pain
head and neck VMs. Although usually asymptomatic, and symptoms, reduced coagulopathy when present,
this anomaly can cause headache. improved function, and increased self-perceived qual-
ity of life. Bleeding in sporadic VM as well as in BRBN
stopped within 24 hours. Side effects were minor and
included mucositis, mild headache, fatigue, and diar-
CLINICAL COURSE rhea. Moreover, a statistically significant reduction in
AND PROGNOSIS volume was observed with MRIs in most patients that
reached 1-year follow-up. A multicentric European
Study (VASE; NCT 02638389) is ongoing to determine
VMs grow proportionately with the patient. They
Part 22
which subtypes of VMs are best suited for rapamycin
never regress spontaneously. Initially, asymptomatic
treatment and for how long the treatment should be
lesions often become painful in response to trauma
continued. Rapamycin should not be considered as
or in altered hormonal states (puberty or pregnancy).
treatment for small, localized, and asymptomatic slow-
::
They may also expand following thrombosis. No
flow vascular malformations that respond to standard
oncogenic transformation of this condition has been
Vascular Diseases
of care.92
described. The major complications are life-threat-
ening GI hemorrhage and airway compression.
Patients with inherited venous anomalies, as well as
Procedures: Percutaneous intralesional sclero-
therapy is the primary treatment for VM, in contrast
BRBN and MVM, tend to develop additional small
to GVM. Absolute ethanol is the most efficient scleros-
lesions with time.15 Patients with Maffucci syndrome
ing agent.93 Local compression or intralesional coils are
have a high incidence of malignancies (40%), mainly
used to prevent dissemination of ethanol into the sys-
chondrosarcoma but also glioma, fibrosarcoma, and
temic circulation. Local complications include inflam-
angiosarcoma.58
mation, edema, blistering, necrosis, chronic drainage,
and temporary or permanent nerve deficit. Systemic
complications, such as renal or pulmonary toxicity,
MANAGEMENT myocardial depression, and cardiac arrest, have been
reported. Multiple sclerotherapy sessions are often
needed because VMs have a propensity to recanalize
Treatment of venous anomalies is warranted when
and recur. Alternatives to ethanol sclerotherapy are
lesions are symptomatic or for cosmetic purposes. The
sodium tetradecyl-sulphate foam or lauromacrogol
most common indications for treatment are pain and
that are effective for small VMs and cause fewer local
functional impairment.54 Their management is often
adverse effects. Positive response was seen in 49.5%
multidisciplinary, involving hematologists, surgeons,
(for pain) and 52.7% (for mass reduction), within the
and interventional radiologists.
86 patients (91 VMs) involved in one study.94 Detergent
sclerosants have been used as microfoams, using air
INTERVENTIONS bubbles or carbon dioxide to increase volume and sur-
face contact with endothelium. However, neurologic
Medical Management: Compression garments complications have been described in 2% of cases.
can be helpful to decrease swelling and pain in VMs of A modified radiopaque ethanol sclerosing agent was
the extremity by decreasing venous pressure. In con- therefore developed. It traps ethanol within a mesh of
trast, compression increases pain in GVM patients and ethylcellulose to increase viscosity.95,96
is not indicated in this situation. Surgical resection is often performed after
Low-molecular-weight heparin (LMWH; LMWH, sclerotherapy.53 In patients with BRBN, endoscopic
100 IU anti-Xa/kg/day) is usually given to patients coagulation with Nd:YAG (neodymium-doped yttrium
with signs of LIC 24 hours before and for another 5 aluminum garnet)laser, bipolar or argon plasma coag-
days after surgical procedures to minimize hemor- ulation, and band-ligation are useful. An open sur-
rhagic risk. The same treatment is given to patients gery allowing surgical resection of all GI lesions can
with painful episodes of local thrombosis. In this eradicate the need for blood transfusion as well as
circumstance, the duration of treatment is usually the common intermittent abdominal pain caused by
2 weeks.54,67,84 intussuception.97,98 In patients with GVM, skin graft is
Bleeding from GI BRBN lesions can be managed often needed if excision is undertaken. VVM is also best
conservatively with iron supplementation and blood treated with surgical resection. Closure often necessi-
transfusions. tates a skin graft.
With the understanding of the underlying patho-
physiologic mechanisms (activation of the PI3K-AKT Counseling: In patients with VMCM or GVM,
2652 signaling), targeted molecular therapies are becom- genetic counseling should be provided for affected
ing possible. Rapamycin (sirolimus) was promising in families, informing patients of a 50% risk of inheriting
Kang_CH147_p2636-2668.indd 2652 08/12/18 3:09 pm
the disease-causing mutation and of the variability
in clinical expression. Psychosocial counseling and
part of a syndrome, such as in Turner and Noonan
syndromes.100
22
patient organizations dedicated to vascular anoma-
lies are also an important asset for patients and their
families.
SYNDROMIC DISORDERS
KLIPPEL-TRENAUNAY SYNDROME
LYMPHATIC ANOMALIES
Klippel-Trenaunay syndrome is a combined capillary–
lymphatic–venous malformation associated with
AT-A-GLANCE hypertrophy of the affected limb. Lower limbs are
affected in 70% of cases. It is characterized by a geo-
■ Worldwide occurrence of LM is unknown, but it is graphic, widespread CM associated with lymphatic
Chapter 147 :: Vascular Malformations
less frequent than that of VM. vesicles. The persistence of a persistent embry-
■ Congenital, sporadic, slow-flow malformations of onic vein located on the lateral side of the thigh is
lymphatic vessels pathognomonic.101,102
■ LMs consist of micro- or macrocysts filled with
lymphatic fluid.
■ Histologically consist of dilated lymphatic CLOVES SYNDROME
channels with flat endothelium that expresses
D2-40. CLOVES syndrome is an eponym for congenital lipo-
matous overgrowth with vascular malformations,
■ Primary lymphedema can be inherited as an
epidermal nevi, and skeletal anomalies.103 This non-
autosomal trait.
hereditary disorder is characterized by progressive
■ Infection is the most common complication that asymmetric hypertrophy, multiple truncal lipomatous
can lead to septicemia. masses with paraspinal fast-flow or slow-flow vascu-
lar anomalies (or both), epidermal nevus or nevi, acral
lesions, and skeletal or spinal anomalies (Fig. 147-15).
INTRODUCTION
LMs are localized morphogenic errors of the lym-
phatic vessels. They consist of small vesicles or large
cysts filled with lymphatic fluid. Macrocystic LM can
be diagnosed in utero as early as the first trimester of
pregnancy.99 However, most LMs are diagnosed dur-
ing infancy, before the age of 2 years. Some can mani-
fest only at puberty or during adulthood. They can be
isolated, combined with other vascular anomalies, or
be part of a syndrome.
Primary lymphedema is not a localized lesion but a
more generalized condition, in which the lymph fluid
accumulates in the interstitial tissue. Lower extremities
are most commonly affected. It can be uni- or bilateral.
Primary lymphedema can be an isolated condition or
part of a syndrome, and it can occur sporadically or as
an inherited disorder.
EPIDEMIOLOGY
LM is a congenital disorder of unknown incidence.
It occurs sporadically in contrast to primary lymph-
edema, which can be inherited, usually as an auto-
somal dominant trait, in up to 20% of cases. Primary
lymphedema is divided into congenital (Milroy dis-
ease, Online Mendelian Inheritance in Man [OMIM] Figure 147-15 Capillary–lymphatic–venous malforma-
#153100) and late onset (presenting at puberty, tion of right lower extremity with soft tissue hypertrophy 2653
Meige disease, OMIM #153200). It can be isolated or (CLOVES syndrome).
Kang_CH147_p2636-2668.indd 2653 08/12/18 3:09 pm
22 The most important differential diagnoses are other
overgrowth syndromes, such as the Proteus syndrome CLINICAL FEATURES
and Proteus-like syndrome.104
CUTANEOUS FINDINGS
GENERALIZED LYMPHATIC LMs are composed of microcysts (Figs. 147-16 and
ANOMALY 147-17), previously termed lymphangioma circumscrip-
tum) or macrocysts (>1 cm in diameter, previously
Generalized lymphatic anomaly (GLA) is a rare condition known as cystic hygroma) that grow proportionally
in which LMs can invade several organs such as the medi- with the child (see Figs. 147-1B, 147-16, and 147-17).
astinum, lungs, pleura, GI tract, bones, and soft tissue.105 They are filled with clear or serosanguineous fluid
(see Fig. 147-17B). Whereas macrocystic LM manifests
as a soft, multilobulated, well-defined mass, micro-
GORHAM-STOUT SYNDROME cystic LMs are ill-defined and often invade adjacent
Part 22
Gorham-Stout syndrome, or “vanishing bone disease,” structures. Dermal LMs can manifest as small, mil-
is an aggressive rare lymphatic disorder characterized limeter-sized vesicles, clear (see Figs. 147-16D and
by progressive demineralization and destruction of 147-17A) or dark red in color (when there is intracys-
bones, which are replaced by lymphatic vessels and tic bleeding), that can bleed and become purple and
::
capillaries.106 nodular.
Vascular Diseases
A B C
2654 Figure 147-16 Various aspects of lymphatic malformation (LM). Bluish discoloration (A) of extensive LM of cheek, as seen
on computed tomography scan (B and C). Small clear vesicles of dermal LM (D).
Kang_CH147_p2636-2668.indd 2654 08/12/18 3:09 pm
22
A B C
Chapter 147 :: Vascular Malformations
Figure 147-17 A, Microcystic, dermal lymphatic malformation (LM) consisting of clear and dark-red vesicles. B, Intraopera-
tive view of a macrocystic LM of axilla showing a well-delineated cyst filled with clear lymph fluid. C, Histologically, LM
consists of dilated lymphatic channels with flat endothelium (hematoxylin and eosin).
Angiokeratoma Circumscriptum: Angio- COMPLICATIONS
keratoma circumscriptum, or capillary-lymphatic
malformation (CLM), is a combined, well-demarcated LM can suddenly enlarge in response to cough, inflam-
lesion often located on an extremity. The lesion is pink mation, fever, viral or bacterial infection, or intral-
to bluish-red in color, slightly raised, and usually esional bleeding (Fig. 147-19). Recurrent cellulitis is
hyperkeratotic. Clinically, angiokeratoma circumscrip- the major complication, especially in patients with
tum, angiokeratoma of Mibelli (circumscribed, dark- KTS, and it can evolve into septicemia if not promptly
red, hyperkeratotic plaques on distal extremities), treated. Local redness and warmth appear, and the
and angiokeratoma of Fordyce (very common hyper- lesion becomes painful.
keratotic, blue-black papules on the scrotum of elderly Facial asymmetry, especially of the mandible,
men) are recognized. They are to be differentiated from is commonly associated with microcystic LM (see
angiokeratomas of Fabry disease (see Chap. 127) and Fig. 147-16). Intraorbital LM is responsible for ocular
from VVM (previously known as verrucous heman- dystopia and exophthalmia, as well as orbital enlarge-
gioma; see earlier VM section). ment. LM on the tongue impairs speech and produces
oozing and halitosis. Airway obstruction is common
Lymphedema: Lymphedema is characterized when the base of the tongue or the cervicofacial area is
by swelling of the affected body part, usually a lower affected.111 Extensive limb LM can cause elephantiasis.
extremity, and caused by accumulation of lymphatic Extension into the pleura can occur and cause chylo-
fluid into the extracellular space caused by intrinsic thorax. Visceral LM can cause protein-losing enteropa-
lymphatic dysfunction (Fig. 147-18). Various pheno- thy and hypoalbuminemia. Patients with KTS as well
types exist depending on the age of onset, location, as patients with CLOVES are at high risk of pulmonary
and associated anomalies.107,108 embolism because of the persistent embryonic vein and
the presence of ectatic thoracic veins, respectively.110
Lymphedema is complicated by cellulitis in
Congenital Lymphedema: Milroy disease is
20% of cases. More rarely, pleural effusion, even in
suspected in the presence of swelling of the dorsum of
utero; hydrops fetalis; and chylous ascites can be
the feet with a family history of lymphedema. Lymph-
observed.108,112,113
edema is present at birth, often bilateral, and affects
the lower limbs below the knees.109 Other features
can be associated with congenital lymphedema, such
as hydrocele (37% of males), prominent veins (23%),
upslanting toenails (14%), papillomatosis (10%), or ETIOLOGY AND
urethral abnormalities in males (4%).
PATHOGENESIS
NONCUTANEOUS FINDINGS LMs, as well as KTS and CLOVES syndrome, are caused
by mosaic or somatic mutations in PIK3CA that acti-
Pulmonary embolism can occur in patients with KTS vate the PI3K/AKT/mTor signaling pathway.11,12,114,115
or CLOVES syndrome.110 Depending on the affected More than 20 genes have been identified to be
organ, symptoms such as pleural effusion, ascites, and mutated in various forms primary lymphedema.108
malabsorption can occur in patients with GLA.105 Pain- Milroy disease is caused by inherited loss-of-function
ful pathological fractures are common in patients with mutations in vascular endothelial growth factor recep- 2655
Gorham-Stout syndrome.106 tor 3 (VEGFR3).116-118 Mutations in the same gene
Kang_CH147_p2636-2668.indd 2655 08/12/18 3:09 pm
22
A B
Part 22
::
Vascular Diseases
C D
Figure 147-18 Various types of congenital primary lymphedema characterized by lymphedema of lower limb, below the
knees (A); with papillomatosis (B); with upslanting toenails (C); that can evolve into elephantiasis (D).
are also responsible for sporadic hydrops fetalis and in the FOXC2 transcription factor.120 Lymphedema
generalized subcutaneous edema.119 Lymphedema- associated with microcephaly, with or without cho-
distichiasis is caused by loss-of-function mutations rioretinopathy or developmental delay, is caused
A B
2656 Figure 147-19 Intraoperative view of an axillary lymphatic malformation that had suddenly increased in size because of
intracystic bleeding.
Kang_CH147_p2636-2668.indd 2656 08/12/18 3:09 pm
by dominantly inherited mutations in KIF11.121,122
Hypotrichosis–lymphedema–telangiectasia, which has TABLE 147-7
22
an autosomal-dominant or -recessive pattern of inheri- Differential Diagnosis of Lymphatic
tance, is caused by mutations in SOX18.118 Hennekam Malformation
syndrome (OMIM #235510) is an autosomal recessive
Most Likely
generalized lymphatic dysplasia characterized by
■ Infantile hemangioma
intestinal lymphangiectasia with severe and progres-
sive lymphedema of the limbs, genitalia, and face, as Consider
well as severe mental retardation.123 Mutations have ■ Venous malformation
■ Teratoma
been identified in CCBE1 (collagen and calcium-
■ Fibrosarcoma or rhabdomyosarcoma
binding EGF domains 1).107,124 Lymphedema is asso-
ciated with hematologic malignancies in Emberger Rule Out
Vulvar
syndrome caused by GATA2 mutations.125 In about
■ Acquired lymphangiectasia caused by radiotherapy
35% to 40% of patients with familial primary lymph-
■ Acquired lymphangiectasia caused by Crohn disease
Chapter 147 :: Vascular Malformations
edema, a germline mutation can be identified.108,126
management. For example, identification of a germ-
DIAGNOSIS line GATA2 mutation indicates specific cancer sur-
veillance programs, and a KIF11 mutation implies a
need for detailed ophthalmologic and developmental
SUPPORTIVE STUDIES examination.
Pathology: LMs are characterized by dilated, flat-
endothelium-lined channels of variable wall thick-
ness (see Fig. 147-8C). No blood cells are seen in these
spaces, except after intracystic bleeding or in the pres- DIFFERENTIAL DIAGNOSIS
ence of a combined lymphatic-venous malformation.
Macrocystic LM consists of a single or multiple lym- See Table 147-7.
phatic cysts surrounded by a thick fibrous membrane.
The cysts do not communicate with each other. Endo-
thelial cells express specific lymphatic markers, such as
podoplanin, D2-40, and VEGFR-3.44,127 Lymphedema is CLINICAL COURSE
characterized by abnormalities in the primary periph-
eral lymphatic capillaries, collecting lymphatic vessels,
AND PROGNOSIS
or lymphatic valves and lymphovenous valves. This is
LM usually grows with the child. It can cause asym-
pronounced in the affected limb but is sometimes also
metry with bony overgrowth. Increases in size can be
seen on the contralateral side. Extensive dermal fibro-
seen during infection or intralesional bleeding. Epi-
sis is often present.44
sodic reports of spontaneous involution exist. Because
Imaging: Doppler ultrasound shows microcysts, regression is commonly seen after local infection, it
macrocysts, or both separated by thin septa. In con- may be caused by postinflammatory autosclerosis.
trast to VMs, they are not compressible by the probe. Patients with Gorham-Stout syndrome have a poor
MRI also demonstrates the cystic nature of the lesion, prognosis; it is lethal in 16% of cases.
with often discernable fluid-fluid levels.128,129 Com-
puted tomography (CT) is the best study to show bony
involvement.
Doppler ultrasonography of the deep venous sys- MANAGEMENT
tem of the affected lower limb and the pelvic area is
needed in patients with KTS and CLOVES. Moreover, INTERVENTIONS
because CLOVES syndrome is characterized by pro-
gressive overgrowth during infancy, careful follow- Medical Management: Systemic antibiotics
up every 6 months is recommended until the end of should be used to treat intralesional bacterial infec-
puberty. Follow-up consists of clinical examination, tion as well as early cellulitis in both isolated and syn-
scaniometry (leg length films), and abdominal ultra- dromic LM. Antiinflammatory drugs may be required
sonography because of the increased risk of cryptor- to alleviate pain. Patients with CLOVES or KTS syn-
chidism, hydrocele, renal atrophy, and Wilms tumor.130 drome need to receive LMWH at prophylactic dosages
Additional investigations should be done according to of 100 anti-Xa/kg/day before any surgical procedure;
symptoms. this should be continued for another 10 to 20 days post-
operatively to avoid perioperative decompensation of
Genetic Testing: Genetic panel–based testing is their coagulation abnormalities, and more importantly,
available in accredited diagnostic laboratories. Such to reduce the risk of life-threatening postoperative pul- 2657
screens help identify germline mutations and can aid monary embolism.110 Rapamycin has been effectively
Kang_CH147_p2636-2668.indd 2657 08/12/18 3:09 pm
22 Part 22
::
Vascular Diseases
A B
Figure 147-20 Dermal lymphatic malformation (A) efficiently treated with carbon dioxide laser (B).
used for extensive LMs resistant to standard treatment, problems, necessitating inclusion in special surveil-
as well as for patients with KTS and GLA. Although lance programs. Psychosocial counseling and patient
none had complete resolution, several patients experi- organizations dedicated to vascular anomalies are also
enced cessation of lymphatic leakage and infection and an important help for patients and their families.
reduction of volume of the malformation. Side effects
were minor.131,132
Lymphedema is best treated with elastic stock- ARTERIOVENOUS
ings, massage, and pneumatic compression devices.
Sildenafil was suggested as effective in the treatment MALFORMATIONS
of LM,133 but further studies have not confirmed its
efficacy and highlighted potential side effects, such as
infections and bleeding.134,135 AT-A-GLANCE
Procedures: Nd:YAG laser or carbon dioxide laser ■ Worldwide occurrence; rare but exact frequency
photocoagulation has been used to treat dermal LM unknown
vesicles that cause oozing (Fig. 147-20). Macrocystic ■ Congenital, fast-flow malformations that can be
LM is treated with fluid aspiration followed by per- occult until puberty
cutaneous, intralesional injection of sclerosing agents ■ Histologically consist of direct communications
performed by an interventional radiologist. Scleros- between arteries and veins
ing agents include sodium tetradecyl sulfate, pure ■ Usually sporadic but can have a genetic predis-
ethanol, OK432 (extract from a killed strain of group position as in CM-AVM, hereditary hemorrhagic
A Streptococcus pyogenes: picibanil), doxycycline, and telangiectasia, or PHTS
bleomycin. Side effects vary from fever and erythema
■ Most difficult vascular malformation to treat; need
to edema.136,137
a multidisciplinary approach
Surgical resection is another alternative for LM,
CLOVES, and KTS syndrome.138,139 Results depend on
the anatomical site and extension of the lesion. Recur-
rence is frequent because it is difficult to differentiate
microcystic LM from adjacent normal tissue. This leads INTRODUCTION
to either incomplete resection or unnecessary sacrifice
of normal structures. Fast-flow vascular malformations, namely AVM and
AVF, are the most severe and devastating malforma-
Counseling: Family and patient education tions. AVF is usually the result of trauma. AVM is char-
regarding the cause and treatment is necessary for acterized by the presence of a “nidus,” the epicenter of
2658 primary lymphedema because it can be inherited the lesion that is composed of direct communications
and associated with other important medical between multiple feeding arteries and draining veins
Kang_CH147_p2636-2668.indd 2658 08/12/18 3:09 pm
without an intervening normal capillary bed. AVMs
are present at birth, although they are not always
combination of any of these AVMs) can be seen.144 In
contrast, in 23% of patients with CM-AVM1 and 13%
22
visible. They can be localized or extensive and evolve of patients with CM-AVM2, an intracerebral or intra-
with age. spinal AVM is present.18,19,22,23,35], but there is no risk for
visceral AVM as in HHT. Vein of Galen aneurysmal
malformation is also part of the phenotype. Recur-
rent epistaxis is typically the initial manifestation of
EPIDEMIOLOGY HHT. The nosebleeds can be severe enough to require
blood transfusions. Pulmonary AVMs affect approxi-
The incidence of AVM is unknown. No sex preponder- mately 30% of patients with HHT and are particularly
ance has been identified. It is a rare, usually sporadic, common in HHT1. These patients are at higher risk of
fast-flow vascular malformation. Hereditary hemor- stroke and brain abscess than in the healthy popula-
rhagic telangiectasia (HHT) is an autosomal inherited tion because the normal filtering function of the lung is
disorder with a prevalence of 1 in 5000 individuals.140 lost. Migraine headaches occur in 13% to 50% of cases.
Chapter 147 :: Vascular Malformations
CM-AVM is another familial form with autoso- Recurrent painless GI bleeding occurs in 10% to 40%
mal dominant inheritance and a similar prevalence of patients. Symptoms may include abdominal pain,
estimate.19-23 jaundice, symptoms of high-output cardiac failure,
and bleeding from esophageal varices.145
SYNDROMIC DISORDERS PARKES WEBER SYNDROME
Parkes Weber syndrome is characterized by a large,
BONNET-DECHAUME-BLANC OR congenital, cutaneous, red vascular stain on an extrem-
ity in association with soft tissue and skeletal hyper-
WYBURN-MASON SYNDROME trophy of the affected limb and underlying multiple
Bonnet-Dechaume-Blanc or Wyburn-Mason syndrome arteriolar-venular microfistulas.146 The affected extrem-
is a sporadic, syndromic AVM located in the centrofa- ity, often the lower one, is longer and larger than the
cial or hemifacial area (or both), with oculo-orbital and contralateral one. Although often sporadic, it can be
cerebral involvement.141 It rarely follows a trigeminal part of CM-AVM. In these cases, there are small, multi-
distribution like SWS. Intracerebral AVMs are common focal CMs located on other parts of the body. The signs
in Bonnet-Dechaume syndrome and can cause epi- and symptoms worsen with age. Affected patients can
staxis, exophthalmos, and hemianopia. Mental retar- develop congestive heart failure.
dation can also occur.
PHOSPHATASE AND TENSIN
COBB SYNDROME HOMOLOG HAMARTOMA
Cobb syndrome is another sporadic, syndromic AVM TUMOR SYNDROME
that associates cutaneous and spinal cord AVMs of the
PHTS is an autosomal-dominant disorder that includes
same metamere.142 The cutaneous lesion masquerades
patients with Bannayan-Riley-Ruvalcaba syndrome
as a CM, although it is warm on palpation. Cobb syn-
and Cowden syndrome because 60% and 81% of them,
drome manifests in childhood with a sudden onset of
respectively, have a mutation in PTEN.147 These patients
back or lower extremity pain associated with sensory
typically have macrocephaly, penile freckling, multiple
disturbance. Other neurologic complications (pain,
developmental venous anomalies in the brain, fast-
sensory and motor disturbances, and neurogenic blad-
flow VMs (54%), and an increased risk of malignancy.
der) can occur depending on the location and exten-
The VMs are often multifocal (57%) and musculoskel-
sion of the AVM.
etal and associated with ectopic fat deposition and dis-
ruption of the normal tissue architecture.147,148
HEREDITARY HEMORRHAGIC
TELANGIECTASIA
Individuals with HHT demonstrate combinations of CLINICAL FEATURES
the following triad: multiple cutaneous and mucosal
telangiectasias, often located on the mucosal lip; epi-
staxis; and a positive family history.143 The diagnosis
CUTANEOUS FINDINGS
is clinical, in accordance with the Curaçao criteria. AVMs usually manifest as cutaneous, faint, red to
The estimated frequencies of manifestations in HHT purple, ill-defined masses with a thrill, a bruit, or
patients are spontaneous, recurrent epistaxis, 90%; a pulsation of increased amplitude (Figs. 147-21 to
skin telangiectasia, 75%; hepatic or pulmonary AVMs, 147-23; see also Figs. 147-1D and 147-1E). About one
30%; and GI bleeding, 15%.144 In 30% of patients with third of AVMs are present at birth, another -third 2659
HHT, a hepatic, pulmonary, or cerebral AVM (or a appear during childhood or at puberty, and the rest
Kang_CH147_p2636-2668.indd 2659 08/12/18 3:09 pm
22 Part 22
::
Vascular Diseases
A B
Figure 147-21 Stage 2 arteriovenous malformation of the left thigh mimicking an infantile hemangioma that did not
disappear at 8 years of age (A). Arteriography showing the nidus confirmed the diagnosis (B).
manifest in adulthood because hormonal changes and CM-AVM (see earlier discussion of CM-AVM) mani-
trauma usually trigger the growth of an AVM. AVMs fests as multiple atypical CMs that are randomly dis-
can affect any tissue and organ. Seventy percent of tributed (see Fig. 147-6) and associated with fast-flow
AVMs are located on the head and neck. They never lesions—AVM, AVF, or Parkes Weber syndrome—in
regress spontaneously and get worse with time.149 30% (CM-AVM1) or 20% (CM-AVM2) of patients. The
In 1985, Schobinger staged AVMs according to their expressivity is highly variable within families, from
severity. Schobinger stage I is a red stain with bruit small, asymptomatic CMs to life-threatening AVMs.
and pulses of increased amplitude. With age, puberty, The fast-flow lesions (AVM or AVF) are either cutane-
or trauma, AVM can worsen, and veins become prom- ous or subcutaneous, with or without intramuscular
inent and tortuous (Schobinger stage II; Fig. 147-22) and intraosseous involvement. About 10% to 15% of
and subsequently darker and painful, and they ulcer- patients with CM-AVM have a Parkes Weber pheno-
ate and bleed (Schobinger stage III; see Fig. 147-23). type affecting the lower (two thirds of such cases) or
The final stage of a large AVM is cardiac failure the upper extremity. The AVMs can be asymptomatic,
(Schobinger stage IV; see Fig. 147-23).150 The patient but complications occur depending on the location
often reports hearing his or her cardiac pulse. and size.19-21
A B C
Figure 147-22 A, Extensive arteriovenous malformation deforming the right buttock. B, Arteriography showing nidus of
2660 the malformation. C, Histology shows direct connection between artery and vein, with thrombotic material secondary to
embolization procedure.
Kang_CH147_p2636-2668.indd 2660 08/12/18 3:09 pm
22
Chapter 147 :: Vascular Malformations
A B
Figure 147-23 Stage 2 arteriovenous malformation (AVM; A) evolving into stage 4 AVM (B).
NONCUTANEOUS FINDINGS higher in patients with HHT2. A similar increased risk
is seen in CM-AVM1 and 2. Patients with HHT with
In 30% of patients with HHT, a hepatic, pulmonary, pulmonary AVMs and telangiectasia of the GI tract are
or cerebral AVM (or a combination of any of these at risk for life-threatening hemorrhage. Other sites of
AVMs).144 In 23% of patients CM-AVM1 and 13% of bleeding may include sites in the kidney, spleen, blad-
patients with CM-AVM2, an intracerebral or intraspi- der, liver, meninges, and brain. Strokes may be either
nal AVM (or both) is present.18,19,22,23,35 hemorrhagic or ischemic.
These patients are at higher risk of stroke and brain
abscess than in the healthy population because the
normal filtering function of the lung is lost. Migraine
COMPLICATIONS headaches occur in 13% to 50% of the cases. Recurrent
painless GI bleeding occurs in 10% to 40% of patients.
AVMs involving the face can as well destroy the bone Symptoms may include abdominal pain, jaundice,
structure underneath and can cause gingival bleeding symptoms of high-output cardiac failure, and bleeding
or epistaxis as well as hypertrophic asymmetry. Bony from esophageal varices.145
hypertrophy is a common feature of facial AVM, which Patients with CM-AVM can exhibit intrauterine life-
causes asymmetry. AVM of an extremity often causes threatening intracerebral bleeding caused by vein of
peripheral ischemia due to blood flow steel phenome- Galen aneurysmal malformation.22,151 The brain and
non. Cardiac failure is rare (Schobinger stage IV), espe- medullary AVMs can also bleed postnatally.22,35,151
cially during childhood. Puberty and trauma as well as
hormonal influence can trigger appearance of AVMs.
Intracerebral AVM such as in Bonnet-Dechaume syn-
drome, can cause epistaxis, exophthalmos, and hemi-
anopia. Mental retardation can also occur.
ETIOLOGY AND
Cobb syndrome manifests in childhood with a sud- PATHOGENESIS
den onset of back or lower extremity pain associated
with sensory disturbance. Other neurologic complica- Underlying genetic defects have been identified for
tions (pain, sensory and motor disturbances, and neu- the fast-flow vascular malformations of CM-AVM
rogenic bladder) can occur depending on the location (OMIM #608354), HHT (OMIM #187300), and PHTS
and extension of the AVM. (OMIM #153480 and 158350). These disorders are
Recurrent epistaxis is typically the initial manifes- inherited as an autosomal-dominant pattern with a
tation of HHT. The nosebleeds can be severe enough penetrance of about 95% for the cutaneous CMs or
to require blood transfusions. The prevalence of brain telangiectasias.18,19,22,151
AVM is 1000-fold higher in patients with HHT1 than HHT is caused by alterations in the transforming 2661
in the general population (1 in 10,000) and 100-fold growth factor-β signaling pathway. The genes implicated
Kang_CH147_p2636-2668.indd 2661 08/12/18 3:09 pm
22 in HHT are ENG, encoding endoglin (HHT type 1), and
ACVRL1 (formerly called ALK1, HHT type 2); SMAD4,
cutaneous CMs (CM-AVM) or telangiectasias (HHT).
Molecular diagnosis helps genetic counseling and
and GDF2 are less frequently involved.152-155 guide further imaging for eventual undetected mal-
Endoglin and ACVL1 are type III and type I trans- formations. Patients with AVM with associated mac-
forming growth factor-β receptors, and both are rocephaly should be screened for PTEN mutations.
well-expressed on vascular endothelial cells. Loss- Identification of a germline mutation would enable
of-function of this signaling concomitantly increases precise counseling and inclusion in cancer surveillance
PI3K-AKT signaling.156 programs.
CM-AVM1 is caused by loss-of-function mutations in
RASA1.18,20 RASA1 encodes P120RASGAP, a GTPase reg-
ulating RAS activity. It converts the active GTP-bound
RAS into its inactive GDP-bound form.61,62,157 The high DIFFERENTIAL DIAGNOSIS
intrafamilial phenotypic variability is explained by the
necessity of a somatic second-hit mutation to occur.21,158 See Table 147-8.
During childhood, AVM is often mistaken for a CM
Part 22
In 30% of patients with CM-AVM1, the mutation is de
novo. CM-AVM2 is caused by loss-of-function mutation or hemangioma because of its presentation as a faint,
in EPHB4, which encodes an endothelial cell receptor ill-defined, macular red stain. However, dispropor-
in venous vessels. The ligand EPHRINB2, in contrast tionate warmth on palpation is a clue to its fast-flow
::
to EPHB4, is expressed in arterial endothelial cells. This component. A pulse, thrill, and bruit are other signs
Vascular Diseases
bidirectional ligand–receptor system is important for supporting AVM. Doppler ultrasonography can help
arteriovenous identity and separation. EPHB4 signals differentiate a slow-flow CM from a fast-flow AVM or
using p120RASGAP, and the loss of function of either hemangioma. Hemangiomas have equatorial feeding
gene causes increased RAS-MAPK signaling.18,159-162 arteries, peripheral veins, variable echogenicity, and
AVM can also be caused by loss-of-function mutation fast flow but no true arteriovenous shunting.
in PTEN, a tumor suppressor gene, as seen in patients Other dermatologic lesions can mimic AVM.
with PHTS.163 PTEN regulates PI3K-AKT activity, and Epithelioid hemangioendothelioma occurs in the
this seems to be regulated at least in part by the HHT extremity as a purple and locally aggressive lesion.
receptor complex (see earlier). Loss of function of one Tumid lupus erythematosus, sarcoidosis of the face,
of the partners of this complex or of PTEN itself leads and Melkersson-Rosenthal syndrome of the lip can
to activation of PI3K. mimic AVM. Dabska tumor can simulate an AVM on a
Sporadic extracranial AVMs are caused by somatic child when it is located in the ear.
activating MAP2K1 (MEK) mutations.14 Brain AVM
are caused by another activating mutation in KRAS,
which is also involved in the RAS-MAPK signaling
pathway.164 CLINICAL COURSE
AND PROGNOSIS
DIAGNOSIS AVMs tend to worsen with time, causing local destruc-
tion or life-threatening bleeding. Puberty and trauma
trigger growth of the lesion. Improper management,
SUPPORTIVE STUDIES often because of misdiagnosis, such as ligation of feed-
ing arteries, or partial resection of the nidus, can have
Pathology: Histologically, AVMs are poorly
demarcated and consist of distorted arteries and veins
with thickened muscle walls caused by arteriovenous
shunting and fibrosis (see Fig. 147-22C). TABLE 147-8
Differential Diagnosis of Arteriovenous
Imaging: Ultrasonography and color Doppler Malformation
show no mass but an aggregation of high-velocity
Most Likely
arterial and pulsatile venous flow with low resistance. At birth
Vessels are tortuous. On the extremities, noninvasive ■ Congenital hemangioma
follow-up is performed by comparing the arterial out- ■ Infantile fibrosarcoma
flow of the affected limb with the unaffected one. MRI ■ Other sarcoma
is preferred over CT to delineate the extent of an AVM Rapid growth after birth
and differentiate AVM from hemangiomas and other ■ Infantile hemangioma
VMs. Flow voids, corresponding to fast-flow vessels, Rule Out
are pathognomonic of AVM. Arteriography is needed Ear, nose
before any treatment to determine feeding arteries and ■ Lupus erythematosus tumidus
the nidus (see Figs. 147-1E and 147-22B). ■ Sarcoidosis
■ Dabska tumor
2662 Genetic Testing: Genetic testing is indicated in Lip
■ Melkersson-Rosenthal syndrome
patients with an AVM that is associated with multifocal
Kang_CH147_p2636-2668.indd 2662 08/12/18 3:09 pm
dramatic consequences and expansion of the AVM (see
Fig. 147-23). The CMs in CM-AVM are harmless and
bleeding.168 Excision should be complete and radical
with carcinologic margins when possible. It is pre-
22
usually of only cosmetic importance, although flow ceded with superselective embolization to occlude
characteristics are commonly abnormal. the nidus and reduce intraoperative bleeding.19,26 The
overlying skin can be saved if normal; otherwise, it is
widely excised. Coverage options include tissue expan-
sion or a local or free flap.29 Cutaneous expansion can
MANAGEMENT be done before embolization or surgery but may act
as a trigger to stimulate growth of the malformation.
This fast-flow VM is the most complex and difficult Early intervention for “quiescent AVM” (Schobinger
vascular anomaly to treat. Its management is multi- stage I) is debatable and should be considered only if
disciplinary. AVMs should be followed by periodic complete resection is possible.169 Follow-up for at least
evaluation. The goal of cure of an AVM is based on 5 years with annual Doppler ultrasonography or MRI
obliteration (by embolization) and complete removal (or both) is mandatory after any treatment.
Chapter 147 :: Vascular Malformations
of the nidus. Partial resection and proximal ligation For patients with Parkes Weber syndrome, treatment
of the feeding arteries lead to severe complications, should be as conservative as possible. Epiphysiodesis
recurrence, and reexpansion over time. Furthermore, may be necessary to control leg-length discrepancy.
ligature of the proximal arteries prevents access to the This procedure can sometimes aggravate the AVM.146
nidus for embolization, stimulates recruitment of new For patients with HHT, multiple treatments have been
feeding arteries, and therefore expands the malforma- tried to reduce bleeding, including topical application
tion. Mismanagement of AVM can lead to amputation.19 of antiinflammatory drugs, laser, or surgery. Because
of the extensiveness of the lesions, they all give limited
symptom-free intervals.
INTERVENTIONS
Counseling: Genetic counseling is mandatory in
Medications: In a limb AVM or multiple AVFs, CM-AVM, HHT, and PHTS.
elastic stockings can stabilize the lesion and protect the
skin, especially in the early stage. Because AVM is dif-
ficult to eradicate, the focus of management is often to
control the evolution of the malformation rather than REFERENCES
to cure the patient.40,45 A pharmacologic approach with
Marimastat, a synthetic matrix metalloproteinase inhib- 1. Boon LM, Ballieux F, Vikkula M. Pathogenesis of
itor, has been used to treat extensive AVMs with good vascular anomalies. Clin Plast Surg. 2011;38(1):7-19.
results. It reduced extension of the malformation and 2. Mulliken JB, Glowacki J. Hemangiomas and vascular
decreased pain and bruit.46 Unfortunately, the drug is malformations in infants and children: a classification
no longer available. Thalidomide was successfully used based on endothelial characteristics. Plast Reconstr
to reduce the frequency and duration of nosebleeds in Surg. 1982;69(3):412-422.
3. Mulliken JB. Mulliken and Young’s Vascular Anomalies:
patients with HHT.165 It has also been used in unresect-
Hemangiomas and Malformations. 2nd ed. New York:
able stage 3 AVMs for which standard treatment had not Oxford University Press; 2013.
been successful. For some patients, it was used in com- 4. Wassef M, Blei F, Adams D, et al. Vascular anomalies
bination with embolization to improve efficacy. classification: recommendations from the Interna-
tional Society for the Study of Vascular Anomalies.
Procedures: Superselective embolization: In contrast Pediatrics. 2015;136(1):e203-e214.
to AVF, superselective arterial embolization alone is 5. Jacobs AH, Walton RG. The incidence of birthmarks in
only palliative and is done only in unresectable, com- the neonate. Pediatrics. 1976;58(2):218-222.
plicated AVM. The embolized particles need to reach 6. Elajmi A, Clapuyt P, Hammer F, et al. Management of
vascular anomalies in children. Ann Chir Plast Esthet.
the epicenter of the lesion to avoid refilling of the nidus
2016;61(5):480-497.
through new collaterals.166 Direct puncture of the nidus 7. Brouillard P, Vikkula M. Genetic causes of vascular
is another possibility in patients with previous arterial malformations. Hum Mol Genet. 2007;16(Spec No. 2):
ligation or embolization.167 The most common materi- R140-R149.
als are liquids (ethanol, isobutylcyanoacrylate), par- 8. Limaye N, Boon LM, Vikkula M. From germline towards
ticles (Ivalon), foam, and implantable devices (coils, somatic mutations in the pathophysiology of vascu-
microspheres). Embolization is rarely curative unless lar anomalies. Hum Mol Genet. 2009;18(R1):R65-R74.
performed for an arteriovenous fistula. Embolization 9. Nguyen HL, Boon LM, Vikkula M. Genetics of vascu-
can be used alone (palliative approach) or followed lar malformations. Semin Pediatr Surg. 2014;23(4):
by complete surgical excision (curative approach). 221-226.
10. Limaye N, Kangas J, Mendola A, et al. Somatic activat-
The site of injection must be as close as possible to the
ing PIK3CA mutations cause venous malformation.
nidus to permit a superselective approach. Proximal Am J Hum Genet. 2015;97(6):914-921.
occlusion is responsible of secondary refilling of the 11. Boscolo E, Coma S, Luks VL, et al. AKT hyper-
nidus through collateral arteries.24 phosphorylation associated with PI3K mutations in
Radical excision: Surgical resection is usually done lymphatic endothelial cells from a patient with lym- 2663
only after embolization to minimize perioperative phatic malformation. Angiogenesis. 2015;18(2):151-162.
Kang_CH147_p2636-2668.indd 2663 08/12/18 3:09 pm
22 12. Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and
other vascular malformative/overgrowth disorders
31. Toriello HV, Mulliken JB. Accurately renaming macro-
cephaly-cutis marmorata telangiectatica congenita
are caused by somatic mutations in PIK3CA. J Pediatr. (M-CMTC) as macrocephaly-capillary malformation
2015;166(4):1048-54 e1-e5. (M-CM). Am J Med Genet A. 2007;143A(24):3009.
13. Shirley MD, Tang H, Gallione CJ, et al. Sturge- 32. Peterman CM, Vadeboncoeur S, Mulliken JB, et al.
Weber syndrome and port-wine stains caused Wilms tumor screening in diffuse capillary malforma-
by somatic mutation in GNAQ. N Engl J Med. tion with overgrowth and macrocephaly-capillary
2013;368(21):1971-1979. malformation: a retrospective study. J Am Acad Der-
14. Couto JA, Huang AY, Konczyk DJ, et al. Somatic matol. 2017;77(5):874-878.
MAP2K1 mutations are associated with extracra- 33. Adams BB, Lucky AW. Acquired port-wine stains and
nial arteriovenous malformation. Am J Hum Genet. antecedent trauma: case report and review of the lit-
2017;100(3):546-554. erature. Arch Dermatol. 2000;136(7):897-899.
15. Soblet J, Kangas J, Natynki M, et al. Blue rubber bleb 34. Larralde M, Abad ME, Luna PC, et al. Capillary
nevus (BRBN) syndrome is caused by somatic TEK (TIE2) malformation-arteriovenous malformation: a clini-
mutations. J Invest Dermatol. 2017;137(1):207-216. cal review of 45 patients. Int J Dermatol. 2014;53(4):
16. Burrows PE, Laor T, Paltiel H, et al. Diagnostic imaging 458-461.
Part 22
in the evaluation of vascular birthmarks. Dermatol 35. Thiex R, Mulliken JB, Revencu N, et al. A novel
Clin. 1998;16(3):455-488. association between RASA1 mutations and spi-
17. Eerola I, Boon LM, Watanabe S, et al. Locus for nal arteriovenous anomalies. Am J Neuroradiol.
susceptibility for familial capillary malformation 2010;31(4):775-779.
::
(“port-wine stain”) maps to 5q. Eur J Hum Genet. 36. Segatto MM, Schmitt EU, Hagemann LN, et al.
Vascular Diseases
2002;10(6):375-380. Phacomatosis pigmentovascularis type IIa—case
18. Eerola I, Boon LM, Mulliken JB, et al. Capillary malfor- report. Ann Bras Dermatol. 2013;88(6 suppl 1):85-88.
mation-arteriovenous malformation, a new clinical 37. Hofer T. Meyerson phenomenon within a nevus flam-
and genetic disorder caused by RASA1 mutations. meus. The different eczematous reactions within
Am J Hum Genet. 2003;73(6):1240-1249. port-wine stains. Dermatology. 2002;205(2):180-183.
19. Boon LM, Mulliken JB, Vikkula M. RASA1: variable 38. Klapman MH, Yao JF. Thickening and nodules in
phenotype with capillary and arteriovenous malfor- port-wine stains. J Am Acad Dermatol. 2001;44(2):
mations. Curr Opin Genet Dev. 2005;15(3):265-269. 300-302.
20. Revencu N, Boon LM, Mulliken JB, et al. Parkes Weber 39. Valeyrie L, Lebrun-Vignes B, Descamps V, et al.
syndrome, vein of Galen aneurysmal malformation, Pyogenic granuloma within port-wine stains: an
and other fast-flow vascular anomalies are caused by alarming clinical presentation. Eur J Dermatol.
RASA1 mutations. Hum Mutat. 2008;29(7):959-965. 2002;12(4):373-375.
21. Revencu N, Boon LM, Mendola A, et al. RASA1 muta- 40. Etchevers HC, Vincent C, Le Douarin NM, et al. The
tions and associated phenotypes in 68 families with cephalic neural crest provides pericytes and smooth
capillary malformation-arteriovenous malformation. muscle cells to all blood vessels of the face and fore-
Hum Mutat. 2013;34(12):1632-1641. brain. Development. 2001;128(7):1059-1068.
22. Revencu N, Boon LM, Mulliken JB, et al. RASA1 and 41. Van Raamsdonk CD, Bezrookove V, Green G,
capillary malformation-arteriovenous malformation. et al. Frequent somatic mutations of GNAQ in uveal
In: Erickson RP, Wynshaw-Boris A, eds. Inborn Errors of melanoma and blue naevi. Nature. 2009;457(7229):
Development: The Molecular Basis of Clinical Disorders 599-602.
of Morphogenesis. New York: Oxford University Press; 42. Thomas AC, Zeng Z, Riviere JB, et al. Mosaic acti-
2016:1423-1428. vating mutations in GNA11 and GNAQ are associ-
23. Amyere M, Revencu N, Helaers R, et al. Germline loss- ated with phakomatosis pigmentovascularis and
of-function mutations in EPHB4 cause a second form extensive dermal melanocytosis. J Invest Dermatol.
of capillary malformation–arteriovenous malforma- 2016;136(4):770-778.
tion (CM-AVM2) deregulating RAS-MAPK signaling. 43. Riviere JB, Mirzaa GM, O’Roak BJ, et al. De novo germ-
Circulation. 2017;136(11):1037-1048. line and postzygotic mutations in AKT3, PIK3R2 and
24. Enjolras O, Boukobza M, Jdid R. Cervical occult spinal PIK3CA cause a spectrum of related megalencephaly
dysraphism: MRI findings and the value of a vascular syndromes. Nat Genet. 2012;44(8):934-940.
birthmark. Pediatr Dermatol. 1995;12(3):256-259. 44. Wassef M, Vanwijck R, Clapuyt P, et al. Vascular tumours
25. Leung AK, Telmesani AM. Salmon patches in Caucasian and malformations, classification, pathology and
children. Pediatr Dermatol. 1989;6(3):185-187. imaging. Ann Chir Plast Esthet. 2006;51(4-5):263-281.
26. Pratt AG. Birthmarks in infants. AMA Arch Derm 45. Finley JL, Clark RA, Colvin RB, et al. Immunofluores-
Syphilol. 1953;67(3):302-305. cent staining with antibodies to factor VIII, fibronec-
27. Ruiz-Maldonado R, Tamayo L, Laterza AM, et al. tin, and collagenous basement membrane protein in
Phacomatosis pigmentovascularis: a new syndrome? normal human skin and port wine stains. Arch Der-
Report of four cases. Pediatr Dermatol. 1987;4(3): matol. 1982;118(12):971-975.
189-196. 46. Breugem CC, Hennekam RC, van Gemert MJ, et al. Are
28. Happle R. Phacomatosis pigmentovascularis revisited capillary malformations neurovenular or purely neu-
and reclassified. Arch Dermatol. 2005;141(3):385-388. ral? Plast Reconstr Surg. 2005;115(2):578-587.
29. Boukobza M, Enjolras O, Cambra M, et al. Sturge- 47. Enjolras O, Riche MC, Merland JJ. Facial port-wine
Weber syndrome. The current neuroradiologic data. stains and Sturge-Weber syndrome. Pediatrics.
J Radiol. 2000;81(7):765-771. 1985;76(1):48-51.
30. Lee MS, Liang MG, Mulliken JB. Diffuse capillary mal- 48. van der Horst CM, Koster PH, de Borgie CA, et al.
formation with overgrowth: a clinical subtype of Effect of the timing of treatment of port-wine stains
2664 vascular anomalies with hypertrophy. J Am Acad Der- with the flash-lamp-pumped pulsed-dye laser. N Engl
matol. 2013;69(4):589-594. J Med. 1998;338(15):1028-1033.
Kang_CH147_p2636-2668.indd 2664 08/12/18 3:09 pm
49. Michel S, Landthaler M, Hohenleutner U. Recur-
rence of port-wine stains after treatment with the
69. Wouters V, Boon LM, Mulliken JB, et al. TIE2 and cuta-
neomucosal venous malformation. In: Epstein C,
22
flashlamp-pumped pulsed dye laser. Br J Dermatol. Erickson RP, Wynshaw-Boris A, eds. Inborn Errors
2000;143(6):1230-1234. of Development: The Molecular Basis of Clinical
50. Passeron T, Salhi A, Mazer JM, et al. Prognosis and Disorders of Morphogenesis. 2nd ed. New York: Oxford
response to laser treatment of early-onset hyper- University Press; 2008:491-494.
trophic port-wine stains (PWS). J Am Acad Dermatol. 70. Limaye N, Wouters V, Uebelhoer M, et al. Somatic
2016;75(1):64-68. mutations in angiopoietin receptor gene TEK cause
51. Ville D, Enjolras O, Chiron C, et al. Prophylactic anti- solitary and multiple sporadic venous malforma-
epileptic treatment in Sturge-Weber disease. Seizure. tions. Nat Genet. 2009;41(1):118-124.
2002;11(3):145-150. 71. Uebelhoer M, Natynki M, Kangas J, et al. Venous
52. Arzimanoglou AA, Andermann F, Aicardi J, et al. Sturge- malformation-causative TIE2 mutations mediate an
Weber syndrome: indications and results of surgery AKT-dependent decrease in PDGFB. Hum Mol Genet.
in 20 patients. Neurology. 2000;55(10):1472-1479. 2013;22(17):3438-3448.
53. Boon LM, Vanwijck R. Medical and surgical treat- 72. Nätynki M, Kangas J, Miinalainen I, et al. Common and
ment of venous malformations. Ann Chir Plast Esthet. specific effects of TIE2 mutations causing venous mal-
Chapter 147 :: Vascular Malformations
2006;51(4-5):403-411. formations. Hum Mol Genet. 2015;24(22):6374-6389.
54. Dompmartin A, Vikkula M, Boon LM. Venous malfor- 73. Brouillard P, Ghassibé M, Boon L, et al. Identification of
mation: update on aetiopathogenesis, diagnosis and novel glomulin gene mutations responsible for inher-
management. Phlebology. 2010;25:224-235. ited glomuvenous malformations (glomangiomas).
55. Boon LM, Mulliken JB, Enjolras O, et al . Glomuvenous Am J Hum Genet. 2002;71(4):521.
malformation (glomangioma) and venous malforma- 74. Amyere M, Aerts V, Brouillard P, et al. Somatic
tion: distinct clinicopathologic and genetic entities. uniparental isodisomy explains multifocality of
Arch Dermatol. 2004;140(8):971-976. glomuvenous malformations. Am J Hum Genet.
56. Brouillard P, Boon LM, Mulliken JB, et al. Mutations in 2013;92(2):188-196.
a novel factor, glomulin, are responsible for glomu- 75. Brouillard P, Ghassibe M, Penington A, et al. Four
venous malformations (“glomangiomas”). Am J Hum common glomulin mutations cause two thirds of
Genet. 2002;70(4):866-874. glomuvenous malformations (“familial glomangio-
57. Ballieux F, Boon LM, Vikkula M. Blue bleb rubber nevus mas”): evidence for a founder effect. J Med Genet.
syndrome. Handb Clin Neurol. 2015;132:223-230. 2005;42(2):e13.
58. Kaplan RP, Wang JT, Amron DM, et al. Maffucci’s syn- 76. Brouillard P, Enjolras O, Boon LM, et al. Glomulin and
drome: two case reports with a literature review. glomuvenous malformation. In: Epstein CJ, Erickson
J Am Acad Dermatol. 1993;29(5 Pt 2):894-899. RP, Wynshaw-Boris A, eds. Inborn Errors of Devel-
59. Boon L, Mulliken J, Enjolras O, et al. Glomuvenous opment: The Molecular Basis of Clinical Disorders of
malformation (“glomangioma”) is a distinct clini- Morphogenesis. 2nd ed. New York: Oxford University
copathologic and genetic entity. Eur J Hum Genet. Press; 2008:1561-1565.
2002;10:123. 77. Brouillard P, Boon LM, Revencu N, et al. Genotypes
60. Boon LM, Vikkula M. Multiple cutaneous and mucosal and phenotypes of 162 families with a glomulin
venous malformations. In: Pagon RA, Bird TD, Dolan mutation. Mol Syndromol. 2013;4(4):157-164.
CR, Stephens K, eds. GeneReviews. Seattle; 1993. 78. Couto JA, Vivero MP, Kozakewich HP, et al. A
61. Vikkula M, Boon LM, Carraway KL 3rd, et al. somatic MAP3K3 mutation is associated with ver-
Vascular dysmorphogenesis caused by an activating rucous venous malformation. Am J Hum Genet.
mutation in the receptor tyrosine kinase TIE2. Cell. 2015;96(3):480-486.
1996;87(7):1181-1190. 79. Couvineau A, Wouters V, Bertrand G, et al. PTHR1
62. Mallory SB, Enjolras O, Boon LM, et al. Congenital mutations associated with Ollier disease result
plaque-type glomuvenous malformations present- in receptor loss of function. Hum Mol Genet.
ing in childhood. Arch Dermatol. 2006;142(7):892-896. 2008;17(18):2766-2775.
63. Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Ver- 80. Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic
rucous hemangioma revisited. Pediatr Dermatol. mosaic IDH1 and IDH2 mutations are associated
2006;23(3):208-215. with enchondroma and spindle cell hemangioma
64. Hein KD, Mulliken JB, Kozakewich HP, et al. Venous in Ollier disease and Maffucci syndrome. Nat Genet.
malformations of skeletal muscle. Plast Reconstr Surg. 2011;43(12):1256-1261.
2002;110(7):1625-1635. 81. Amyere M, Dompmartin A, Wouters V, et al. Common
65. Berenguer B, Burrows PE, Zurakowski D, et al. Sclero- somatic alterations identified in Maffucci syn-
therapy of craniofacial venous malformations: drome by molecular karyotyping. Mol Syndromol.
complications and results. Plast Reconstr Surg. 2014;5(6):259-267.
1999;104(1):1-11; discussion 12-15. 82. Mazoyer E, Enjolras O, Laurian C, et al. Coagulation
66. Rodriguez-Manero M, Aguado L, Redondo P. abnormalities associated with extensive venous
Pulmonary arterial hypertension in patients with malformations of the limbs: differentiation from
slow-flow vascular malformations. Arch Dermatol. Kasabach-Merritt syndrome. Clin Lab Haematol.
2010;146(12):1347-1352. 2002;24(4):243-51.
67. Dompmartin A, Acher A, Thibon P, et al. Association 83. Hermans C, Dessomme B, Lambert C, et al. Venous
of localized intravascular coagulopathy with venous malformations and coagulopathy. Ann Chir Plast
malformations. Arch Dermatol. 2008;144(7):873-877. Esthet. 2006;51(4-5):388-393.
68. Wouters V, Limaye N, Uebelhoer M, et al. Hereditary 84. Dompmartin A, Ballieux F, Thibon P, et al.
cutaneomucosal venous malformations are caused by Elevated D-dimer level in the differential diag-
TIE2 mutations with widely variable hyper-phosphor- nosis of venous malformations. Arch Dermatol. 2665
ylating effects. Eur J Hum Genet. 2010;18(4):414-420. 2009;145(11):1239-1244.
Kang_CH147_p2636-2668.indd 2665 08/12/18 3:09 pm
22 85. Wassef M, Enjolras O. Superficial vascular malforma-
tions: classification and histopathology. Ann Pathol.
106. Moller G, Priemel M, Amling M, et al. The Gorham-
Stout syndrome (Gorham’s massive osteolysis).
1999;19(3):253-264. A report of six cases with histopathological findings.
86. Kato N, Kumakiri M, Ohkawara A. Localized form of J Bone Joint Surg Br. 1999;81(3):501-506.
multiple glomus tumors: report of the first case show- 107. Connell F, Kalidas K, Ostergaard P, et al. Linkage and
ing partial involution. J Dermatol. 1990;17(7):423-428. sequence analysis indicate that CCBE1 is mutated in
87. Goodman TF, Abele DC. Multiple glomus tumors. recessively inherited generalised lymphatic dyspla-
A clinical and electron microscopic study. Arch sia. Hum Genet. 2010;127(2):231-241.
Dermatol. 1971;103(1):11-23. 108. Brouillard P, Boon L, Vikkula M. Genetics of lymphatic
88. Paltiel HJ, Burrows PE, Kozakewich HP, et al. Soft- anomalies. J Clin Invest. 2014;124(3):898-904.
tissue vascular anomalies: utility of US for diagnosis. 109. Brice G, Child AH, Evans A, et al. Milroy disease and
Radiology. 2000;214(3):747-754. the VEGFR-3 mutation phenotype. J Med Genet.
89. Konez O, Burrows PE. Magnetic resonance of vascu- 2005;42(2):98-102.
lar anomalies. Magn Reson Imaging Clin North Am. 110. Alomari AI, Burrows PE, Lee EY, et al. CLOVES syn-
2002;10(2):363-388, vii. drome with thoracic and central phlebectasia:
90. Boscolo E, Limaye N, Huang L, et al. Rapamycin increased risk of pulmonary embolism. J Thorac Car-
Part 22
improves TIE2-mutated venous malformation in diovasc Surg. 2010;140(2):459-463.
murine model and human subjects. J Clin Invest. 111. Edwards PD, Rahbar R, Ferraro NF, et al. Lymphatic
2015;125(9):3491-3504. malformation of the lingual base and oral floor. Plast
91. Boon LM, Hammer J, Seront E, et al. Rapamycin as Reconstr Surg. 2005;115(7):1906-1915.
::
novel treatment for refractory-to-standard-care 112. Ghalamkarpour A, Morlot S, Raas-Rothschild A,
Vascular Diseases
slow-flow vascular malformations. Plast Reconstr et al. Hereditary lymphedema type I associated with
Surg. 2015;136(4 suppl):38. VEGFR3 mutation: the first de novo case and atypical
92. Yuksekkaya H, Ozbek O, Keser M, et al. Blue rubber presentations. Clin Genet. 2006;70(4):330-335.
bleb nevus syndrome: successful treatment with siro- 113. Daniel-Spiegel E, Ghalamkarpour A, Spiegel R, et al.
limus. Pediatrics. 2012;129(4):e1080-e1084. Hydrops fetalis: an unusual prenatal presentation of
93. Hammer FD, Boon LM, Mathurin P, et al. Ethanol hereditary congenital lymphedema. Prenat Diagn.
sclerotherapy of venous malformations: evaluation 2005;25(11):1015-1018.
of systemic ethanol contamination. J Vasc Interv 114. Osborn AJ, Dickie P, Neilson DE, et al. Activating
Radiol. 2001;12(5):595-600. PIK3CA alleles and lymphangiogenic phenotype
94. Park HS, Do YS, Park KB, et al. Clinical outcome and of lymphatic endothelial cells isolated from lym-
predictors of treatment response in foam sodium phatic malformations. Hum Mol Genet. 2015;24(4):
tetradecyl sulfate sclerotherapy of venous malforma- 926-938.
tions. Eur Radiol. 2016;26(5):1301-1310. 115. Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic
95. Sannier K, Dompmartin A, Théron J, et al. A new activating mutations in PIK3CA cause CLOVES syn-
sclerosing agent in the treatment of venous mal- drome. Am J Hum Genet. 2012;90(6):1108-1115.
formations. Study on 23 cases Intervent Radiol. 116. Irrthum A, Karkkainen MJ, Devriendt K, et al.
2004;10:113-127. Congenital hereditary lymphedema caused by a
96. Dompmartin A, Blaizot X, Théron J, et al. Radio- mutation that inactivates VEGFR3 tyrosine kinase.
opaque ethylcellulose-ethanol is a safe and efficient Am J Hum Genet. 2000;67(2):295-301.
sclerosing agent for venous malformations. Eur 117. Evans AL, Bell R, Brice G, et al. Identification of
Radiol. 2011;21(12):2647-2656. eight novel VEGFR-3 mutations in families with
97. Fishman SJ, Fox VL. Visceral vascular anomalies. primary congenital lymphoedema. J Med Genet.
Gastrointest Endosc Clin North Am. 2001;11(4): 2003;40(9):697-703.
813-834, viii. 118. Irrthum A, Devriendt K, Chitayat D, et al. Mutations in
98. Fishman SJ, Smithers CJ, Folkman J, et al. Blue rubber the transcription factor gene SOX18 underlie recessive
bleb nevus syndrome: surgical eradication of gastro- and dominant forms of hypotrichosis-lymphedema-
intestinal bleeding. Ann Surg. 2005;241(3):523-528. telangiectasia. Am J Hum Genet. 2003;72(6):1470-1478.
99. Marler JJ, Fishman SJ, Upton J, et al. Prenatal 119. Ghalamkarpour A, Holnthoner W, Saharinen P, et al.
diagnosis of vascular anomalies. J Pediatr Surg. Recessive primary congenital lymphoedema caused
2002;37(3):318-326. by a VEGFR3 mutation. J Med Genet. 2009;46(6):
100. Sybert VP, McCauley E. Turner’s syndrome. N Engl J 399-404.
Med. 2004;351(12):1227-1238. 120. Mansour S, Brice GW, Jeffery S, et al. Lymphedema-
101. Cohen MM Jr. Klippel-Trenaunay syndrome. Am J Med distichiasis syndrome. In: Pagon RA, Adam MP,
Genet. 2000;93(3):171-175. Ardinger HH, et al, eds. GeneReviews. Seattle; 1993.
102. Oduber CE, van der Horst CM, Hennekam RC. Klippel- 121. Ostergaard P, Simpson MA, Mendola A, et al. Mutations
Trenaunay syndrome: diagnostic criteria and hypoth- in KIF11 cause autosomal-dominant microcephaly vari-
esis on etiology. Ann Plast Surg. 2008;60(2):217-223. ably associated with congenital lymphedema and cho-
103. Alomari AI, Thiex R, Mulliken JB. Hermann Friedberg’s rioretinopathy. Am J Hum Genet. 2012;90(2):356-362.
case report: an early description of CLOVES syn- 122. Schlogel MJ, Mendola A, Fastre E, et al. No evidence
drome. Clin Genet. 2010;78(4):342-347. of locus heterogeneity in familial microcephaly with
104. Biesecker LG, Happle R, Mulliken JB, et al. Proteus syn- or without chorioretinopathy, lymphedema, or men-
drome: diagnostic criteria, differential diagnosis, and tal retardation syndrome. Orphanet J Rare Dis. 2015;
patient evaluation. Am J Med Genet. 1999;84(5):389-395. 10:52.
105. Lala S, Mulliken JB, Alomari AI, et al. Gorham-Stout 123. Hennekam RC, Geerdink RA, Hamel BC, et al. Autosomal
disease and generalized lymphatic anomaly— recessive intestinal lymphangiectasia and lymph-
2666 clinical, radiologic, and histologic differentiation. edema, with facial anomalies and mental retardation.
Skeletal Radiol. 2013;42(7):917-924. J Med GenetAm J Med Genet. 1989;34(4):593-600.
Kang_CH147_p2636-2668.indd 2666 08/12/18 3:09 pm
124. Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1
cause generalized lymph vessel dysplasia in humans.
143. Letteboer TG, Mager HJ, Snijder RJ, et al. Genotype-
phenotype relationship for localization and age
22
Nat Genet. 2009;41(12):1272-1274. distribution of telangiectases in hereditary hemor-
125. Ostergaard P, Simpson MA, Connell FC, et al. rhagic telangiectasia. J Med GenetAm J Med Genet A.
Mutations in GATA2 cause primary lymphedema 2008;146A(21):2733-2739.
associated with a predisposition to acute myeloid 144. Morgan T, McDonald J, Anderson C, et al. Intracranial
leukemia (Emberger syndrome). Nat Genet. hemorrhage in infants and children with hereditary
2011;43(10):929-931. hemorrhagic telangiectasia (Osler-Weber-Rendu
126. Mendola A, Schlogel MJ, Ghalamkarpour A, et al. syndrome). Pediatrics. 2002;109(1):E12.
Mutations in the VEGFR3 signaling pathway explain 145. Govani FS, Shovlin CL. Hereditary haemorrhagic tel-
36% of familial lymphedema. Mol Syndromol. angiectasia: a clinical and scientific review. Eur J Hum
2013;4(6):257-266. Genet. 2009;17(7):860-871.
127. Galambos C, Nodit L. Identification of lymphatic 146. Enjolras O, Chapot R, Merland JJ. Vascular anomalies
endothelium in pediatric vascular tumors and mal- and the growth of limbs: a review. J Pediatr Orthop B.
formations. Pediatr Dev Pathol. 2005;8(2):181-189. 2004;13(6):349-357.
128. Dubois J, Garel L, Abela A, et al. Lymphangiomas in 147. Tan WH, Baris HN, Burrows PE, et al. The spectrum of
Chapter 147 :: Vascular Malformations
children: percutaneous sclerotherapy with an alco- vascular anomalies in patients with PTEN mutations:
holic solution of zein. Radiology. 1997;204(3):651-654. implications for diagnosis and management. J Med
129. Dubois J, Garel L, Grignon A, et al. Imaging of heman- Genet. 2007;44(9):594-602.
giomas and vascular malformations in children. Acad 148. Cohen MM Jr. Vasculogenesis, angiogenesis, heman-
Radiol. 1998;5(5):390-400. giomas, and vascular malformations. J Med GenetAm
130. Peterman CM, Fevurly RD, Alomari AI, et al. Sonographic J Med Genet. 2002;108(4):265-274.
screening for Wilms tumor in children with CLOVES 149. Enjolras O, Logeart I, Gelbert F, et al. Arteriovenous
syndrome. Pediatr Blood Cancer. 2017;64(12). malformations: a study of 200 cases. Ann Dermatol
131. Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for Venereol. 2000;127(1):17-22.
the treatment of complicated vascular anomalies in 150. Kohout MP, Hansen M, Pribaz JJ, et al. Arteriovenous
children. Pediatr Blood Cancer. 2011;57(6):1018-1024. malformations of the head and neck: natural
132. Adams DM, Trenor CC 3rd, Hammill AM, et al. Effi- history and management. Plast Reconstr Surg.
cacy and safety of sirolimus in the treatment 1998;102(3):643-654.
of complicated vascular anomalies. Pediatrics. 151. Revencu N, Boon LM, Mulliken JB, et al. RASA1 and
2016;137(2):1-10. capillary malformation-arteriovenous malforma-
133. Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil tion. In: Epstein C, Erickson R, Wynshaw-Boris A,
for severe lymphatic malformations. N Engl J Med. eds. Inborn Errors of Development: The Molecular
2012;366(4):384-386. Basis of Clinical Disorders of Morphogenesis. 2nd ed.
134. Rankin H, Zwicker K, Trenor CC 3rd. Caution is recom- Oxford, UK: Oxford University Press; 2008:647-650.
mended prior to sildenafil use in vascular anomalies. 152. Johnson DW, Berg JN, Baldwin MA, et al. Mutations
Pediatr Blood Cancer. 2015;62(11):2015-2017. in the activin receptor-like kinase 1 gene in heredi-
135. Koshy JC, Eisemann BS, Agrawal N, et al. Sildenafil tary haemorrhagic telangiectasia type 2. Nat Genet.
for microcystic lymphatic malformations of the head 1996;13(2):189-195.
and neck: a prospective study. Int J Pediatr Otorhino- 153. McAllister KA, Grogg KM, Johnson DW, et al. Endoglin,
laryngol. 2015;79(7):980-982. a TGF-beta binding protein of endothelial cells, is the
136. Mathur NN, Rana I, Bothra R, et al. Bleomycin sclero- gene for hereditary haemorrhagic telangiectasia
therapy in congenital lymphatic and vascular type 1. Nat Genet. 1994;8(4):345-351.
malformations of head and neck. Int J Pediatr Otorhi- 154. Gallione CJ, Repetto GM, Legius E, et al. A combined
nolaryngol. 2005;69(1):75-80. syndrome of juvenile polyposis and hereditary
137. Claesson G, Kuylenstierna R. OK-432 therapy for lym- haemorrhagic telangiectasia associated with muta-
phatic malformation in 32 patients (28 children). Int J tions in MADH4 (SMAD4). Lancet. 2004;363(9412):
Pediatr Otorhinolaryngol. 2002;65(1):1-6. 852-859.
138. Harsha WJ, Perkins JA, Lewis CW, et al. Pediatric 155. Wooderchak-Donahue WL, McDonald J, O’Fallon
admissions and procedures for lymphatic malforma- B, et al. BMP9 mutations cause a vascular-anomaly
tions in the United States: 1997 and 2000. Lymphat syndrome with phenotypic overlap with heredi-
Res Biol. 2005;3(2):58-65. tary hemorrhagic telangiectasia. Am J Hum Genet.
139. Ballieux F, Modarressi A, Hammer F, et al. 2013;93(3):530-537.
Reconstructive surgery in the management of a 156. Ola R, Dubrac A, Han J, et al. PI3 kinase inhibition
patient with CLOVES syndrome. J Plast Reconstr improves vascular malformations in mouse models
Aesthet Surg. 2013;66(12):1813-1815. of hereditary haemorrhagic telangiectasia. Nat Com-
140. Hosman AE, Devlin HL, Silva BM, et al. Specific cancer mun. 2016;7:13650.
rates may differ in patients with hereditary haemor- 157. Blume-Peytavi U, Adler YD, Geilen CC, et al. Multiple
rhagic telangiectasia compared to controls. Orphanet familial cutaneous glomangioma: a pedigree of 4
J Rare Dis. 2013;8:195. generations and critical analysis of histologic and
141. Lester J, Ruano-Calderon LA, Gonzalez-Olhovich I. genetic differences of glomus tumors. J Am Acad Der-
Wyburn-Mason syndrome. J Neuroimaging. 2005; matol. 2000;42(4):633-639.
15(3):284-285. 158. Macmurdo CF, Wooderchak-Donahue W, Bayrak-
142. Rodesch G, Hurth M, Alvarez H, et al. Classification Toydemir P, et al. RASA1 somatic mutation and
of spinal cord arteriovenous shunts: proposal for a variable expressivity in capillary malformation/
reappraisal—the Bicetre experience with 155 con- arteriovenous malformation (CM/AVM) syndrome.
secutive patients treated between 1981 and 1999. J Med GenetAm J Med Genet A. 2016;170(6):1450- 2667
Neurosurgery. 2002;51(2):374-379; discussion 379-380. 1454.
Kang_CH147_p2636-2668.indd 2667 08/12/18 3:09 pm
22 159. van der Geer P, Henkemeyer M, Jacks T, et al.
Aberrant Ras regulation and reduced p190 tyrosine
164. Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic activat-
ing KRAS mutations in arteriovenous malformations
phosphorylation in cells lacking p120-Gap. Mol Cell of the brain. N Engl J Med. 2018;378(3):250-261.
Biol. 1997;17(4):1840-1847. 165. Lebrin F, Srun S, Raymond K, et al. Thalidomide stimu-
160. Henkemeyer M, Rossi DJ, Holmyard DP, et al. lates vessel maturation and reduces epistaxis in indi-
Vascular system defects and neuronal apoptosis in viduals with hereditary hemorrhagic telangiectasia.
mice lacking ras GTPase-activating protein. Nature. Nat Med. 2010;16(4):420-428.
1995;377(6551):695-701. 166. Burrows PE, Fellows KE. Techniques for manage-
161. Kawasaki J, Aegerter S, Fevurly RD, et al. RASA1 ment of pediatric vascular anomalies. In: Cope C, ed.
functions in EPHB4 signaling pathway to sup- Current Techniques in Interventional Radiology. Phila-
press endothelial mTORC1 activity. J Clin Invest. delphia: Current Medicine; 1995:12-27.
2014;124(6):2774-2784. 167. Yakes WF, Rossi P, Odink H. How I do it. Arteriovenous
162. Xiao Z, Carrasco R, Kinneer K, et al. EphB4 promotes malformation management. Cardiovasc Intervent
or suppresses Ras/MEK/ERK pathway in a context- Radiol. 1996;19(2):65-71.
dependent manner: implications for EphB4 as a can- 168. Wu JK, Bisdorff A, Gelbert F, et al. arteriovenous mal-
cer target. Cancer Biol Ther. 2012;13(8):630-637. formation: evaluation, management, and outcome.
Part 22
163. Zhou XP, Marsh DJ, Hampel H, et al. Germline and Plast Reconstr Surg. 2005;115(4):985-995.
germline mosaic PTEN mutations associated with a 169. Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial
Proteus-like syndrome of hemihypertrophy, lower arteriovenous malformations: natural progression
limb asymmetry, arteriovenous malformations and and recurrence after treatment. Plast Reconstr Surg.
::
lipomatosis. Hum Mol Genet. 2000;9(5):765-768. 2010;125(4):1185-1194.
Vascular Diseases
2668
Kang_CH147_p2636-2668.indd 2668 08/12/18 3:09 pm
S-ar putea să vă placă și
- ISSVA Ijms-23-02358Document13 paginiISSVA Ijms-23-02358Henrique PeresÎncă nu există evaluări
- Low Flow Vascular Malformations ManagementDocument23 paginiLow Flow Vascular Malformations ManagementVedarth DashÎncă nu există evaluări
- Imaging of Vascular MalformationsDocument16 paginiImaging of Vascular MalformationsHector Hernandez-SoriaÎncă nu există evaluări
- Venous Malformations: Constantino S. Peña and Guilherme DabusDocument13 paginiVenous Malformations: Constantino S. Peña and Guilherme DabusAsyurÎncă nu există evaluări
- Vascular Malformations: A Review: Joshua A. Cox, MD Erica Bartlett, MD Edward I. Lee, MDDocument6 paginiVascular Malformations: A Review: Joshua A. Cox, MD Erica Bartlett, MD Edward I. Lee, MDYusmiatiÎncă nu există evaluări
- Lymphatic Malformations: Current Status: EditorialDocument2 paginiLymphatic Malformations: Current Status: EditorialDr Basant Kumar GuptaÎncă nu există evaluări
- Update On The Classification of Hemangioma: Antony George, Varghese Mani, Ahammed NoufalDocument4 paginiUpdate On The Classification of Hemangioma: Antony George, Varghese Mani, Ahammed NoufalyanuararipratamaÎncă nu există evaluări
- Imaging and Management of Vascular MalformationsDocument11 paginiImaging and Management of Vascular MalformationsMessah V DianaÎncă nu există evaluări
- C24 LymphaticsDocument23 paginiC24 LymphaticsErneslito LucasÎncă nu există evaluări
- Updateontheclassifiationofhemangioma PDFDocument5 paginiUpdateontheclassifiationofhemangioma PDFDella Elvina RoeslandÎncă nu există evaluări
- Vascular StainsDocument15 paginiVascular StainsIlse PalaciosÎncă nu există evaluări
- Vascular Tumors: Infantile HemangiomaDocument4 paginiVascular Tumors: Infantile HemangiomavicenteturasÎncă nu există evaluări
- Deep Venous Thrombosis: Anne M. Aquila, APRNDocument20 paginiDeep Venous Thrombosis: Anne M. Aquila, APRNTan RøbìñÎncă nu există evaluări
- Strokeaha 117 018152Document8 paginiStrokeaha 117 018152Mikha ManurungÎncă nu există evaluări
- Review ArticleDocument8 paginiReview ArticleOctavianmdÎncă nu există evaluări
- Aas AhaDocument12 paginiAas AhaArick Frendi AndriyanÎncă nu există evaluări
- Spinal Vascular Malformations: Michelle J. Clarke William E. Krauss Mark A. PichelmannDocument9 paginiSpinal Vascular Malformations: Michelle J. Clarke William E. Krauss Mark A. Pichelmannmetasoniko81Încă nu există evaluări
- AtvbahaDocument16 paginiAtvbahaapi-654112138Încă nu există evaluări
- HematomyeliaDocument8 paginiHematomyeliaJessica Seunghye ParkÎncă nu există evaluări
- Pain Mechanisms in Vascular Disease - Dor VascularDocument12 paginiPain Mechanisms in Vascular Disease - Dor VascularAllsÎncă nu există evaluări
- Varicose Veins: BSC Mbbs Mrcs PHDDocument9 paginiVaricose Veins: BSC Mbbs Mrcs PHDNelly ElizabethÎncă nu există evaluări
- Cervicofacial Vascular Anomalies. I. Hemangiomas and Other Benign Vascular TumorsDocument9 paginiCervicofacial Vascular Anomalies. I. Hemangiomas and Other Benign Vascular TumorsBikash ShresthaÎncă nu există evaluări
- Genetics of Hemangiomas, Vascular Malformations, and Primary LymphedemDocument7 paginiGenetics of Hemangiomas, Vascular Malformations, and Primary LymphedemMiradz 'demmy' MuhidinÎncă nu există evaluări
- Week 11 - Cardiac Microbes, Tumors, Heart FailureDocument20 paginiWeek 11 - Cardiac Microbes, Tumors, Heart Failureshivani patelÎncă nu există evaluări
- Avm TheraphyDocument11 paginiAvm TheraphynurminsyahÎncă nu există evaluări
- Tiempos en Malformaciones VascularesDocument7 paginiTiempos en Malformaciones VascularesJosue AyalaÎncă nu există evaluări
- Genetics of Intracranial AneurysmsDocument8 paginiGenetics of Intracranial AneurysmsAnand KumarÎncă nu există evaluări
- Translate SARAF2Document7 paginiTranslate SARAF2anon_174720041Încă nu există evaluări
- Vascular MalformationsDocument28 paginiVascular Malformationswindi_martika2803100% (1)
- Brain Arteriovenous MalformationsDocument20 paginiBrain Arteriovenous MalformationsTony NgÎncă nu există evaluări
- Baharvadat Et Al svn-2019-000269Document9 paginiBaharvadat Et Al svn-2019-000269Bobby VarkeyÎncă nu există evaluări
- Vascular Skin LesionsDocument39 paginiVascular Skin LesionsApril Mae Magos LabradorÎncă nu există evaluări
- Week 10 - Hypertension, Atherosclerosis, ArrhythmiaDocument14 paginiWeek 10 - Hypertension, Atherosclerosis, Arrhythmiashivani patel100% (1)
- Table 1. 2 Settings of MyxomaDocument8 paginiTable 1. 2 Settings of MyxomaGlenda DavisÎncă nu există evaluări
- Chronic Venous Disorders New TemplateDocument43 paginiChronic Venous Disorders New TemplateamrÎncă nu există evaluări
- Al Referat - Luka BakarDocument3 paginiAl Referat - Luka BakarhanifaÎncă nu există evaluări
- Vascular Malformations of The Central Nervous SystemDocument9 paginiVascular Malformations of The Central Nervous SystemFranklin Aranda100% (2)
- PBC 23124 PDFDocument7 paginiPBC 23124 PDFBalaguer RodolfoÎncă nu există evaluări
- Cap 14Document49 paginiCap 14Saul RivasÎncă nu există evaluări
- Intracranial Dural Arteriovenous Fistulae: Presentation and Natural History ClassificationDocument3 paginiIntracranial Dural Arteriovenous Fistulae: Presentation and Natural History ClassificationFinger JempolÎncă nu există evaluări
- Chronic Ulceration of The Leg 2013 Surgery OxfordDocument5 paginiChronic Ulceration of The Leg 2013 Surgery OxfordSanjaya SenevirathneÎncă nu există evaluări
- Modified Alvarado Score and Its Application in The Diagnosis of Acute AppendicitisDocument2 paginiModified Alvarado Score and Its Application in The Diagnosis of Acute AppendicitisanunadÎncă nu există evaluări
- Hemodynamics and Diagnosis Venous Disease-Jvs 1207Document21 paginiHemodynamics and Diagnosis Venous Disease-Jvs 1207Fahrudin ŠabanovićÎncă nu există evaluări
- Vascular Malformation and LymphoedemaDocument44 paginiVascular Malformation and LymphoedemaWorku KifleÎncă nu există evaluări
- Vascular TumorDocument14 paginiVascular Tumordicky achmad ansyariÎncă nu există evaluări
- Definition, Presentation, Treatment, and Histology: by Dr. Martin Mihm, Jr. and Linda Rozell-Shannon, M.SDocument17 paginiDefinition, Presentation, Treatment, and Histology: by Dr. Martin Mihm, Jr. and Linda Rozell-Shannon, M.SAriandindi AriandiÎncă nu există evaluări
- Managing Systemic Venous Occlusions in Children: Reviewarticle Open AccessDocument11 paginiManaging Systemic Venous Occlusions in Children: Reviewarticle Open AccessakshayajainaÎncă nu există evaluări
- AneurysmDocument40 paginiAneurysmRichardÎncă nu există evaluări
- Alvarez2007 Veine de GalienDocument18 paginiAlvarez2007 Veine de GalienModou NianeÎncă nu există evaluări
- Pathology Week9 BloodVesselsDocument4 paginiPathology Week9 BloodVesselsSalifyanji SimpambaÎncă nu există evaluări
- Dilatasi VenaDocument14 paginiDilatasi Venaworo winantiÎncă nu există evaluări
- Seminars in Diagnostic Pathology Volume 30 Issue 1 2013 (Doi 10.1053 - J.semdp.2012.01.003) Requena, Luis Kutzner, Heinz - HemangioendotheliomaDocument16 paginiSeminars in Diagnostic Pathology Volume 30 Issue 1 2013 (Doi 10.1053 - J.semdp.2012.01.003) Requena, Luis Kutzner, Heinz - HemangioendotheliomazaimmuhtarÎncă nu există evaluări
- Boroumand 2016Document3 paginiBoroumand 2016Winston FontesÎncă nu există evaluări
- Malignant Renal Tumors 2Document1 paginăMalignant Renal Tumors 2iqz saniomeÎncă nu există evaluări
- BloodVessels 3.ppsDocument32 paginiBloodVessels 3.ppssiddarth reddyÎncă nu există evaluări
- Arterial Fibromuscular Dysplasia - JMCPDocument22 paginiArterial Fibromuscular Dysplasia - JMCPKarla Castro PereyraÎncă nu există evaluări
- jgf2 4Document7 paginijgf2 4Permata GBKP Runggun SurabayaÎncă nu există evaluări
- Vasculitesdiagdiferencial 20220511173912Document7 paginiVasculitesdiagdiferencial 20220511173912Isabelle JácomeÎncă nu există evaluări
- 6th Year AVM, Anurysm, Venous and LymphaticDocument103 pagini6th Year AVM, Anurysm, Venous and LymphaticamrÎncă nu există evaluări
- Hair-Grooming Syncope SeizuresDocument5 paginiHair-Grooming Syncope Seizurestototm4480Încă nu există evaluări
- Glossary EyeDocument228 paginiGlossary EyeCarolus AugustusÎncă nu există evaluări
- Cis Self-Study Lesson Plan: Otolaryngological InstrumentationDocument4 paginiCis Self-Study Lesson Plan: Otolaryngological Instrumentationjerimiah_manzonÎncă nu există evaluări
- Al Ameen Medical CollegeDocument13 paginiAl Ameen Medical CollegeRakeshKumar1987Încă nu există evaluări
- Respiratory ArrestDocument4 paginiRespiratory Arrestbrain_warming25Încă nu există evaluări
- Traction SDocument5 paginiTraction SJessa BorreÎncă nu există evaluări
- CircumcisionDocument4 paginiCircumcisionapi-244757499Încă nu există evaluări
- Chranjivi HospitalsDocument12 paginiChranjivi HospitalsRATI RAMÎncă nu există evaluări
- EszopicloneDocument1 paginăEszopicloneapi-300103989Încă nu există evaluări
- Atlas NeuroanatomyDocument874 paginiAtlas NeuroanatomyBilge Yilmaz100% (3)
- Hospital ProposalDocument5 paginiHospital ProposalSushant Sharma100% (1)
- @anesthesia Books 2016 Principles-Ana PDFDocument358 pagini@anesthesia Books 2016 Principles-Ana PDFsilvanaÎncă nu există evaluări
- Guíe ADHA Adult (2011) PDFDocument15 paginiGuíe ADHA Adult (2011) PDFmanoilocÎncă nu există evaluări
- Persistent Pulmonary Hypertension of The NewbornDocument4 paginiPersistent Pulmonary Hypertension of The NewbornMarwa Adly100% (1)
- Dopamine Vs EpinephrineDocument11 paginiDopamine Vs EpinephrineAyiek WicaksonoÎncă nu există evaluări
- Neuromuscular Junction by Dr. RoomiDocument20 paginiNeuromuscular Junction by Dr. RoomiMudassar Roomi100% (1)
- Posterior Crossbite in Primary and Mixed Dentition - Etiology and Management PedoDocument41 paginiPosterior Crossbite in Primary and Mixed Dentition - Etiology and Management PedoFourthMolar.comÎncă nu există evaluări
- 7 FTPDocument6 pagini7 FTPhaddig8Încă nu există evaluări
- Charge Nurse ResponsibilitiesDocument4 paginiCharge Nurse ResponsibilitiesdocpanchuÎncă nu există evaluări
- (HeartSim 200 DfU) - 982Document86 pagini(HeartSim 200 DfU) - 982edgargadoÎncă nu există evaluări
- Effective SLPDocument10 paginiEffective SLPRené Ignacio Guzmán SalgadoÎncă nu există evaluări
- AVPUDocument2 paginiAVPUAnissa Citra DewiÎncă nu există evaluări
- AprobacijaDocument4 paginiAprobacijaMario Marco Del TintorettoÎncă nu există evaluări
- The Correlation Between Osteophorosis and Tooth LossDocument10 paginiThe Correlation Between Osteophorosis and Tooth LossAgeng BudianantiÎncă nu există evaluări
- Otitis MediaDocument7 paginiOtitis MediaNorminaKiramAkmadÎncă nu există evaluări
- DERM EXAM Clinical Skills Practical Rubric UPDATED-1Document3 paginiDERM EXAM Clinical Skills Practical Rubric UPDATED-1AlaaDaghlasÎncă nu există evaluări
- Molar Incisor Hypomineralisation MIH - An OverviewDocument9 paginiMolar Incisor Hypomineralisation MIH - An OverviewBabaÎncă nu există evaluări
- Fink, Carson & DeVellis - Adult Circumcision Outcomes Study PDFDocument4 paginiFink, Carson & DeVellis - Adult Circumcision Outcomes Study PDFOmar MohammedÎncă nu există evaluări
- Chinook Medical CatalogDocument52 paginiChinook Medical CatalogsullivbtÎncă nu există evaluări
- Guideline Hypoglycemia PDFDocument13 paginiGuideline Hypoglycemia PDFleslyjanet100% (1)