Documente Academic
Documente Profesional
Documente Cultură
A. Latimer Diagrams in Acid E Vs She in 1 M H
Încărcat de
Nadia Maharani Chadiza0 evaluări0% au considerat acest document util (0 voturi)
22 vizualizări2 paginiVg
Titlu original
Latimer 09
Drepturi de autor
© © All Rights Reserved
Formate disponibile
PDF, TXT sau citiți online pe Scribd
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentVg
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
0 evaluări0% au considerat acest document util (0 voturi)
22 vizualizări2 paginiA. Latimer Diagrams in Acid E Vs She in 1 M H
Încărcat de
Nadia Maharani ChadizaVg
Drepturi de autor:
© All Rights Reserved
Formate disponibile
Descărcați ca PDF, TXT sau citiți online pe Scribd
Sunteți pe pagina 1din 2
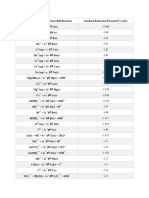
A.
LATIMER DIAGRAMS IN ACID Eo½ vs SHE in 1 M H+
+0.10 - 0.37 -1.63 +1.0 +0.36 -0.25 -0.2
TiO2+ ----- Ti3+ ------- Ti2+ -------Ti VO2+ ------- VO2+ ------- V3+ --------V2+ ---------V
+1.33 -0.41 -0.91 1.82 -0.28 1.68 -0.25
Cr2O72- ------ Cr3+ --------Cr2+ --------Cr Co3+ --------- Co2+ --------- Co NiO2 ------Ni2+ -------- Ni
↓ ------------------------↑
-0.74
+0.564 +2.26 +1.0 +1.5 -1.20 2.20 0.77 -0.41
MnO4- ------ MnO42- ------ MnO2 (s)------Mn+3 ----- Mn2+ ----- Mn (s) FeO42- -----Fe3+ -------Fe2+ ------- Fe
↓ -------------------------------------------------------------↑
+1.51
0.361 0.73 0.40 0.26 -0.4 2 1.26 0.99
Fe(CN)63- -------Fe(CN)64- ReO4- ------- ReO3 ----------ReO2 -------- Re ----- Re- PdO3 -------Pd4+ ---------Pd2+ --------Pd
0.9 1.6 1.3 0.45 1.8 0.153 0.521
RuO4 ------- RuO4- ---------- RuO42- ------- Ru2+ -------Ru CuO+ -------Cu2+ --------Cu+ -------Cu
↓ -----------------------↑
0.34
2.1 1.98 0.799 0.22 -0.152 0.92 0.789
AgO+ ------ Ag2+ ------ Ag+ -------Ag AgCl (S) -----Ag AgI (s)------- Ag Hg2+ ------- Hg22+ ------- Hg
1.36 1.83 1.00 0.84 1.19
Au3+ --------- Au+ -----------Au AuCl4- ------------Au PtO2 --------Pt2+ --------Pt
-0.76 -0.40 -2.7 -2.35 +0.267
Zn2+ ------- Zn Cd2+ ---------- Cd Na+ --------Na Mg2+ --------Mg Hg2Cl2 (S) -------- Hg
0.15 -0.136 1.46 -0.126 1.25 -0.336 -1.68
Sn4+ ------- Sn2+ ---------Sn PbO2 (S) --------Pb2+ -------- Pb Tl3+ --------Tl+ -------- Tl Al3+ -------- Al
-0.106 0.517 0.132 -0.848 -0.143 -0.25 -0.42
CO2 -------CO ------- C ---------CH4 SiO2 ------- Si ----------SiH4 GeO2 ------------Ge --------GeH4
0.79 1.07 1.00 1.59 1.77 -1.87 +1.41 +1.28
NO3- -----N2O4 (g) ------- HNO2 --------NO (g) -------N2O (g) ------N2 (g) ------NH3OH+ ------ N2H5+ ------ NH4+
↓----------------------------↑ ----------------------------- --------------------------- ------------------------------
0.94 1.29 - 0.050 1.35
-0.28 -0.50 -0.51 -0.05 0.559 0.247 -0.38
H3PO4 --------H3PO3 ----------H3PO2 -------P4 (s) --------PH3 (g) H3AsO4 -------H3AsO3 --------As ------- AsH3 (g)
0.58 0.212 -0.51 1.6 0.32 1.15 0.74 -0.32
Sb2O5 ------ SbO+ -------Sb ------SbH3 (g) Bi2O5 ---- BiO+ ----- Bi SeO42- -----H2SeO3 ------Se------- H2Se
2.075 0.68 1.77 -0.22 0.57 0.51 0.08 0.50 0.14
O3 ------- O2 ------ H2O2 ------ H2O SO42- --------S2O62- -------SO2 (g) ------S4O62- ------S2O32- -------S (s) ------ H2S (g)
----------------------- ---------------------------- -----------------------------------------
+1.23 0.17 0.45
1.51 1.1 0.37 -1.0 0.17 0.380 -0.52 -1.66
PoO3 ---------PoO2 ---------Po2+ ----------Po -------H2Po UO22+ --------UO2+ --------U4+ --------U3+ --------U
1.19 1.21 1.64 1.63 1.36 1.82 1.49 1.59 1.07
ClO4- ------- ClO3- ------ HClO2 ------HOCl -----Cl2 (g) ------ Cl- BrO4- ----BrO3- -----HOBr-----Br2 -----–Br-
1.7 1.14 1.45 0.536 1.23 1.06 3.06 2.87
H5IO6 ------IO3- ------HOI --------I2 (s) --------I- IO3- ------- ICl2- -------I2 F2 -------- HF F2 -------- F-
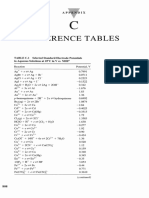
B. LATIMER DIAGRAMS IN BASIC SOLUTION ( 1 M OH-)
-1.69 0.13 -0.13 -1.1 -1.4
3- 2-
TiO2 (s) -------Ti VO4 -------- V CrO4 ----- Cr(OH)3 (s) -------Cr(OH)2 (s) ------- Cr
0.558 0.603 0.1 -0.2 -1.58 -1.285
- 2- 2-
MnO4 ------- MnO4 --------MnO2 (S) ----- Mn(OH)3 (S) ---- Mn(OH)2 (S) ------ Mn ZnO2 ------- Zn
0.72 -0.56 -0.86 0.14 -0.72 -2.31
FeO42- --------Fe(OH)3 (S) -----Fe(OH)2 (S) ------Fe Co(OH)3 --------Co(OH)2 ---------Co H2AlO3- ------- Al
0.49 -0.72 -0.73 0.07 -0.08 -0.36
NiO2 -------- Ni(OH)2 ----------Ni Pd(OH)4 --------Pd(OH)2 --------Pd Cu(OH)2 -------Cu2O ------ Cu
0.74 0.57 0.344 0.098 -0.90 -0.91 0.28 -0.54
Ag2O3 ------AgO ------ Ag2O------ Ag HgO -------Hg Sn(OH)62- -----HSnO2- ------Sn PbO2 ------PbO-----Pb
-0.86 0.88 -0.46 0.76 0.94 -3.04 0.73 0.1
NO3- ----- N2O4 ------ NO2- -------NO ------N2O ------ N2------ NH2OH ------N2H4 -------NH3
-------------------------- ---------------------- ----------------------- ---------------------------
0.01 -0.14 -0.76 0.42
-1.12 -1.57 -2.05 -0.89 -0.67 -0.68 -1.21
3- 2- -
PO4 ------HPO2 ---------H2PO2 -------P4 -------PH3 (g) AsO43- -
-------H2AsO3 -------As --------- AsH3 (g)
-0.93 -1.12 -0.04 -0.74 -0.48 0.05 -0.37 -0.84
2- 2- 2- 2- -2
SO4 ------SO3 ------S2O4 ------S2O3 -------S ------S 2- 2-
SeO4 ------SeO3 ------Se -------Se2-
-------------------------------------------
-0.66
0.36 0.33 0.66 0.40 1.36 0.99 0.54 0.45 1.07
- - - - -
ClO4 ------ClO3 ---------ClO2 -------- ClO -----Cl2 (g) -----Cl - -
BrO4 ------BrO3 -----BrO -----Br2 (l) ------Br-
-
0.7 0.14 0.45 0.54 -0.828 -0.08 0.87
H3IO62- -----IO3- --------IO- -------I2 (S) ------ I- OH- --------H2 (g) O2 ------HO2- ------OH-
---------------------- ---------------------
0.49 0.401
-1.01 - 0.52 -0.70 -1.69 -0.93
CO32- -------- HCO2- -------C -------CH4 (g) SiO32- --------- Si ---------SiH4
* SKIP STEP POTENTIALS E = (n1E1 + n2E2)/(n1 + n2) ; the weighted average of connecting potentials
S-ar putea să vă placă și
- Tables of Common IonsDocument2 paginiTables of Common IonsAmirul MukhlisÎncă nu există evaluări
- OZONE - Allotrope of OxygenDocument4 paginiOZONE - Allotrope of OxygenMatthew LeeÎncă nu există evaluări
- Appendix EDocument1 paginăAppendix EYrhon IbanezÎncă nu există evaluări
- Standard Reduction PotentialsDocument3 paginiStandard Reduction PotentialsjaverfrivÎncă nu există evaluări
- Nguyên tố Dạng oxi hoá +ne Dạng khử E, VDocument12 paginiNguyên tố Dạng oxi hoá +ne Dạng khử E, VNhat KhanhÎncă nu există evaluări
- Standard Reduction PotentialDocument8 paginiStandard Reduction PotentialMateus CostaÎncă nu există evaluări
- Standard Reduction PotentialDocument7 paginiStandard Reduction Potentialyoyotoonzone1Încă nu există evaluări
- Standard Electrode and Reduction Potentials at 298 K PrintableDocument3 paginiStandard Electrode and Reduction Potentials at 298 K Printablecarina_yii9690Încă nu există evaluări
- EMF SeriesDocument5 paginiEMF Seriesmike rosaÎncă nu există evaluări
- Chemical EquationsDocument10 paginiChemical Equationsme-elormÎncă nu există evaluări
- P2 Standard Reduction Potentials by ValueDocument6 paginiP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZÎncă nu există evaluări
- Standard Reduction Potentials Data Extended PDFDocument2 paginiStandard Reduction Potentials Data Extended PDFAceÎncă nu există evaluări
- Standard Reduction PotentialsDocument5 paginiStandard Reduction PotentialsnathaloaÎncă nu există evaluări
- Tablas Redox 2146Document15 paginiTablas Redox 2146TshikoÎncă nu există evaluări
- Standard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Document2 paginiStandard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Alexander RodriguezÎncă nu există evaluări
- WS Balancing KeyDocument2 paginiWS Balancing Keyapi-3706290100% (1)
- Electrochemical Series PDFDocument10 paginiElectrochemical Series PDFheitorpcents496Încă nu există evaluări
- ElectrodeDocument2 paginiElectrodeThatcher PanchoÎncă nu există evaluări
- Standard Reduction Potentials at 25Document1 paginăStandard Reduction Potentials at 25Beverly RamosÎncă nu există evaluări
- Kim Loại: Nguyên TỐ Nguyên Tử Khối Hóa Trị Số Oxi HóaDocument2 paginiKim Loại: Nguyên TỐ Nguyên Tử Khối Hóa Trị Số Oxi HóaQuốc Tiên SinhÎncă nu există evaluări
- Chapter 4 Oxidation-ReductionDocument68 paginiChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNÎncă nu există evaluări
- Potencial EletroquimicoDocument13 paginiPotencial EletroquimicoMatheus EduardoÎncă nu există evaluări
- Chemistry Balancing EquationsDocument3 paginiChemistry Balancing EquationsApple Bottom JeansÎncă nu există evaluări
- Tabla de PotencialesDocument6 paginiTabla de PotencialesLuis AntonioÎncă nu există evaluări
- Tabla Potencial Reduccion PDFDocument13 paginiTabla Potencial Reduccion PDFFóxel ArgÎncă nu există evaluări
- Standard Redox Potential Table PDFDocument10 paginiStandard Redox Potential Table PDFFercho LotudoÎncă nu există evaluări
- E° HBCPDocument10 paginiE° HBCPFelipe FariaÎncă nu există evaluări
- Electrochemical Series - CRC Handbook of Chemistry and PhysicsDocument11 paginiElectrochemical Series - CRC Handbook of Chemistry and Physicsmiguel reynagaÎncă nu există evaluări
- Tabla de Potenciales Redox PDFDocument14 paginiTabla de Potenciales Redox PDFAna Altamirano100% (1)
- CRC Electrode PotentialsDocument10 paginiCRC Electrode PotentialsMohamedou ThiamÎncă nu există evaluări
- Exp1 Prelab Day1Document2 paginiExp1 Prelab Day1Aaron OrtegaÎncă nu există evaluări
- Redox Workbook HoDocument43 paginiRedox Workbook HoMuhammad TauseefÎncă nu există evaluări
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 paginiStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaÎncă nu există evaluări
- Chapter 3 Oxidation ReductionDocument68 paginiChapter 3 Oxidation Reductionlong.vuongbz188Încă nu există evaluări
- Formation Constants of Complex IonsDocument2 paginiFormation Constants of Complex IonsAerial TranÎncă nu există evaluări
- Write The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Document2 paginiWrite The Formulas For The Following Ionic Compounds:: Bonding and Naming WS 4Bea Lha Zandra BesingaÎncă nu există evaluări
- SRP Table Chem DataDocument1 paginăSRP Table Chem Dataapi-222503660Încă nu există evaluări
- Chemical Formula ListDocument9 paginiChemical Formula ListViola Voon Li WeiÎncă nu există evaluări
- Serie ElectroquímicaDocument10 paginiSerie ElectroquímicaÁngeles LópezÎncă nu există evaluări
- Polyatomic IonsDocument1 paginăPolyatomic IonsJames PerriamÎncă nu există evaluări
- Electrochemical Series: Petr VanýsekDocument10 paginiElectrochemical Series: Petr VanýsekMycoLogist4LifeÎncă nu există evaluări
- Nota Ringkas KimiaDocument2 paginiNota Ringkas KimiaZulhilmil Zul100% (7)
- Kims CopiesDocument17 paginiKims Copieszafarchem_iqbalÎncă nu există evaluări
- MSDS Leadframe (16 Items)Document8 paginiMSDS Leadframe (16 Items)bennisg8Încă nu există evaluări
- Review On Redox ReactionDocument6 paginiReview On Redox ReactionLes SampolloÎncă nu există evaluări
- Theoretical Preliminary Test ResultsDocument5 paginiTheoretical Preliminary Test ResultsThea Viktoria OgalescoÎncă nu există evaluări
- Unusual Structures and Physical Properties in Organometallic ChemistryDe la EverandUnusual Structures and Physical Properties in Organometallic ChemistryÎncă nu există evaluări
- Solution Manual for The Elements of Polymer Science and EngineeringDe la EverandSolution Manual for The Elements of Polymer Science and EngineeringEvaluare: 4 din 5 stele4/5 (3)
- Materials Data for Cyclic Loading: Low-Alloy SteelsDe la EverandMaterials Data for Cyclic Loading: Low-Alloy SteelsEvaluare: 5 din 5 stele5/5 (2)
- Materials Data for Cyclic Loading: Cast and Welded MetalsDe la EverandMaterials Data for Cyclic Loading: Cast and Welded MetalsÎncă nu există evaluări
- CZT Vs NaIDocument5 paginiCZT Vs NaIsamÎncă nu există evaluări
- Experiment 8A Formal ReportDocument4 paginiExperiment 8A Formal ReportEj RempilloÎncă nu există evaluări
- ORGANIC ANALYSIS - PDF Class 12Document3 paginiORGANIC ANALYSIS - PDF Class 12Rekha LalÎncă nu există evaluări
- Oxygen CycleDocument11 paginiOxygen CycleMarc Jonas DiazÎncă nu există evaluări
- HL Paper 1: Two Species Are Isotopes of The Same Element?Document8 paginiHL Paper 1: Two Species Are Isotopes of The Same Element?fuduÎncă nu există evaluări
- 8Document2 pagini8Sridevaphani ChellapillaÎncă nu există evaluări
- Analysis of Some Metals in Human Hair by The AAS MethodDocument8 paginiAnalysis of Some Metals in Human Hair by The AAS MethodhaurathyaÎncă nu există evaluări
- Chemical and Physical Hydrogeology of CoalDocument389 paginiChemical and Physical Hydrogeology of CoalsalahudinÎncă nu există evaluări
- Turbine Parts MOC Blog 2Document15 paginiTurbine Parts MOC Blog 2kattukoluÎncă nu există evaluări
- Aqa 74041 QP Jun16Document28 paginiAqa 74041 QP Jun16Attique IftikharÎncă nu există evaluări
- Mark Scheme (Results) January 2007: GCE Chemistry (6244/01)Document23 paginiMark Scheme (Results) January 2007: GCE Chemistry (6244/01)raaaaaawrÎncă nu există evaluări
- Recipe For Success!: Ingredients Reactants Chemical ReactionDocument4 paginiRecipe For Success!: Ingredients Reactants Chemical ReactionNatalie MartinezÎncă nu există evaluări
- Acids Bases and Salts Notes PDFDocument7 paginiAcids Bases and Salts Notes PDFMoghanram JÎncă nu există evaluări
- Soalan Kimia Pertengahan Tahun Form 4Document11 paginiSoalan Kimia Pertengahan Tahun Form 4Ridzuan Mohd AliÎncă nu există evaluări
- Partial Roasting of Copper ConcentrateDocument12 paginiPartial Roasting of Copper ConcentrateMarceloValeriaÎncă nu există evaluări
- SLMR 1912 11 15Document20 paginiSLMR 1912 11 15Russell HartillÎncă nu există evaluări
- Half-Life Centium LabDocument6 paginiHalf-Life Centium LabjohnosborneÎncă nu există evaluări
- Metal p4 Ws 4Document10 paginiMetal p4 Ws 4NonnoonoÎncă nu există evaluări
- Module Electron Configuration Chemical PeriodicityDocument31 paginiModule Electron Configuration Chemical PeriodicityEllah Iracielli TevesÎncă nu există evaluări
- Exp 4Document14 paginiExp 4Farhatul Abrar AnandaÎncă nu există evaluări
- RaginiDocument8 paginiRaginikrimps4uÎncă nu există evaluări
- STPM 2018 Sem 1 Mock AnsDocument2 paginiSTPM 2018 Sem 1 Mock Ansm-7319562Încă nu există evaluări
- TheoryDocument4 paginiTheoryDarin Rocky SumairÎncă nu există evaluări
- Part1 Icho 1 5 PDFDocument64 paginiPart1 Icho 1 5 PDFManuel GuilhermeÎncă nu există evaluări
- Organic Laboratory Preparation Class 12: Nepal APF School DP Paneru MS.C ChemistryDocument25 paginiOrganic Laboratory Preparation Class 12: Nepal APF School DP Paneru MS.C ChemistryDamaru Paneru100% (1)
- HSC June 2009 Paper and Marking Scheme On Same Paper Word DocumentDocument26 paginiHSC June 2009 Paper and Marking Scheme On Same Paper Word DocumentreekoyeÎncă nu există evaluări
- Ncert Important Chemistry Points-1 For Jee-Main 2023Document99 paginiNcert Important Chemistry Points-1 For Jee-Main 2023PrinceÎncă nu există evaluări
- B Osg-1Document22 paginiB Osg-1harisÎncă nu există evaluări
- Chemical Compatibility Reference Chart: SteelDocument15 paginiChemical Compatibility Reference Chart: SteelSreesanth SaruvilÎncă nu există evaluări