Documente Academic
Documente Profesional
Documente Cultură
Biotechnology Quality Manual v.7
Încărcat de
Julie DeckerDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Biotechnology Quality Manual v.7
Încărcat de
Julie DeckerDrepturi de autor:
Formate disponibile
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
Draft Template
Quality Manual
For
Biotechnology Quality Management System v1b
Printed copies are uncontrolled Page 1 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
Table of contents:
Section Page
4.0 Biotechnology Quality Management System …………………………. 3-4
5.0 Management Responsibility …………………………………………... 4-6
6.0 Resource Management …………………………………………………. 6-7
7.0 Planning and Process Realization ……………………………………… 7-13
8.0 Monitoring, Analysis, and Improvement ……………………………… 13-16
Disclaimer: This document provides general guidelines for the development of a quality
manual. Use of this template does not guarantee conformance to BQMS Audit Standard
requirements. Auditors will seek to verify the processes described in the quality manual
created from this template.
Instructions for use of this template:
Items appearing in normal Times New Roman font should remain as part of your quality manual.
If you prefer to restate them, you may do so. Be careful not to omit important sections.
Items that appear in italics are either information or instructions for completing the section.
They should all be removed from your quality manual prior to submission.
"We" or "Our" may be substituted for the name of your organization.
As you are completing your own quality manual by using this template, be certain that you have
the BQMS Standard open to the same section you are working on. This will ensure that there are
no omissions.
The quality manual begins with a brief introduction in which you state a brief summary of your
organization's history and the reason you are applying for certification under the BQMS. Insert
the information here.
Add the address of the main office, the name of a contact person, and relevant contact
information
Printed copies are uncontrolled Page 2 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
4.0 Biotechnology Quality Management System
4.1 This is a general section. By addressing all requirements in the standard, you address
this section by default.
4.2 Documentation Requirements.
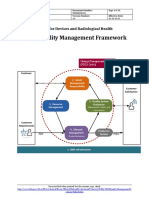
4.2.1 General
[Process Interrelation Map]
4.2.2 State the scope of your quality management system. Where does it begin and end? Who
is your customer (In other words, who requested the work you are doing, and who will
receive the result.) When do your processes begin and when do they end? Also include
any exclusion(s) from section 7 of the standard.
[Your organization’s] procedures, documents, and records required by this standard are
referenced in this Quality Manual and in the Document Status form.
Reference:
Quality Manual QM-01b
Document Status Form 4.2.1c
4.2.3 Control of documents.
How will your organization provide evidence of controlled documents?
[Your organization] has a procedure [insert document identification] to approve each
document's adequacy prior to use and review and to update documents periodically for
re-approval. We ensure that changes and revision status are current.
We ensure that applicable documents are available at point of use and are legible.
Obsolete documents are archived and/or marked to prevent their unintended use.
External documents retain the original identification and are filed in either electronic
and/or paper form.
The Document Status form [which should be attached to the procedure] lists each
controlled document, its version, the date of the document that should be in use, and any
other information you feel is important to document control.
Reference:
Procedure 4.2.3 Document Control
Record 4.2.1c Document Status Form
Printed copies are uncontrolled Page 3 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
4.2.4 Control of records.
How will your organization provide evidence of control of records?
[Your organization] has established procedure [insert procedure identification] to define
the controls needed for the identification, storage, protection, retrieval, and disposition of
records. Records have been established and maintained to provide evidence of
conformity to the requirements of this standard and of the effective operation of a quality
management system. Records remain legible, readily identifiable, and retrievable
because [Insert a statement of how you ensure this will happen.]
Records that indicate the regulated GE organism(s) have arrived at their intended
destinations are retained for a minimum of two (2) years, and
Records associated with approved field trials are retained for a minimum of five (5) years
after relevant regulatory obligations for the permit(s) have been fulfilled.
Records relating to the quality management system are kept for [insert the amount of time
you wish to retain records not related to APHIS requirements].
Reference:
Procedure 4.2.4 Control of Records
Record 4.2.1c Document Status Form
5 Management Responsibility
5.1 Management commitment.
Top management [identified in an organization chart or by some other means] is
committed to the development and implementation of the BQMS by communicating the
importance of meeting regulatory requirements [by doing what? Conducting meetings,
posting signs, providing training, etc].
[Your organization] ensures the availability of resources for implementation of the
BQMS and continually improves its effectiveness [by doing what? Address
management’s contribution to resources, especially financial resources].
Quality Policy
Our Quality Policy is:
[Put Statement here]
Our Objectives are:
[Put Objectives here]
Printed copies are uncontrolled Page 4 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
Objectives are communicated and understood within the organization, and they are
reviewed for continuing suitability at the annual management review meeting.
Explain how you communicate the quality policy and objectives so that they will be
understood and that relevant personal can relate to them. This could be by posting them
in work areas, discussing them at meetings, posting them on the web site or other means.
5.3 Quality planning.
Top management ensures that planning of quality management system conformance is
carried out to meet requirements given in BQMS section 4.1 as well as the objectives in
section 5.2, to achieve compliance with relevant applicable rules and regulations and to
ensure the integrity of the quality management system. Compliance with applicable
regulations or BQMS procedures or SOPs is maintained when changes to the system are
planned and implemented by [describe how you maintain compliance when change
occurs].
5.4 Responsibility and authority.
Top management ensures that responsibilities and authorities are defined and
communicated within the organization to ensure the effective operation and maintenance
of the quality management system.
The organization chart [or a similar document] [ Insert name and identification of the
document] lists relevant personnel assigned to managerial positions within the quality
management system. [It is best to create a document and reference it in the quality
manual.]
Persons listed must have their responsibilities and authorities outlined in an auditable
model. [It is best to create a document and reference it in the quality manual. Position
descriptions may be used for this purpose and can be referenced.]
Reference:
Reference 5.4 Organization chart or similar document
Job descriptions of relevant personal
5.5 BQMS manager (team leader).
Top management appointed [identify the person or position] as the Quality Management
Representative (QMR) who, irrespective of other responsibilities, has the responsibility
and authority to manage the quality management system; to ensure relevant training and
education of personnel (see 6.2.1); and to ensure that the quality management system is
established, implemented, maintained and updated. The QMR will report to top
management on the effectiveness and suitability of the quality management system at the
annual management review meeting.
Printed copies are uncontrolled Page 5 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
5.6 Communication
5.6.1 External communication.
Your organization is to establish, implement and maintain effective arrangements for
communicating with relevant external associates that may influence the effectiveness of
the BQMS.
[Designated personnel: list the personnel or their position] have responsibility and
authority to communicate externally any information concerning conformance to the
BQMS. Relevant information obtained through external communication is included as
input to the BQMS system updating and management review processes. [Explain how
the information is transmitted, from whom, to whom and how you ensure that it is
transmitted.]
Reference:
Record 5.6.1 External communication
5.6.2 Internal communication.
[Your organization] has established, implemented and maintains effective arrangements
for communicating with internal personnel on issues having an impact on conformance.
[Explain how you ensure internal communication.]
5.7 Management review
5.7.1 General.
[Your Organization’s] top management reviews our BQMS annually. We will review the
system more frequently if needed to ensure its continuing suitability, adequacy and
effectiveness. The review will include the opportunities for continuous improvement,
objective(s) and our quality policy. Records of management review inputs and outputs
are stated in the Management Review Meeting minutes.
Reference:
Record 5.7.1 Management Review meeting
6 Resource Management
6.1 Provision of resources.
Your organization is to determine and provide the following resources needed to
implement and maintain a BQMS, continually improve its effectiveness, and to meet
regulatory requirements.
[Your organization] provides the resources necessary to implement and maintain the
BQMS and meet regulatory requirements.
Printed copies are uncontrolled Page 6 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
6.2 Human resources.
6.2.1 General.
[Your organization] hires competent personnel to perform work affecting conformance to
your BQMS based on appropriate education, training, skills, technical knowledge and
experience. [You may qualify current personal be ‘grandfathering’ them as of the date of
your quality manual].
Reference:
Job description(s)
6.2.2 Competence, awareness and training.
Procedure [insert procedure identification] ensures that relevant personnel perform work
affecting conformance to BQMS. They are trained in the relevant aspects of the quality
management system.
[ Explain how your organization determines:
a) the necessary competency for personnel performing work affecting regulatory
compliance;
b) provides training or takes other actions to satisfy these needs;
c) ensures that personnel responsible for monitoring, corrections, and corrective actions
of the BQMS are trained;
d) evaluates the implementation and the effectiveness of competency, training, and
monitoring correction or corrective actions;
e)and ensures that the persons are aware of the relevance and importance of their
activities and how they contribute to the achievement of the quality objectives. ]
Reference:
Procedure 6.2.2 Competence, awareness and training
Record 6.2.2.a Training
Record 6.2.2.b Competency
6.3 Infrastructure.
[Your organization] provides resources for infrastructure which may include buildings,
workspace, associated utilities, process equipment, and supporting services, transport, or
communication. [Explain briefly how these are provided; for example, is your work unit
part of a larger organization?]
6.3 Work environment.
[Your organization] provides resources for the establishment, management and
maintenance of the work environment. [Explain briefly how these are provided; for
example, is there special lighting, equipment, or climate control?]
Printed copies are uncontrolled Page 7 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
7 Planning and Process Realization
7.1 General.
[Your organization] has developed a documented procedure [or procedures] [Identify the
procedure] for planning and process realization of [clearly identify the specific species
you are releasing or intend to release]. The required critical control points have been
addressed during the release of regulated GE organisms. [Your organization] monitors
and verifies the effectiveness of the planned activities and any changes to those activities.
Records of training for personnel performing tasks are maintained and controlled (see
4.2.4 and 6.2.2).
7.2 Critical Control Points and Procedures.
Use this space to describe how you plan to address critical control points in clause 7.
Will it be one organized procedure or several procedures to address each critical control
point? This sub clause could be used to provide an overview of how you manage clause 7
requirements from your company’s prospective.
7.2.1 Storage.
Procedure [insert procedure identification] addresses containment and storage of
regulated GE organisms. The procedure you write must address: storage, segregation of
regulated material from non-regulated material, the method used to mark and/or label
containers, the type of containers, limiting access and pest control. Please refer to the
standard for specific requirements.
Reference:
Procedure 7.2.1 Storage
7.2.2 Transport, movement and import of regulated GE organisms.
Procedure [Insert procedure identification] addresses the identification of regulated GE
organisms in storage, movement, import, transfers and field location. The transport
procedure you write must address your method(s) of storage to prevent mixing, marking
and/or labeling, and physical packaging. This procedure also must address tracking
from shipment to receipt for verification, marking and/or labeling of packages for
verification and inspection of contents for containment integrity in shipping and from
storage to environmental field site. Finally, the procedure must address proper disposal
and/or return to use of packaging. Please refer to the standard for specific requirements.
Reference: (You may have more than one procedure and/or record)
Procedure 7.2.2 Transport and movement and import of regulated GE Organism
Record 7.2.2 Transport movement and import of regulated GE Organism
7.2.3 Environmental release planning and monitoring.
Procedure(s) [insert procedure identification] addresses the planning and monitoring of
environmental field tests of regulated GE organisms. Procedures are to be sufficiently
Printed copies are uncontrolled Page 8 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
detailed to describe the methods the organization will use to achieve planned activities
and conduct regulated activities. You may have several procedures or you may combine
them to fit the need of this clause.
The procedure you write must sufficiently describe:
a) site selection planning to address the following: surrounding land and sexually
compatible species; the site’s historical land use and topography; land ownership rights
to allow access for monitoring volunteers and/or mitigation for an appropriate length of
time; and any local threaten and endangered species.
b) pre-planting handling of GE organisms and transfer to environmental field release
sites.
c) preparation and cleaning of planting equipment. (This would include planters or
planting equipment, transfer and conveying equipment. Remember to include small
equipment and hand planting if appropriate.)
d) use of physical markers and/or global positioning system (GPS) coordinates to identify
the field release sites, including boundaries, where applicable.
e) the environmental field release site maps indicating surrounding land uses, isolation
method [distances] employed, and border rows, where applicable.
f) verification of reproductive growth control and/or isolation.
g) harvest preparation and equipment cleaning which addresses the verification of which
environmental field release site to harvest and the cleaning of relevant equipment used
for harvest on the field site. (This would include the combine or harvest equipment,
baskets, conveying and transport equipment used for harvest.)
h) Volunteer monitoring of environmental field release sites including communication of
post-harvest land use restrictions and the monitoring of the regulated environmental field
release site including border rows for volunteer plants.
Please refer to the Standard for specific requirements.
References: (Your organization may have one or more procedures and/or records)
Procedure(s) 7.2.3 a-h Environmental Release Planning and Monitoring
Record(s) 7.2.3 a-h Environmental Release Planning and Monitoring
Training 6.2.2
Roles and responsibilities 6.2.1
Printed copies are uncontrolled Page 9 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
7.2.4 Post-Harvest Handling and Transfer.
Procedure [Insert procedure identification] describes in detail the post-harvest handling
activities and the methods employed to maintain the identity of the regulated GE
material.
The procedure you write must include, as applicable:
a) the method that regulated GE material is transferred to storage and/or further
processing and is contained during transfer.
b) the way in which regulated GE material is marked and/or labeled during post-harvest
handling and transfer.
c) the method of maintaining the identity of regulated GE material while being used
and/or processed;
d) the method of maintaining the identity regulated GE material during transfer to a
subsequent facility for further evaluation and/or use;
e) the way in which equipment is cleaned prior to use for processing/conditioning and/or
evaluation of regulated GE material. This equipment may be seed cleaning, sorting,
extruding, testing, or inspecting).
f) the way in which misidentified GE material is handled.
References; (Your organization may have one or more procedures and/or records)
Record 7.2.4 Post-Harvest Handling and transfer
Procedure 7.2.4 Post-Harvest Handling and Transfer
7.2.5 Devitalization and disposition.
[This may be written as one or two procedures.]
Procedure [Insert identification of the procedure] addresses the devitalization and
disposition of regulated GE organisms in the field. [This procedure must explain how
regulated organisms are rendered nonviable according to relevant applicable rules,
regulations, and guidance.]
Procedure [Insert procedure identification] addresses the methods used to devitalize and
dispose of regulated GE organisms when they are in storage and/or other means of
containment [This procedure must explain how regulated organisms are rendered
nonviable according to relevant applicable rules, regulations and guidance.]
Printed copies are uncontrolled Page 10 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
Reference:
Record 7.2.5.a Field devitalization and disposition
Record 7.2.5.b Storage and or containment devitalization and disposition
Procedures 7.2.5 Devitalization and disposition
7.2.6 Regulatory compliance incident self-reporting requirements.
Procedure [Insert procedure identification] addresses the submission of regulatory
compliance incidents to appropriate regulatory authorities.
The procedure you write must describe:
a) personnel roles and responsibilities for regulatory compliance within the organization
that conducts regulated movements or environmental field releases.
b) A description of the way in which you document the nature and extent of the regulatory
compliance incident.
c) how you communicate internal requirements for the prompt resolution and
remediation of the regulatory compliance incident.
d) the method of reporting the regulatory compliance incident to appropriate regulatory
authorities according to applicable rules and regulations; and
e) training of relevant individuals involved in regulatory compliance reporting
requirements.
Reference:
Procedure 7.2.6 Regulatory compliance incident self-reporting
Record 6.2.2 Training
Records 7.2.6.internal and external (may be same record or two)
Personal roles and responsibilities 6.2.1
7.2.7 Resolution of compliance incidents and control of non-conforming regulated
material.
[This may be written as one or two procedures.]
Procedure [Insert procedure identification] ensures that regulated material which does
not conform to regulatory requirements is identified and controlled to prevent its
unintended use or delivery; a loss of confinement/containment; mixing with conventional
or commercial commodities; persistence in the environment.
The procedure you write to address resolution of compliance incidents must address:
Printed copies are uncontrolled Page 11 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
a) personnel roles and responsibilities for communicating and implementing resolution
activities.
b) personal roles and responsibilities for dealing with non-conforming regulated
material.
c) documentation of resolution activities used for the prompt remediation of regulatory
compliance incidents.
d) records of communication between the organization and applicable regulatory entities
during the resolution and remediation of regulatory compliance incidents.
Procedure (Insert procedure identification] addresses the way in which you deal with
non-conforming regulated material.
The procedure you write must state the these materials are dealt with in one or more of
the following ways;
a) By taking action to eliminate the detected non-conformity
b) By authorizing its use, release, or acceptance under concession by a relevant
authority; or
c) By authorizing its use or acceptance for another purpose.
The procedure also must address:
a) re-verification to demonstrate conformity to the requirements, after non-conforming
regulated material is corrected or used for another purpose.
b) the action taken, appropriate to the effect or potential effect of non-conforming
regulated material, when release of the material has been detected after delivery or use
has started.
Reference:
Procedure 7.2.7.a Nonconforming Product
Procedure 7.2.7.b Resolution of compliance incident
Record(s) 7.2.7
7.3 Management of External Associates.
[Your organization] ensures that the activities of relevant service providers, cooperators,
contractors are in conformance with the BQMS procedures in Clause 7 and its sub-
clauses. [In this space describe how you can make this assurance. This may be done
through training, contracts, agreements, certification to some type of management system
such as BQMS or another equivalent system that you would approve. You may include
Printed copies are uncontrolled Page 12 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
that all current external associates are approved by “grandfathering” as of the date of
your quality manual. ]
8 Monitoring, Analysis and Improvement
8.1 General.
[Your organization] plans and implements the monitoring, measurement, analysis and
improvement processes needed to demonstrate conformity with quality management
system and the quality policy and objectives. [We] ensure conformity with the
requirements of the BQMS audit standard, as described in this document and continually
improve the effectiveness of the organization’s quality management system.
8.2 Monitoring and Measurement
8.2.1 Internal audit.
Internal audits are used to determine [Your Organization's] ability to meet requirements
of planned arrangements, to the requirements of this standard, and to BQMS
requirements that [we have] established. The internal audit also demonstrates that the
BQMS is effectively implemented and maintained. Audits are performed at least
annually.
Procedure [insert procedure identification] is used when performing internal audits.
The procedure you write must address:
a) planning of an audit program. This is to consider the status and importance of the
processes and areas to be audited, as well as the results of previous internal and
external audits.
b) The audit criteria, scope, frequency, and methods of the audit.
c) The selection of the auditors and conduct of auditors which must ensure objectivity
and impartiality of the audit process to ensure auditors do not audit their own work.
d) The responsibilities for planning and conducting audits.
e) Reporting of results and follow-up activities to include the verification of the actions
taken and the reporting of verification results.
[Your organization’s] management ensures that actions are taken without undue delay to
eliminate detected nonconformities and their causes within the area audited. [You should
use corrective action procedure.]
The results of internal audits are discussed during management reviews. [This will be an
agenda item.]
Printed copies are uncontrolled Page 13 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
Reference:
Procedure 8.2.1 Internal Audit
Record 8.2.1 Internal Audit
Record 5.8.1 Management Review
Procedure 8.4.2 Corrective Action
8.2.2 Monitoring of Process
[Your organization] applies suitable methods for monitoring and, where applicable,
measurement of the quality management system processes. [Write an explanation of these
methods and how they demonstrate the ability of your processes to achieve planned
results.]
When planned results are not achieved, appropriate correction and corrective action is to
be taken.
8.2.3 Monitoring of Service
[Your organization] monitors and verifies the activity of services that may impact the
quality management system. [Write an explanation of the way you verify that service
providers conform with planned arrangements. This can include cost, timeliness of
delivery, quality of the product, contract and deliverable oversight and so on.]
Reference: (this may be several references that you already may be monitoring. If so
make reference to those records or documents)
Record 8.2.3 Monitoring of Service
8.3 Analysis of data
[Your organization] determines, collects, and analyzes appropriate data to demonstrate
the suitability and effectiveness of the BQMS. [Your organization’s] analysis of data
provides information relating to compliance with applicable rules and regulations,
conformity to BQMS requirements and characteristics and trends of processes and
external associate performance including opportunities for preventive action.
[Your organization] evaluates where continual improvement of the effectiveness of the
BQMS can be made. This includes data generated as a result of monitoring and
measurement and from other relevant sources.
[In most cases, the quality objectives lend themselves to fulfilling this requirement;
however, there may be other areas that you wish to monitor and measure. If you are
Printed copies are uncontrolled Page 14 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
uncertain about the performance of any of your processes, they may become the object of
this requirement.]
Reference: (this may be several references that you already may be monitoring. If so
make reference to those records or documents)
Record 8.2.3 Monitoring of Service
8.4 Improvement
8.4.1 Continual improvement.
[Your organization] continually improves the effectiveness of the quality management
system through the use of the quality policy, quality objectives, internal and external
audit results, analysis of data, corrective and preventive actions, and management review.
[If you can think of anything you've done recently to improve your operation, it should be
listed here.]
8.4.2 Corrective Action.
[Your organization] takes corrective action to eliminate the cause of non-conformance
within their quality management system in order to prevent recurrence. The actions are
appropriate to the effects of the nonconformities encountered.
Procedure [insert procedure identification] addresses corrective action.
The procedure your write must include a method to:
a) review nonconformities with the BQMS as well as non-compliance with regulatory
requirements communicated by APHIS or other regulatory agencies.
b) determine the root causes of nonconformities
c) evaluate the need for action to ensure that nonconformities with the BQMS do not
recur.
d) determine and implement action needed and
e) review corrective action taken to determine its effectiveness.
Reference:
Procedure 8.4.2 Corrective action
Record 8.4.2 Corrective action
Printed copies are uncontrolled Page 15 of 16
Effective date Month/day/Year
Your Logo can go here: Company address of Corporate headquarters can go
here:
Document Number: Quality Manual
Approved By: Quality Management Representative or position that
this document would be primary responsible for.
Revision Details: Document Status Form
8.5 Preventive Action
[Your organization] determines action to eliminate the cause of potential nonconformities
within the BQMS or regulatory non-compliance and their causes in order to prevent their
occurrence. The actions are appropriate to the effects of potential problems.
Procedure [insert procedure identification] addresses preventive action.
The procedure you write must address the following methods to:
a) determine potential nonconformities and their causes
b) evaluate the need for action to prevent occurrence of nonconformities
c) determine and implement action needed and
d) reviewing preventive action taken to determine its effectiveness.
Reference:
Procedure 8.5 Preventative action
Record 8.4.2 Preventative action
Printed copies are uncontrolled Page 16 of 16
Effective date Month/day/Year
S-ar putea să vă placă și
- P003 Internal Communication ProcedureDocument6 paginiP003 Internal Communication ProcedureFloreid75% (4)
- Change Control Board A Complete Guide - 2020 EditionDe la EverandChange Control Board A Complete Guide - 2020 EditionÎncă nu există evaluări
- Document Control Procedure ExampleDocument6 paginiDocument Control Procedure ExampleISO 9001 Checklist90% (30)
- Regulatory Governance A Complete Guide - 2020 EditionDe la EverandRegulatory Governance A Complete Guide - 2020 EditionÎncă nu există evaluări
- Basic - INTERNAL AUDIT REPORTDocument10 paginiBasic - INTERNAL AUDIT REPORTsamrinÎncă nu există evaluări
- Administrative Controls A Complete Guide - 2020 EditionDe la EverandAdministrative Controls A Complete Guide - 2020 EditionÎncă nu există evaluări
- BQMS Template Procedure 8.2.1 Internal Audit v3.0Document4 paginiBQMS Template Procedure 8.2.1 Internal Audit v3.0Tria Meildha GustinÎncă nu există evaluări
- Document Control A Complete Guide - 2020 EditionDe la EverandDocument Control A Complete Guide - 2020 EditionÎncă nu există evaluări
- Document Control Procedure ExampleDocument3 paginiDocument Control Procedure ExampleErich Kadow33% (3)
- ISO 9001 2008 Quality ManualDocument38 paginiISO 9001 2008 Quality ManualWillyanto Lee100% (1)
- Final Quality Audit A Complete Guide - 2020 EditionDe la EverandFinal Quality Audit A Complete Guide - 2020 EditionÎncă nu există evaluări
- Section - 6 API Spec Q1 Ninth Edition Requirement (Clause: 1-4)Document21 paginiSection - 6 API Spec Q1 Ninth Edition Requirement (Clause: 1-4)JohnÎncă nu există evaluări
- Revision Control System A Complete Guide - 2020 EditionDe la EverandRevision Control System A Complete Guide - 2020 EditionÎncă nu există evaluări
- Final AssignmentDocument6 paginiFinal Assignmentmhk665Încă nu există evaluări
- Quality Manual: M. Barbisotti & Sons LTDDocument20 paginiQuality Manual: M. Barbisotti & Sons LTDengsam777100% (1)
- 2.1 Document Control and ManagementDocument34 pagini2.1 Document Control and Managementaymenmoataz100% (2)
- Internal Communication ProcedureDocument6 paginiInternal Communication ProcedureRhea SimoneÎncă nu există evaluări
- Sample Iso 9001-08 QSMDocument22 paginiSample Iso 9001-08 QSMLove100% (1)
- Iso 9001 2015Document21 paginiIso 9001 2015Sapan Kansara100% (1)
- ISO 9001-2015 Client-Transition-Checklist - Add Your Co NameDocument11 paginiISO 9001-2015 Client-Transition-Checklist - Add Your Co NameTomBerendsenÎncă nu există evaluări
- ISO 9001 Client Transition ChecklistDocument11 paginiISO 9001 Client Transition Checklisttimisite247Încă nu există evaluări
- SYS 003 A D1 Management Review ProcedureDocument5 paginiSYS 003 A D1 Management Review ProcedureAnonymous BVyI6frEJ100% (1)
- ISO 9001 Clause 4.1Document6 paginiISO 9001 Clause 4.1sasi10000Încă nu există evaluări
- ISO 9001 Requirements ExplainedDocument18 paginiISO 9001 Requirements ExplaineddesurkarbÎncă nu există evaluări
- ICE Quality MAnagement System - DraftDocument7 paginiICE Quality MAnagement System - DraftMark Roger Huberit IIÎncă nu există evaluări
- Container Consulting Services Quality Manual - ISO 9001Document7 paginiContainer Consulting Services Quality Manual - ISO 9001Richard TroianoÎncă nu există evaluări
- Total Cleaning and Housekeeping Services PDFDocument31 paginiTotal Cleaning and Housekeeping Services PDFsaadÎncă nu există evaluări
- The Four Main Components of A Quality Management SystemDocument9 paginiThe Four Main Components of A Quality Management SystemShishir GyawaliÎncă nu există evaluări
- ISO 9001 Quality ManualDocument29 paginiISO 9001 Quality ManualRaja Sufyan Minhas88% (8)
- QMS-010 SampleDocument6 paginiQMS-010 SampleMostafa FawzyÎncă nu există evaluări
- Quality ManualDocument30 paginiQuality Manualpj_villegas24Încă nu există evaluări
- Total Cleaning and Housekeeping ServicesDocument31 paginiTotal Cleaning and Housekeeping ServicesKeemeÎncă nu există evaluări
- Iso 9001 - 2015Document7 paginiIso 9001 - 2015Project m7070% (1)
- Effective and Efficient Internal and Supplier Quality System Auditing For Medical DevicesDocument3 paginiEffective and Efficient Internal and Supplier Quality System Auditing For Medical Devicesapi-252950791Încă nu există evaluări
- Internal AuditsDocument9 paginiInternal Audits李哲祥100% (1)
- As9100 InterpretationsDocument61 paginiAs9100 InterpretationsNestor Czerwacki100% (1)
- Quality PolicyDocument8 paginiQuality PolicyFiroz KhanÎncă nu există evaluări
- Iso 9001 10 Clauses InterpretationDocument34 paginiIso 9001 10 Clauses InterpretationJojo DollolasaÎncă nu există evaluări
- Iso 9002 International StandardDocument6 paginiIso 9002 International Standardjojumathew100% (1)
- ISO 9001 Training MaterialDocument248 paginiISO 9001 Training MaterialBuddharaju Kiran100% (2)
- Iso 9001Document27 paginiIso 9001Sagar DaundÎncă nu există evaluări
- 6.1 Risks & OpportunitiesDocument11 pagini6.1 Risks & OpportunitiesAbigail Tshabalala100% (3)
- ISO9001 2008comparisonDocument2 paginiISO9001 2008comparisonsubhash_savitaÎncă nu există evaluări
- ISO 9001:2015 Procedure For Control of Documented InformationDocument9 paginiISO 9001:2015 Procedure For Control of Documented InformationQualtic Certifications100% (6)
- Iso 9001 2015 Quality Manual-ExposedDocument23 paginiIso 9001 2015 Quality Manual-ExposedJobair Alam100% (1)
- Configuring Invoice Approval Workflow-R12Document32 paginiConfiguring Invoice Approval Workflow-R12kriole130% (1)
- ISO 9001:2015 - Client Transition ChecklistDocument8 paginiISO 9001:2015 - Client Transition ChecklistYulissaPazÎncă nu există evaluări
- Management ReviewsDocument9 paginiManagement Reviews李哲祥100% (1)
- Welcome To Internal Auditing Training Course: Certification Services Pakistan (PVT) LTDDocument248 paginiWelcome To Internal Auditing Training Course: Certification Services Pakistan (PVT) LTDFarhan Saleem100% (1)
- How Many Clauses Are in ISO 9001Document32 paginiHow Many Clauses Are in ISO 9001Pavel Solis PerezÎncă nu există evaluări
- Calidad TotalDocument25 paginiCalidad TotallarissamatanoÎncă nu există evaluări
- TS Gap AuditDocument17 paginiTS Gap Auditputu_adiÎncă nu există evaluări
- ISO 9001-2000 ClauseDocument6 paginiISO 9001-2000 ClausesrkadaliÎncă nu există evaluări
- 2015 Process Audit ChecklistDocument17 pagini2015 Process Audit ChecklistAde Kurniawan100% (1)
- Introduction To ISO 9001:2008: Clause 1: ScopeDocument58 paginiIntroduction To ISO 9001:2008: Clause 1: ScopeMula SrikantÎncă nu există evaluări
- What Is A Document?: Tips On ISO 9001 Quality Management System DocumentationDocument5 paginiWhat Is A Document?: Tips On ISO 9001 Quality Management System DocumentationMohammad Jaid AlamÎncă nu există evaluări
- Company X Inc. Quality System Manual: Typical Top Level Operations Flowchart Main Processes / SystemsDocument15 paginiCompany X Inc. Quality System Manual: Typical Top Level Operations Flowchart Main Processes / SystemskadirikakaÎncă nu există evaluări
- ISO 9001-2015 Process Audit ChecklistDocument17 paginiISO 9001-2015 Process Audit ChecklistAhmed Samir Salim100% (12)
- Assembly and RiggingDocument52 paginiAssembly and RiggingPokemon Go0% (1)
- Module 1 Dynamics of Rigid BodiesDocument11 paginiModule 1 Dynamics of Rigid BodiesBilly Joel DasmariñasÎncă nu există evaluări
- Dark Energy Survey DES CollaborationDocument38 paginiDark Energy Survey DES CollaborationgeorgcantorÎncă nu există evaluări
- Bhagwan Mahavir College of Architecture: Topic: Lacing, Batteneing, BracingDocument14 paginiBhagwan Mahavir College of Architecture: Topic: Lacing, Batteneing, BracingJai MenDparaÎncă nu există evaluări
- List of Institutions With Ladderized Program Under Eo 358 JULY 2006 - DECEMBER 31, 2007Document216 paginiList of Institutions With Ladderized Program Under Eo 358 JULY 2006 - DECEMBER 31, 2007Jen CalaquiÎncă nu există evaluări
- Case StudyDocument15 paginiCase StudyChaitali moreyÎncă nu există evaluări
- System of Linear Equation and ApplicationDocument32 paginiSystem of Linear Equation and Applicationihsaanbava0% (1)
- KV4BBSR Notice ContractuaL Interview 2023-24Document9 paginiKV4BBSR Notice ContractuaL Interview 2023-24SuchitaÎncă nu există evaluări
- Ebook Computer Forensics Principles and Practices 1St Edition Volonino Test Bank Full Chapter PDFDocument29 paginiEbook Computer Forensics Principles and Practices 1St Edition Volonino Test Bank Full Chapter PDFmundifycoucheefnhgl100% (10)
- EHVACDocument16 paginiEHVACsidharthchandak16Încă nu există evaluări
- DocsDocument4 paginiDocsSwastika SharmaÎncă nu există evaluări
- Chapter 2.2 Quantitative Analysis NewDocument44 paginiChapter 2.2 Quantitative Analysis NewMinase TilayeÎncă nu există evaluări
- Reclaimer PDFDocument8 paginiReclaimer PDFSiti NurhidayatiÎncă nu există evaluări
- Vacon NX, Non-Regenerative Front End FI9 UD01217B PDFDocument48 paginiVacon NX, Non-Regenerative Front End FI9 UD01217B PDFSilvian IonescuÎncă nu există evaluări
- Ccie R&s Expanded-BlueprintDocument12 paginiCcie R&s Expanded-BlueprintAftab AlamÎncă nu există evaluări
- Grade 6 q2 Mathematics LasDocument151 paginiGrade 6 q2 Mathematics LasERIC VALLE80% (5)
- Age and Gender Detection Using Deep Learning: HYDERABAD - 501 510Document11 paginiAge and Gender Detection Using Deep Learning: HYDERABAD - 501 510ShyamkumarBannuÎncă nu există evaluări
- Practical Organic ChemistryDocument598 paginiPractical Organic ChemistryGerardo Estrada99% (127)
- WT&D (Optimization of WDS) PDFDocument89 paginiWT&D (Optimization of WDS) PDFAbirham TilahunÎncă nu există evaluări
- Synthesis Essay Final DraftDocument5 paginiSynthesis Essay Final Draftapi-283802944Încă nu există evaluări
- ParaphrasingDocument11 paginiParaphrasingAntiiSukmaÎncă nu există evaluări
- Study On The Form Factor and Full-Scale Ship Resistance Prediction MethodDocument2 paginiStudy On The Form Factor and Full-Scale Ship Resistance Prediction MethodRaka AdityaÎncă nu există evaluări
- Trainer Manual Internal Quality AuditDocument32 paginiTrainer Manual Internal Quality AuditMuhammad Erwin Yamashita100% (5)
- Advocating For Appropriate Educational ServicesDocument32 paginiAdvocating For Appropriate Educational ServicesTransverse Myelitis AssociationÎncă nu există evaluări
- PST SubjectDocument2 paginiPST SubjectCarol ElizagaÎncă nu există evaluări
- Read The Dialogue Below and Answer The Following QuestionDocument5 paginiRead The Dialogue Below and Answer The Following QuestionDavid GainesÎncă nu există evaluări
- Registration Form - Synergies in Communication - 6th Edition - 2017-Drobot AnaDocument3 paginiRegistration Form - Synergies in Communication - 6th Edition - 2017-Drobot AnaAna IrinaÎncă nu există evaluări
- What Is Public RelationsDocument52 paginiWhat Is Public RelationsMarwa MoussaÎncă nu există evaluări
- Digital Speed Control of DC Motor For Industrial Automation Using Pulse Width Modulation TechniqueDocument6 paginiDigital Speed Control of DC Motor For Industrial Automation Using Pulse Width Modulation TechniquevendiÎncă nu există evaluări
- Starex Is BTSDocument24 paginiStarex Is BTSKLÎncă nu există evaluări
- The 7 Habits of Highly Effective PeopleDe la EverandThe 7 Habits of Highly Effective PeopleEvaluare: 4 din 5 stele4/5 (2567)
- High Road Leadership: Bringing People Together in a World That DividesDe la EverandHigh Road Leadership: Bringing People Together in a World That DividesÎncă nu există evaluări
- Summary of Noah Kagan's Million Dollar WeekendDe la EverandSummary of Noah Kagan's Million Dollar WeekendEvaluare: 5 din 5 stele5/5 (2)
- The Coaching Habit: Say Less, Ask More & Change the Way You Lead ForeverDe la EverandThe Coaching Habit: Say Less, Ask More & Change the Way You Lead ForeverEvaluare: 4.5 din 5 stele4.5/5 (186)
- Scaling Up: How a Few Companies Make It...and Why the Rest Don't, Rockefeller Habits 2.0De la EverandScaling Up: How a Few Companies Make It...and Why the Rest Don't, Rockefeller Habits 2.0Evaluare: 5 din 5 stele5/5 (2)
- The First Minute: How to start conversations that get resultsDe la EverandThe First Minute: How to start conversations that get resultsEvaluare: 4.5 din 5 stele4.5/5 (57)
- How to Talk to Anyone at Work: 72 Little Tricks for Big Success Communicating on the JobDe la EverandHow to Talk to Anyone at Work: 72 Little Tricks for Big Success Communicating on the JobEvaluare: 4.5 din 5 stele4.5/5 (37)
- Billion Dollar Lessons: What You Can Learn from the Most Inexcusable Business Failures of the Last Twenty-five YearsDe la EverandBillion Dollar Lessons: What You Can Learn from the Most Inexcusable Business Failures of the Last Twenty-five YearsEvaluare: 4.5 din 5 stele4.5/5 (52)
- Transformed: Moving to the Product Operating ModelDe la EverandTransformed: Moving to the Product Operating ModelEvaluare: 4.5 din 5 stele4.5/5 (2)
- How to Lead: Wisdom from the World's Greatest CEOs, Founders, and Game ChangersDe la EverandHow to Lead: Wisdom from the World's Greatest CEOs, Founders, and Game ChangersEvaluare: 4.5 din 5 stele4.5/5 (95)
- The Power of People Skills: How to Eliminate 90% of Your HR Problems and Dramatically Increase Team and Company Morale and PerformanceDe la EverandThe Power of People Skills: How to Eliminate 90% of Your HR Problems and Dramatically Increase Team and Company Morale and PerformanceEvaluare: 5 din 5 stele5/5 (22)
- Spark: How to Lead Yourself and Others to Greater SuccessDe la EverandSpark: How to Lead Yourself and Others to Greater SuccessEvaluare: 4.5 din 5 stele4.5/5 (132)
- The Introverted Leader: Building on Your Quiet StrengthDe la EverandThe Introverted Leader: Building on Your Quiet StrengthEvaluare: 4.5 din 5 stele4.5/5 (35)
- Unlocking Potential: 7 Coaching Skills That Transform Individuals, Teams, & OrganizationsDe la EverandUnlocking Potential: 7 Coaching Skills That Transform Individuals, Teams, & OrganizationsEvaluare: 4.5 din 5 stele4.5/5 (28)
- Transformed: Moving to the Product Operating ModelDe la EverandTransformed: Moving to the Product Operating ModelEvaluare: 4 din 5 stele4/5 (1)
- 7 Principles of Transformational Leadership: Create a Mindset of Passion, Innovation, and GrowthDe la Everand7 Principles of Transformational Leadership: Create a Mindset of Passion, Innovation, and GrowthEvaluare: 5 din 5 stele5/5 (52)
- The 7 Habits of Highly Effective People: 30th Anniversary EditionDe la EverandThe 7 Habits of Highly Effective People: 30th Anniversary EditionEvaluare: 5 din 5 stele5/5 (337)
- The E-Myth Revisited: Why Most Small Businesses Don't Work andDe la EverandThe E-Myth Revisited: Why Most Small Businesses Don't Work andEvaluare: 4.5 din 5 stele4.5/5 (709)
- The Little Big Things: 163 Ways to Pursue ExcellenceDe la EverandThe Little Big Things: 163 Ways to Pursue ExcellenceÎncă nu există evaluări
- Management Mess to Leadership Success: 30 Challenges to Become the Leader You Would FollowDe la EverandManagement Mess to Leadership Success: 30 Challenges to Become the Leader You Would FollowEvaluare: 4.5 din 5 stele4.5/5 (27)
- Good to Great by Jim Collins - Book Summary: Why Some Companies Make the Leap...And Others Don'tDe la EverandGood to Great by Jim Collins - Book Summary: Why Some Companies Make the Leap...And Others Don'tEvaluare: 4.5 din 5 stele4.5/5 (64)
- Leadership Skills that Inspire Incredible ResultsDe la EverandLeadership Skills that Inspire Incredible ResultsEvaluare: 4.5 din 5 stele4.5/5 (11)
- Summary: Choose Your Enemies Wisely: Business Planning for the Audacious Few: Key Takeaways, Summary and AnalysisDe la EverandSummary: Choose Your Enemies Wisely: Business Planning for the Audacious Few: Key Takeaways, Summary and AnalysisEvaluare: 4.5 din 5 stele4.5/5 (3)
- Summary of Marshall Goldsmith & Mark Reiter's What Got You Here Won't Get You ThereDe la EverandSummary of Marshall Goldsmith & Mark Reiter's What Got You Here Won't Get You ThereEvaluare: 3 din 5 stele3/5 (2)
- Leading with Character: 10 Minutes a Day to a Brilliant Legacy SetDe la EverandLeading with Character: 10 Minutes a Day to a Brilliant Legacy SetÎncă nu există evaluări
- 300+ PMP Practice Questions Aligned with PMBOK 7, Agile Methods, and Key Process Groups - 2024: First EditionDe la Everand300+ PMP Practice Questions Aligned with PMBOK 7, Agile Methods, and Key Process Groups - 2024: First EditionEvaluare: 5 din 5 stele5/5 (1)
- Sun Tzu: The Art of War for Managers; 50 Strategic RulesDe la EverandSun Tzu: The Art of War for Managers; 50 Strategic RulesEvaluare: 5 din 5 stele5/5 (5)