Documente Academic
Documente Profesional
Documente Cultură
Alkenes Reaction Worksheet PDF
Încărcat de
Leslie HernandezDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Alkenes Reaction Worksheet PDF
Încărcat de
Leslie HernandezDrepturi de autor:
Formate disponibile
UNIVERSITY OF SANTO TOMAS
FACULTY OF PHARMACY
PHA 615 LECTURE
PHARMACEUTICAL ORGANIC CHEMISTRY
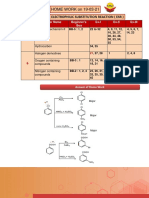
Alkenes Reaction Worksheet
Instructions. Given are the reactants together with their respective catalysts. Draw the product to be
formed and write the specific reaction of the electrophilic addition.
Note: Start to memorize the co-reactants, catalysts, and the type of reaction for the Shifting Examination.
Reactants with catalyst Product formed Specific
Reaction of
Electrophilic
Addition
H3PO4
H3PO4
H3PO4
HgCH3COO, H2O
NaBH4
H3PO4 (anti-
Markovnikov)
BH3/THF
H2O2, NaOH, H2O
+ CH3CH2OH
+ CH3CH2CH2OH
University of Santo Tomas
Faculty of Pharmacy
PHA 615 Lec - Alkene Reactions 1/4 | P a g e
Reactants with catalyst Product formed Specific
Reaction of
Electrophilic
Addition
CH
H3C CH2 + Br DCM/CCl4
2
+ Cl2
DCM/CCl4
+ Cl2 DCM/CCl4
+ Cl2/H2O
+ Br2/H2O
+ Cl2/H2O
Reactants with catalyst Product formed Specific
Reaction of
Electrophilic
Addition
+ H2 Pd/PtO2
NH
+ 2H2 Pd/PtO2
+ 2H2 Pd/PtO2
University of Santo Tomas
Faculty of Pharmacy
PHA 615 Lec - Alkene Reactions 2/4 | P a g e
Reactants with catalyst Product formed Specific
Reaction of
Electrophilic
Addition
+ mCPBA
NH
+ mCPBA
+ mCPBA
+ mCPBA
Reactants with catalyst Product formed Specific
Reaction of
Electrophilic
Addition
+ KMnO4 H2O
NaOH
+ KMnO4 H2O
NaOH
+ KMnO4 H2O
NaOH
CH2
+ KMnO4 H3O+
CH2
+ KMnO4 H3O+
University of Santo Tomas
Faculty of Pharmacy
PHA 615 Lec - Alkene Reactions 3/4 | P a g e
H2C
+ KMnO4 H3O+
+ KMnO4 H3O+
+ KMnO4 H3O+
+ KMnO4 H3O+
Reactants with catalyst Product formed Specific
Reaction of
Electrophilic
Addition
O3, Zn
O3, Zn
O3, Zn
O3, Zn
O3, Zn
6 BzO• + 3CH2=CH2
University of Santo Tomas
Faculty of Pharmacy
PHA 615 Lec - Alkene Reactions 4/4 | P a g e
S-ar putea să vă placă și
- EXPERIMENT 1 - Reactions of Aliphatic and Aromatic HydrocarbonsDocument2 paginiEXPERIMENT 1 - Reactions of Aliphatic and Aromatic HydrocarbonsASYRANI ZULAIKHAÎncă nu există evaluări
- Enrtl-Rk Rate Based Nh3 ModelDocument22 paginiEnrtl-Rk Rate Based Nh3 ModelsamandondonÎncă nu există evaluări
- Distinction Test (Organic Chemistry)Document14 paginiDistinction Test (Organic Chemistry)Ashish Kumar100% (3)
- 2018 PU2 H2 05 Halogen Derivatives Tutorial AnswerDocument12 pagini2018 PU2 H2 05 Halogen Derivatives Tutorial AnswerNeil SharmaÎncă nu există evaluări
- Chem (Final)Document17 paginiChem (Final)Jaynie Lee VillaranÎncă nu există evaluări
- Astm A449Document1 paginăAstm A449Vitor Rigueira de GodoyÎncă nu există evaluări
- Brosur Evo FranklinDocument2 paginiBrosur Evo FranklinFargan ThanÎncă nu există evaluări
- Alkenes WS PDFDocument4 paginiAlkenes WS PDFMARIANELE JAN COSINASÎncă nu există evaluări
- 16.0 Halogen DerivativesDocument21 pagini16.0 Halogen DerivativesKudzayi TusaumweÎncă nu există evaluări
- (11.07.22)Document20 pagini(11.07.22)Micheelle JeannethÎncă nu există evaluări
- Chapter 3a (Compatibility Mode)Document71 paginiChapter 3a (Compatibility Mode)HaiÎncă nu există evaluări
- Exp Addition Substitution Ver03Document2 paginiExp Addition Substitution Ver03clappedÎncă nu există evaluări
- Unit 6 Reaction of Functional Groups - A SummaryDocument3 paginiUnit 6 Reaction of Functional Groups - A SummaryklahaÎncă nu există evaluări
- (Materi Pak Ari) Asn - AdditionDocument8 pagini(Materi Pak Ari) Asn - AdditionlilisÎncă nu există evaluări
- Block 4 Functional Groups 1Document22 paginiBlock 4 Functional Groups 1Cheng FuÎncă nu există evaluări
- Polunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Document21 paginiPolunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Ronak Modi100% (1)
- Chem 3125 Experiment #2 - The Study of Elimination Reactions Using Gas ChromatographyDocument14 paginiChem 3125 Experiment #2 - The Study of Elimination Reactions Using Gas ChromatographyRalston King Stulla ChambersÎncă nu există evaluări
- Chem 1: Chemistry of CarbonDocument7 paginiChem 1: Chemistry of CarbonApple GonzalesÎncă nu există evaluări
- Halogenalkanes: Unit 2 Chemistry C. Bailey PolackDocument23 paginiHalogenalkanes: Unit 2 Chemistry C. Bailey PolackBritney PattersonÎncă nu există evaluări
- Halogenalkanes: Unit 2 Chemistry C. Bailey PolackDocument23 paginiHalogenalkanes: Unit 2 Chemistry C. Bailey PolackBritney PattersonÎncă nu există evaluări
- Notes 02Document67 paginiNotes 02Christine FernandezÎncă nu există evaluări
- Pyrolysis: Mathematical Modeling of Hydrocarbon Pyrolysis ReactionsDocument8 paginiPyrolysis: Mathematical Modeling of Hydrocarbon Pyrolysis ReactionsBahar MeschiÎncă nu există evaluări
- Lec 1 Aldehydes Ketones CH 19 1 ModifiedDocument54 paginiLec 1 Aldehydes Ketones CH 19 1 ModifiedpolinaÎncă nu există evaluări
- Estudio Cinetico de La Descomposicion Catalizada DDocument6 paginiEstudio Cinetico de La Descomposicion Catalizada DBryan Sait De León ReyesÎncă nu există evaluări
- 7.0 Haloalkanes 20212022Document88 pagini7.0 Haloalkanes 20212022ZULAIKA BINTI SALLEH A22BE0415Încă nu există evaluări
- A New Seminar On CatalysisDocument36 paginiA New Seminar On CatalysisPMHÎncă nu există evaluări
- L12 - (JLD 3.0) - Reaction Mechanisms - 25 AugDocument54 paginiL12 - (JLD 3.0) - Reaction Mechanisms - 25 AugkisuisoffbeatÎncă nu există evaluări
- Deb2005 PDFDocument4 paginiDeb2005 PDFjohn doeÎncă nu există evaluări
- Summary of Organic ReactionsDocument6 paginiSummary of Organic ReactionsAbudi Alsagoff100% (5)
- Slide 1Document26 paginiSlide 1ShreyaÎncă nu există evaluări
- Для Просмотра Статьи Разгадайте КапчуDocument24 paginiДля Просмотра Статьи Разгадайте КапчуTIẾN NGUYỄN MINHÎncă nu există evaluări
- Sample Study Material: IIT-JAM ChemistryDocument74 paginiSample Study Material: IIT-JAM ChemistryPradeep PrajapatiÎncă nu există evaluări
- The University of Zambia School of Natural Sciences: Chemistry DepartmentDocument48 paginiThe University of Zambia School of Natural Sciences: Chemistry Departmentmartin mulengaÎncă nu există evaluări
- Accepted Manuscript: 10.1016/j.energy.2017.05.146Document29 paginiAccepted Manuscript: 10.1016/j.energy.2017.05.146bambang_teknikkimiaÎncă nu există evaluări
- Additions of Organomagnesium Halides to α‑Alkoxy Ketones: Revision of the Chelation-Control ModelDocument4 paginiAdditions of Organomagnesium Halides to α‑Alkoxy Ketones: Revision of the Chelation-Control Modelulul dwi yuliandaÎncă nu există evaluări
- Chemical Reactions of AlkynesDocument20 paginiChemical Reactions of AlkynesElizabeth AngaritaÎncă nu există evaluări
- Organic Acids and BasesDocument33 paginiOrganic Acids and BasesWill KnaebleÎncă nu există evaluări
- Orsms l07 & l08 - Fall 2022Document57 paginiOrsms l07 & l08 - Fall 2022Yousef EssamÎncă nu există evaluări
- Organic Chemistry Chapter 4Document58 paginiOrganic Chemistry Chapter 4Laiba KhanÎncă nu există evaluări
- 9930 PTC by Using Phosphonium CompoundsDocument9 pagini9930 PTC by Using Phosphonium Compoundsabrar hassanÎncă nu există evaluări
- Markov Niko VsDocument9 paginiMarkov Niko VsPipit Aditia ListiyaniÎncă nu există evaluări
- More Carboxylic Acids: R C O OH C O O-R + H+Document34 paginiMore Carboxylic Acids: R C O OH C O O-R + H+sungyeon heoÎncă nu există evaluări
- Wittig Reaction SibiDocument4 paginiWittig Reaction SibivinaybharadwajbsÎncă nu există evaluări
- Trans Vicinal: Halogenation DihalidesDocument4 paginiTrans Vicinal: Halogenation DihalidesKarla PereraÎncă nu există evaluări
- Chapter 5 McmurryDocument23 paginiChapter 5 McmurryCarolina XavierÎncă nu există evaluări
- AAACatalysis - IntroductionDocument35 paginiAAACatalysis - IntroductionYoussef AliÎncă nu există evaluări
- 4102609923604691Document538 pagini4102609923604691Vishal MÎncă nu există evaluări
- 6.2 S - Organic C 30 - ReactionsDocument40 pagini6.2 S - Organic C 30 - Reactionsk.7330167Încă nu există evaluări
- Mechanism of Organic ReactionsDocument45 paginiMechanism of Organic ReactionsBapu ThoratÎncă nu există evaluări
- Alkana, Sikloalkana Dan ReaksiDocument58 paginiAlkana, Sikloalkana Dan ReaksiMohammad Rangga Adrian FirdausÎncă nu există evaluări
- L13 - (JLD 3.0) - Reaction Mechanisms - 28 AugDocument46 paginiL13 - (JLD 3.0) - Reaction Mechanisms - 28 AugkisuisoffbeatÎncă nu există evaluări
- (L13) - (JLD 3.0) - Reaction Mechanisms - 28 AugDocument46 pagini(L13) - (JLD 3.0) - Reaction Mechanisms - 28 Augydouneed2012Încă nu există evaluări
- Synthesis of AlkynesDocument31 paginiSynthesis of AlkynesttinbddinÎncă nu există evaluări
- Organic Synthesis Bysir FaheemDocument57 paginiOrganic Synthesis Bysir FaheemSalim ChohanÎncă nu există evaluări
- HaloalkanesDocument218 paginiHaloalkanesVidhan PatniÎncă nu există evaluări
- 13.2 Characteristic Organic ReactionsDocument45 pagini13.2 Characteristic Organic Reactionssafiya_91Încă nu există evaluări
- Polunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Document22 paginiPolunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Ronak ModiÎncă nu există evaluări
- GCE Alevel Chem Topic 23 - SampleDocument5 paginiGCE Alevel Chem Topic 23 - Sampletony hoÎncă nu există evaluări
- L14 - (JLD 3.0) - Reaction Mechanisms - 02 SeptDocument62 paginiL14 - (JLD 3.0) - Reaction Mechanisms - 02 SeptkisuisoffbeatÎncă nu există evaluări
- CATALYSISDocument7 paginiCATALYSISJudy Ann Leyco BartolataÎncă nu există evaluări
- Organic Chemistry IDocument10 paginiOrganic Chemistry Iscribblerofnonsense80% (5)
- Handbook of Coordination Catalysis in Organic ChemistryDe la EverandHandbook of Coordination Catalysis in Organic ChemistryÎncă nu există evaluări
- Graphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1De la EverandGraphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1Încă nu există evaluări
- Production of X-RAYS Using X-RAY Tube: Journal of Physics: Conference SeriesDocument13 paginiProduction of X-RAYS Using X-RAY Tube: Journal of Physics: Conference SeriesATWIJUKIRE DICKENSÎncă nu există evaluări
- Question Paper CodeDocument4 paginiQuestion Paper CodeBalaji ArunÎncă nu există evaluări
- Mole Concept 11 PDFDocument26 paginiMole Concept 11 PDFSamyak Jha100% (1)
- Module 2: Infrared SpectrosDocument5 paginiModule 2: Infrared SpectrosAngela ReyesÎncă nu există evaluări
- Lab 4: An Acyclic Process ObjectivesDocument1 paginăLab 4: An Acyclic Process ObjectivesFnur FatihahÎncă nu există evaluări
- Presented By: Group C Sanjay Kr. Yadav Deepankar Ghosh Natraj S. AdhikariDocument40 paginiPresented By: Group C Sanjay Kr. Yadav Deepankar Ghosh Natraj S. AdhikariBandameedi RamuÎncă nu există evaluări
- Sci3 Q1mod5 Changes in Materials Brenda Quilladas Bgo v1Document25 paginiSci3 Q1mod5 Changes in Materials Brenda Quilladas Bgo v1MArkÎncă nu există evaluări
- Spek Dan Gambar Lelang 2Document3 paginiSpek Dan Gambar Lelang 2Fajar Pandu WijayaÎncă nu există evaluări
- 025welding Engineering An Introduction - (2.5 Plasma Arc Welding)Document3 pagini025welding Engineering An Introduction - (2.5 Plasma Arc Welding)Kamarul NizamÎncă nu există evaluări
- PH Calculation With CO2 AdditionDocument12 paginiPH Calculation With CO2 AdditionPraveen KhatriÎncă nu există evaluări
- D 3002 DesignDocument20 paginiD 3002 DesignmargaretramosÎncă nu există evaluări
- Ultramet 2507: Stainless Steel Electrodes Product Data SheetDocument1 paginăUltramet 2507: Stainless Steel Electrodes Product Data Sheetshaan1001gbÎncă nu există evaluări
- Enhanced Oil Recovery: SyllabusDocument24 paginiEnhanced Oil Recovery: SyllabusAkmuhammet MammetjanovÎncă nu există evaluări
- CEP Refresher Problem Set5Document6 paginiCEP Refresher Problem Set5Alyssa Camille Malig-onÎncă nu există evaluări
- NiSlip 520ADocument8 paginiNiSlip 520ASTI InspiredÎncă nu există evaluări
- Formation Evaluation and Petrophysics MR D. G. BowenDocument225 paginiFormation Evaluation and Petrophysics MR D. G. BowenAimiWani100% (2)
- EmileorConcerningEducation 10106815Document165 paginiEmileorConcerningEducation 10106815Túlio Coelho SampaioÎncă nu există evaluări
- Aade 11 Ntce 23Document10 paginiAade 11 Ntce 23Kinni ShenoldÎncă nu există evaluări
- Alloy 6201 Product Specification Rev.4Document3 paginiAlloy 6201 Product Specification Rev.4Ly PhongÎncă nu există evaluări
- 2nd COTdetailedDocument6 pagini2nd COTdetailedfe delgadoÎncă nu există evaluări
- BS 3604-2 - 1991 Steel Pipes and Tubes For Pressure Purposes Ferritic Alloy Steel With Specified ElevatedDocument24 paginiBS 3604-2 - 1991 Steel Pipes and Tubes For Pressure Purposes Ferritic Alloy Steel With Specified ElevatedtienlamÎncă nu există evaluări
- Artikel Bahasa Inggris Tentang Kesehatan Dan TerjemahannyaDocument5 paginiArtikel Bahasa Inggris Tentang Kesehatan Dan Terjemahannyairwan es ye teÎncă nu există evaluări
- Gasha International School Homework PolicyDocument12 paginiGasha International School Homework PolicyOmar KhidhirÎncă nu există evaluări
- Benzene: Training On The Hazards of Benzene in The WorkplaceDocument26 paginiBenzene: Training On The Hazards of Benzene in The WorkplaceAgung RahmadaniÎncă nu există evaluări
- D 2295 - 96 R02 - RdiyotuDocument3 paginiD 2295 - 96 R02 - RdiyotuJorgeÎncă nu există evaluări
- Raman SpectrosDocument10 paginiRaman SpectrosSruthiÎncă nu există evaluări