Documente Academic
Documente Profesional
Documente Cultură
Agfa CR 75 en PDF
Încărcat de
thaimedicaltrading THTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Agfa CR 75 en PDF
Încărcat de
thaimedicaltrading THDrepturi de autor:
Formate disponibile
Computed Radiography
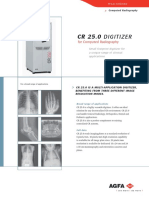
CR 75.0™ DIGITIZER
for Computed Radiography
Maximizing productivity for
the complete range of clinical
applications
CR 75.0 IS A MULTI-USER DIGITIZER FEATURING A
UNIQUE DROP-AND-GO BUFFER THAT ELIMINATES
WAITING TIMES AND MAXIMIZES PRODUCTIVITY
CR 75.0 IS A MULTI-APPLICATION DIGITIZER,
BENEFITING FROM THREE DIFFERENT IMAGE
RESOLUTION MODES
Highest productivity
The cassette buffer eliminates waiting times and allows
for a continuous workflow within the department.
Zero-button operation with automated cassette handling
• No waiting times
makes CR 75.0 a highly productive and user-friendly
for improved patient care
system with a throughput of up to 115 plates an hour,
• Input/output buffer depending on size and application.
for maximized productivity
• For a broad range of applications
No waiting
The CR 75.0 digitizer requires no manual interaction
and all the user has to do is to deposit the cassettes
in the input buffer (up to 10 cassettes). The digitizer
automatically takes cassettes from the input buffer and
reads the demographic data from the memory on the
cassette. It then scans the imaging plate, digitizes the
CR 75.0 DIGITIZER
for Computed Radiography
image and returns the cassette to the output buffer for
new exposures.
Full data
CR 75.0 reads imaging plates at a standard resolution of
6 pixels/mm. 10 pixels/mm high resolution capability
is available for all image plate sizes. 20 pixels/mm
resolution will be available for dedicated 18 x 24 cm and
24 x 30 cm extremities cassettes and plates.
Integrated CR User Station for time-saving
identification and optimized workflow
Compact footprint & optimal accessibility
CR 75.0 occupies a very small floorspace and at the same
time provides unhindered access to several users, both
at the input and the output buffer, resulting in a smooth
flow of operations. This concept makes CR 75.0 the
state-of-the-art solution for centralized CR environments.
Universal CR User Station
Optionally, a fully integrated CR User Station is available.
The CR User Station is suitable for all CR environments:
- Decentralized CR
(Surgery, Intensive Care Unit, Emergency Room,...)
- Personal CR
- In-room CR solutions.
Its modular and ergonomic design includes:
- Cassette identification functions
- Space for:
• Workstation for image handling, processing and
dispatching
• Monitor, network switches and UPS
• Cassette storage
An economical way to go digital
CR is compatible with all existing X-ray systems allowing
X-ray departments to go digital without significant
additional investments and workflow adaptations.
CASSETTE SIZES
ACCEPTED SPATIAL PIXEL
CASSETTE SIZES RESOLUTION MATRIX SIZE
Standard resolution
35 x 43 cm (14 x 17 in) 6 pixels / mm 2320 x 2826
35 x 35 cm (14 x 14 in) 6 pixels / mm 2320 x 2320
High resolution
35 x 43 cm (14 x 17 in) 10 pixels / mm (option) 3480 x 4240
35 x 35 cm (14 x 14 in) 10 pixels / mm (option) 3480 x 3480
35 x 43 cm 10 pixels / mm 2020 x 4240
(automatic collimation
to 21 x 43 cm)
24 x 30 cm 10 pixels / mm 2320 x 2920
18 x 24 cm 10 pixels / mm 1720 x 2320
15 x 30 cm 10 pixels / mm 1420 x 2920
8 x 10 in 10 pixels / mm 1950 x 2460
10 x 12 in 10 pixels / mm 2460 x 2970
Extremities
24 x 30 cm 20 pixels / mm 4760 x 5840
18 x 24 cm 20 pixels / mm 3560 x 4640
SAFETY
REGION REGULATION X-RAY LASER
Europe EN 60601-1: 1990 + A1: Regulation: 1987 EN 60825 - 1:2001
1993 + A2: 1995
EN 60601-1-2: 2001
USA UL 2601 DHHS/FDA 21 CFR DHHS/FDA 21
21CFR part 820: good manu- part 1002, subchapter B CFR parts 1040, 10
facturing practice for medical and 1040, 11
devices
Canada CSA22.2 No.601.1 No.601.1.2
TECHNICAL SPECIFICATIONS
General
Cassette buffer capacity and performance Environmental conditions
• 10 cassettes of mixed sizes, • Temperature: 20 - 30 ˚C (68 - 86 ˚F)
both in input and output buffer • Humidity: 10 - 80% RH
• Throughput: up to 115 plates/h • Magnetic fields: max. 12.60 µT
(depending on size and application) • Rate of change of temperature: 0.5 ˚C/minute
LCD display Environmental effects
Machine status and error conditions • Noise level: max. 65 dB (A)
• Heat dissipation: standby 350 W,
max. 2000 W
Greyscale resolution
• Data acquisition: 12 bits/pixel
• Output to processor: 12 bits/pixel
Safety
Dimensions and weight
Approvals
• W x D x H: 84 x 115 x 142 cm (33 x 45 x 56 in)
TüV, UL, cUL, CE
• At foot: 84 cm (33 in)
• At buffer: 142 cm (56 in)
• Weight: Approx. 320 kg (750.5 lbs) Transport details
• Temperature: -25 to +55 °C (-4 to 131 °F),
-25 °C for max. 72 hours, +55 °C for max. 96 hours
Power • Humidity: 5 - 95% RH
50/60 Hz single phase
240V +10%, max. fuse 16A
230V ±10%, max. fuse 16A
208V ±10%, max. fuse 15A (e.g. USA)
200V ±10%, max. fuse 15A (e.g. Japan)
Agfa-Gevaert has been approved by Lloyd’s Register Quality Assurance limited to the following Quality Management System Agfa Corporation
Standards: ISO 9001:1994; EN ISO 9001:1994, and ANSI/ASQC Q9001-1994. The Quality Management System is applicable to HealthCare Headquarters
the development, production and distribution of Agfa Medical Films. 10 South Academy Street,
Greenville, SC 29601
Agfa-Gevaert has been awarded the Approval of Conformity Certificate by Lloyd’s Register Quality Assurance. It certifies that the Quality
Management System for our X-Ray films conforms to the requirements of Annex V of the EEC Directive 93/42 and Medical Devices
Tel.: 1-864-421-1600
Regulation 1994:3017. Fax: 864-421-1622
Agfa HealthCare has been approved by Lloyd’s Register Quality Assurance limited to the following Quality Management System Standards: Canada
ISO 9001:1994; EN ISO 9001:1994, and ANSI/ASQC Q9001-1994. Agfa, Inc.
The Quality Management System is applicable to: Selling, Servicing, Distribution, and Design of Marketing of Agfa’s Product Assortment 77 Belfield Road,
of Equipement and Sensitive Materials Used in Medical Diagnositic Imaging, Non-destructive Testing, Microfilm and Motion Picture Etobicoke, M9W 1G6
Applications.
Tel.: 416-241-1110
Agfa-Gevaert had been awarded the ISO 9001 certificate by TÜV Zertifizierungsgemeinschaft e.V. This is applicable to Agfa’s Fax: 416-240-7359
Quality Management System for design, production and servicing of Agfa Medical Equipment.
Mexico
Products distributed in North America are manufactured by/for Agfa Corporation, 10 South Academy St., Greenville, South Carolina 29601. Agfa de México, S.A. de C.V.
Agfa, the Agfa rhombus, Point of Knowledge and See More. Do More. are trademarks of Agfa-Gevaert N.V., Belgium or its affiliates. All
Benjamin Franklin #98,
other trademarks are held by their respective owners and are used in an editorial fashion with no intention of infringementt. Colonia Escandon,
C.P. 11800, México, D.F.
The data in this publication are for illustration purposes only and do not necessarily represent standards or specifications which must be Tel.: +52 (55) 5276 7611
met by Agfa. All information contained herein is intended for guidance purposes only, and characteristics of the products described in Fax: +52 (55) 5515 8719
this publication can be changed at any time without notice. Products may not be available for your local area. Please contact your local
sales representative for availability information. Agfa diligently strives to provide as accurate information as possible, but shall not be
responsible for any typographical error.
© Copyright 2004 Agfa-Gevaert N.V. All rights reserved.
MI 00761 10/04
S-ar putea să vă placă și
- Digitizer: For Computed RadiographyDocument4 paginiDigitizer: For Computed RadiographyNikolay PenevÎncă nu există evaluări
- Digitizer: Maximizing Productivity For The Complete Range of Clinical ApplicationsDocument4 paginiDigitizer: Maximizing Productivity For The Complete Range of Clinical ApplicationsTanveerÎncă nu există evaluări
- Digitizer: Maximizing Productivity For The Complete Range of Clinical ApplicationsDocument4 paginiDigitizer: Maximizing Productivity For The Complete Range of Clinical ApplicationsAristarhÎncă nu există evaluări
- Digitizer: Small Footprint Digitizer For The Complete Range of Clinical ApplicationsDocument4 paginiDigitizer: Small Footprint Digitizer For The Complete Range of Clinical ApplicationsHabibÎncă nu există evaluări
- Digitizer: Small Footprint Digitizer For The Complete Range of Clinical ApplicationsDocument4 paginiDigitizer: Small Footprint Digitizer For The Complete Range of Clinical ApplicationsshoaibÎncă nu există evaluări
- Agfa CR 10XDocument4 paginiAgfa CR 10Xwisateru Inti niagaÎncă nu există evaluări
- Agfa CR 10 XDocument4 paginiAgfa CR 10 XSandyKarittokÎncă nu există evaluări
- Konica Xpress CR 190Document6 paginiKonica Xpress CR 190Syed Khawar MukhtarÎncă nu există evaluări
- Laser Imager Carestream Kodak DryView 5800 - BrochureDocument4 paginiLaser Imager Carestream Kodak DryView 5800 - BrochureWisal AhdabÎncă nu există evaluări
- Atlas 9000Document6 paginiAtlas 9000tung NguyenÎncă nu există evaluări
- DV5800Document4 paginiDV5800Mohd SabirÎncă nu există evaluări
- Edge Quix Digital RadiographyDocument6 paginiEdge Quix Digital Radiographyfse4.diasedinisÎncă nu există evaluări
- Digi Super: XJ25 6.8B IE 6.8-170mm 1:1.5Document4 paginiDigi Super: XJ25 6.8B IE 6.8-170mm 1:1.5Supun SupunÎncă nu există evaluări
- Agfa CR 10 X User ManualDocument4 paginiAgfa CR 10 X User ManualpietrokoÎncă nu există evaluări
- Digitizer: No Quality CompromisesDocument4 paginiDigitizer: No Quality CompromisesVo Hong VinhÎncă nu există evaluări
- AGFA CR - 10 XDocument6 paginiAGFA CR - 10 Xrtkraajan1961Încă nu există evaluări
- Digitizer: No Quality CompromisesDocument4 paginiDigitizer: No Quality CompromisesEmanuel FranciscoÎncă nu există evaluări
- Computed Radiography-Crx25p PDFDocument4 paginiComputed Radiography-Crx25p PDFasedeÎncă nu există evaluări
- Wpro100018 HD-CR 35 NDT enDocument8 paginiWpro100018 HD-CR 35 NDT enMuthu PalanisamyÎncă nu există evaluări
- Adc C D: Ompact IgitizerDocument2 paginiAdc C D: Ompact Igitizerthanh sơn trầnÎncă nu există evaluări
- FireCRmed Brochure WebDocument2 paginiFireCRmed Brochure WebTony IuratoÎncă nu există evaluări
- Konica Minolta Srx101a SpecificationsDocument2 paginiKonica Minolta Srx101a SpecificationsWolaé Mathurin Edmond AmegandjinÎncă nu există evaluări
- Small Wonder.: Digital. Compact. AffordableDocument4 paginiSmall Wonder.: Digital. Compact. AffordableAvan Setyo PratamaÎncă nu există evaluări
- Kodak Dryview 6800Document4 paginiKodak Dryview 6800ASM SMÎncă nu există evaluări
- Titan 2000Document16 paginiTitan 2000Maryi Rocio Galeano SanchezÎncă nu există evaluări
- Cios Select BV - 03-06-2020 - 152081960Document16 paginiCios Select BV - 03-06-2020 - 152081960sandra rodriguezÎncă nu există evaluări
- uploadsbrochure15273103556AGFA CR30-XM PDFDocument4 paginiuploadsbrochure15273103556AGFA CR30-XM PDFantoniod179237Încă nu există evaluări
- D D P X-R S: Irect Igital Ortable AY YstemDocument4 paginiD D P X-R S: Irect Igital Ortable AY YstemJairo ManzanedaÎncă nu există evaluări
- DRYSTAR AXYS-DatasheetDocument4 paginiDRYSTAR AXYS-DatasheetNguyen AnhÎncă nu există evaluări
- Brochure Q Rad DRX Radiographic Systems 10 2017Document16 paginiBrochure Q Rad DRX Radiographic Systems 10 2017Ing Gerardo De Gyves AvilaÎncă nu există evaluări
- Carestream DirectView Classic CR System - CM - InfoDocument8 paginiCarestream DirectView Classic CR System - CM - InfoNur Alam0% (1)
- Kodak CR Itx 560 X Ray SystemDocument4 paginiKodak CR Itx 560 X Ray SystemAbbas AbbasÎncă nu există evaluări
- Brochure n33Document4 paginiBrochure n33vladimir.peretyaginÎncă nu există evaluări
- Brilliance iCT Confi GurationDocument12 paginiBrilliance iCT Confi GurationpeymanÎncă nu există evaluări
- Philips Brilliance CT ScannerDocument12 paginiPhilips Brilliance CT ScannerAliali MohamedÎncă nu există evaluări
- 2-4 - 4 Lp-Carestream (Kodak) Dryview 5800Document5 pagini2-4 - 4 Lp-Carestream (Kodak) Dryview 5800nanu_gomezÎncă nu există evaluări
- Ficha Técnica Elite CR SystemDocument6 paginiFicha Técnica Elite CR SystemSebastian RodriguezÎncă nu există evaluări
- As 102176 CV-X C 600S15 GB WW 1039-2Document60 paginiAs 102176 CV-X C 600S15 GB WW 1039-2Alexandru IonutÎncă nu există evaluări
- Smart: Expanding The Horizons of Digital RadiographyDocument6 paginiSmart: Expanding The Horizons of Digital RadiographymrscribdÎncă nu există evaluări
- Canon Aquilion Start 32 S - Technical OfferDocument10 paginiCanon Aquilion Start 32 S - Technical OfferWaheed Mido100% (4)
- AGFA CR-30X Premium Brochure 2nd Generation Dec 2011 With CM Info PDFDocument6 paginiAGFA CR-30X Premium Brochure 2nd Generation Dec 2011 With CM Info PDFyahiateneÎncă nu există evaluări
- CT Neuviz 64 - 220115 PDFDocument4 paginiCT Neuviz 64 - 220115 PDFyanuar100% (1)
- Pengumuman KemenkeuDocument2 paginiPengumuman Kemenkeuasik downloadÎncă nu există evaluări
- Va797a 17 Q 0005 A00005003 PDFDocument4 paginiVa797a 17 Q 0005 A00005003 PDFsahar1344Încă nu există evaluări
- CR 10-X (English - Brochure)Document8 paginiCR 10-X (English - Brochure)Vinay SharmaÎncă nu există evaluări
- SS 22e PDFDocument2 paginiSS 22e PDFDilsher SinghÎncă nu există evaluări
- Adc Solo enDocument2 paginiAdc Solo enNikolay PenevÎncă nu există evaluări
- Line # Description Qty EPIQ 7G System 1 1Document7 paginiLine # Description Qty EPIQ 7G System 1 1AmirÎncă nu există evaluări
- For Digital Radiography: DX-S Is A High-Throughput, Decentralized Digitizer With State-Of-The-Art Image QualityDocument4 paginiFor Digital Radiography: DX-S Is A High-Throughput, Decentralized Digitizer With State-Of-The-Art Image QualityAmilcar BureloÎncă nu există evaluări
- Xineos 2329 Datasheet PDFDocument2 paginiXineos 2329 Datasheet PDFRodrigo Dos Santos SilvaÎncă nu există evaluări
- Xineos 2329 Datasheet PDFDocument2 paginiXineos 2329 Datasheet PDFRodrigo Dos Santos SilvaÎncă nu există evaluări
- Philips Idt16 2Document9 paginiPhilips Idt16 2atmroo9Încă nu există evaluări
- Titan 2000 Vet SpecDocument10 paginiTitan 2000 Vet SpecSrecko Stokanovic50% (2)
- Electronic CircuitsDocument91 paginiElectronic Circuitsvinay Shastry100% (6)
- Siemens ManualDocument10 paginiSiemens ManualFrancisco AvilaÎncă nu există evaluări
- Brochure CXDI 401CDocument6 paginiBrochure CXDI 401CSrecko StokanovicÎncă nu există evaluări
- Microprocessor Architectures and Systems: RISC, CISC and DSPDe la EverandMicroprocessor Architectures and Systems: RISC, CISC and DSPEvaluare: 4 din 5 stele4/5 (1)
- Anti Aliasing: Enhancing Visual Clarity in Computer VisionDe la EverandAnti Aliasing: Enhancing Visual Clarity in Computer VisionÎncă nu există evaluări
- No Rotation No Integration: Simple FunctionDocument2 paginiNo Rotation No Integration: Simple FunctionAwadhÎncă nu există evaluări
- Resolving Our Uncertainty About Oxygen TherapyDocument7 paginiResolving Our Uncertainty About Oxygen TherapyAwadhÎncă nu există evaluări
- 74 300ma X Ray MachineDocument2 pagini74 300ma X Ray MachineAwadhÎncă nu există evaluări
- What Is EOS X-Ray Imaging?Document2 paginiWhat Is EOS X-Ray Imaging?AwadhÎncă nu există evaluări
- Chesneys Equipment For Student Radiographers by P H Carter PDFDocument5 paginiChesneys Equipment For Student Radiographers by P H Carter PDFAwadhÎncă nu există evaluări
- The Epic Arm: Nia Christian, Lindsey Milisits, Jeffrey Nemes, and Dustin SchneiderDocument1 paginăThe Epic Arm: Nia Christian, Lindsey Milisits, Jeffrey Nemes, and Dustin SchneiderAwadhÎncă nu există evaluări
- Oxygen Concentrators For District Hospitals: Michael B Dobson, John Radcliffe Hospital, Oxford, UKDocument3 paginiOxygen Concentrators For District Hospitals: Michael B Dobson, John Radcliffe Hospital, Oxford, UKAwadhÎncă nu există evaluări
- Chemistry OGS ETHOS OGSDocument10 paginiChemistry OGS ETHOS OGSAwadhÎncă nu există evaluări
- The Oxygen Concentrator: DR Roger Eltringham, Consultant Anaesthetist, Gloucestershire Royal Hospital, GloucesterDocument2 paginiThe Oxygen Concentrator: DR Roger Eltringham, Consultant Anaesthetist, Gloucestershire Royal Hospital, GloucesterAwadhÎncă nu există evaluări
- CS 3D Scanner Family: CS 3500 & CS 3600: Device Not Recognized After Cumulative Updates On Windows 10Document3 paginiCS 3D Scanner Family: CS 3500 & CS 3600: Device Not Recognized After Cumulative Updates On Windows 10AwadhÎncă nu există evaluări
- Dual Energy CT Aortography With 50 Reduced Iodine Dose Versus Single Energy CT Aortography With Standard Iodine DoseDocument8 paginiDual Energy CT Aortography With 50 Reduced Iodine Dose Versus Single Energy CT Aortography With Standard Iodine DoseAwadhÎncă nu există evaluări
- Resource Manual For Compliance Test Parameters of Diagnostic X Ray Systems (PDF Version) PDFDocument45 paginiResource Manual For Compliance Test Parameters of Diagnostic X Ray Systems (PDF Version) PDFAwadhÎncă nu există evaluări
- # Medison System Passwords: Debug Mode Windows (DOS) Admin Mode Booting Password Product Setup ModeDocument1 pagină# Medison System Passwords: Debug Mode Windows (DOS) Admin Mode Booting Password Product Setup ModeAwadh100% (3)
- Hitachi AIRIS II Open Tech SpecsDocument2 paginiHitachi AIRIS II Open Tech SpecsAwadhÎncă nu există evaluări
- DB101 (DF005) THRU DB107 (DF10) : Glass Passivated Bridge RectifierDocument2 paginiDB101 (DF005) THRU DB107 (DF10) : Glass Passivated Bridge RectifierAwadhÎncă nu există evaluări
- DAA NotesDocument97 paginiDAA Notesanish.t.pÎncă nu există evaluări
- Parrot: by Alan BrownjohnDocument2 paginiParrot: by Alan BrownjohnPADUMI SASIKALA50% (2)
- CEC CategoryDocument4 paginiCEC CategoryQ8123Încă nu există evaluări
- Seal Kit Seal KitDocument61 paginiSeal Kit Seal KitМаксим Стратила0% (1)
- Sex Quiz ResultsDocument1 paginăSex Quiz Resultsapi-285789748Încă nu există evaluări
- A Comparative Study of Indian and Western Music FormsDocument6 paginiA Comparative Study of Indian and Western Music FormsSophieÎncă nu există evaluări
- Edi WowDocument11 paginiEdi WowfantasighÎncă nu există evaluări
- Precooling and Storage FacilitiesDocument136 paginiPrecooling and Storage FacilitiesspurwonofjpÎncă nu există evaluări
- To Parts CatalogDocument18 paginiTo Parts CatalogaliÎncă nu există evaluări
- 10D Unit-Coral Reefs PDFDocument14 pagini10D Unit-Coral Reefs PDFIWAN KUNCOROÎncă nu există evaluări
- TAPCON® 240: Voltage Regulator For Regulating TransformersDocument6 paginiTAPCON® 240: Voltage Regulator For Regulating TransformerscastrojpÎncă nu există evaluări
- Gerador #LotofácilDocument10 paginiGerador #LotofácilEmanuel BezerraÎncă nu există evaluări
- Sternberg TheoryDocument20 paginiSternberg TheoryKhadijah ElamoreÎncă nu există evaluări
- MSA 516 Application Controls PowerPoint PresentationDocument21 paginiMSA 516 Application Controls PowerPoint PresentationPrince Ric100% (1)
- Ludo (Sorry) - Game StrategyDocument8 paginiLudo (Sorry) - Game Strategysuperyoopy100% (1)
- Sheena HSMDocument8 paginiSheena HSMdesign12Încă nu există evaluări
- LGIT CatalogueDocument11 paginiLGIT CatalogueArjun SharmaÎncă nu există evaluări
- 4UIE - AB - Nov 23Document2 pagini4UIE - AB - Nov 23aaaÎncă nu există evaluări
- ARIMI Et Al-2010-Journal of Texture Studies PDFDocument21 paginiARIMI Et Al-2010-Journal of Texture Studies PDFRomaric OuetchehouÎncă nu există evaluări
- 193-Cable Tray QtyDocument4 pagini193-Cable Tray QtyprkshshrÎncă nu există evaluări
- Battista Mondin, Saint Thomas Aquinas' Philosophy. in The Commentary To The Sentences (Inglés) PDFDocument134 paginiBattista Mondin, Saint Thomas Aquinas' Philosophy. in The Commentary To The Sentences (Inglés) PDFFray Daniel Sisa NiñoÎncă nu există evaluări
- Analysis of The Flow Induced by Air-Bubble SystemsDocument16 paginiAnalysis of The Flow Induced by Air-Bubble SystemsStephany CamacaroÎncă nu există evaluări
- Cover Modul 4 TMJ 2012Document7 paginiCover Modul 4 TMJ 2012Haura Nadya AmaliaÎncă nu există evaluări
- GLS Poland Z Tytuem Lidera Logistyki ENGDocument3 paginiGLS Poland Z Tytuem Lidera Logistyki ENGFyikgfjijgÎncă nu există evaluări
- CDI1Document40 paginiCDI1Leonino Angelica Aiko S.Încă nu există evaluări
- Modul Customer ServiceDocument5 paginiModul Customer ServiceFandy Bestario HarlanÎncă nu există evaluări
- Python Cheat SheetDocument11 paginiPython Cheat SheetWasswa OpioÎncă nu există evaluări
- HPC Module 1Document48 paginiHPC Module 1firebazzÎncă nu există evaluări
- NF EN 10028-3-EnglishDocument17 paginiNF EN 10028-3-Englishhakan gecerÎncă nu există evaluări
- Omb ValvesDocument52 paginiOmb ValvesCesar SotoÎncă nu există evaluări