Documente Academic
Documente Profesional
Documente Cultură
Flu Specimen Collection Poster
Încărcat de
568563Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Flu Specimen Collection Poster
Încărcat de
568563Drepturi de autor:
Formate disponibile
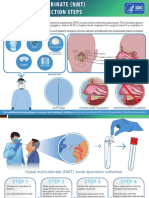
Influenza Specimen Collection
Nasopharyngeal Swab Nasopharyngeal/Nasal Aspirate Nasopharyngeal/Nasal Wash Deep Nasal Swab Combined Nasal & Throat Swab
Materials • Sterile Dacron/nylon swab • Sterile suction catheter/suction • Sterile suction catheter/suction • Sterile polyester swab (aluminum • 2 dry sterile polyester swabs
• Viral transport media tube apparatus apparatus or plastic shaft preferred) (aluminum or plastic shafts preferred)
(should contain 1-3 ML of sterile • Viral transport media tube • Sterile normal saline • Viral transport media tube • Viral transport media tube
viral transport medium) (should contain 1-3 ML of sterile (should contain 1-3 ML of sterile (should contain 1-3 ML of sterile
viral transport medium) viral transport medium) viral transport medium)
Procedure
1 Tilt patient’s head back 70 degrees. 1 Attach catheter to suction apparatus. 1 Attach catheter to suction apparatus. 1 Tilt patient’s head back 70 degrees. 1 Tilt patient’s head back 70 degrees.
2 Insert swab into nostril. (Swab should 2 Tilt patient’s head back 70 degrees. 2 Tilt patient’s head back 70 degrees. 2 While gently rotating the swab, insert 2 While gently rotating the swab, insert
reach depth equal to distance from swab less than one inch into nostril swab less than one inch into nostril
nostrils to outer opening of the ear.) (until resistance is met at turbinates). (until resistance is met at turbinates).
Leave swab in place for several
seconds to absorb secretions.
3 Insert catheter into nostril. (Catheter 3 Insert several drops of sterile normal
should reach depth equal to distance saline into each nostril.
from nostrils to outer opening of ear.) 3 Rotate the swab several times against 3 Rotate the swab several times against
nasal wall and repeat in other nostril nasal wall and repeat in other nostril
3 Slowly remove swab while rotating it. using the same swab. using the same swab.
(Swab both nostrils with same swab.)
4 Insert catheter into nostril. (Catheter
4 Begin gentle suction. Remove catheter should reach depth equal to distance
while rotating it gently. from nostrils to outer opening of ear.)
4 Place tip of the swab into sterile 4 Place tip of the swab into sterile viral
4 Place tip of swab into sterile viral sterile viral transport media tube and transport media tube and cut off the
transport media tube and snap/cut off cut off the applicator stick. applicator stick.
the applicator stick.
5 Place specimen in sterile viral 5 Begin gentle suction. Remove
transport media tube. catheter while rotating it gently.
Note: NP aspirate may not be possible 5 For throat swab, take a second dry
to conduct in infants polyester swab, insert into mouth,
and swab the posterior pharynx and
tonsillar areas. (Avoid the tongue.)
6 Place specimen in sterile viral
transport media tube.
Note: NP aspirate may not be possible
to conduct in infants
6 Place tip of swab into the same tube
and cut off the applicator tip.
Packing: Shipping:
• Label the specimen on viral transport media tube and ensure cap on tube is tightly sealed. • Ship specimens for testing as soon as possible.
(Do not use a pencil or pen for labeling, as they can rub off or smear. Instead, use a bar code • If delivery will be delayed for more than 3-4 days, specimen should be frozen at -70 degrees Celsius (-94 degrees Fahrenheit).
or permanent marker).
• Ensure specimen will be received by the public health laboratory during normal business hours.
• Fill out paperwork in accordance with state health department guidelines.
• Include a frozen cold pack with the specimen(s).
Considerations:
• A nasopharyngeal (NP) swab is the optimal upper respiratory tract specimen collection method for influenza testing.
• Pack specimens in accordance with U.S. Department of Transportation regulations regarding However, such specimens cannot be collected from infants and many older patients may not allow an NP specimen to be
shipment of biological substances, see www.cdc.gov/flu/professionals/diagnosis/index.htm. collected. Alternatively, a combined nasal and throat swab specimen or aspirate specimens can provide good influenza
Storing: virus yield.
• Specimens should be placed into sterile viral transport media and immediately placed on • Some influenza tests are approved only for use with certain kinds of respiratory tract specimens, so follow guidelines
refrigerant gel packs or at 4 degrees Celsius (refrigerator) for transport to the state public provided by test. Also, some tests (e.g., rapid influenza diagnostic tests) are only approved for certain kinds of respiratory
health laboratory. tract specimens.
• Keep specimens refrigerated (2-8 degrees Celsius, 26-46 degrees Fahrenheit) prior to shipping. • For best results (i.e., highest influenza virus yield), collect respiratory tract specimens within four days of illness onset.
• Most sensitive and accurate tests for influenza virus detection are molecular or nucleic acid amplification tests (RT-PCR).
• Negative test results obtained from rapid influenza diagnostic tests (RIDTs) that detect influenza viral antigens do not
exclude influenza virus infection in patients with signs and symptoms of influenza. A negative test result could be a false
negative and should not preclude further diagnostic testing (such as RT-PCR) and starting empiric antiviral treatment.
• A surgical mask and gloves are recommended at a minimum for all procedures. For some patients and procedures,
additional precautions may be indicated, see Standard Precautions at www.cdc.gov/hicpac/2007IP/2007ip_part4.html#a4.
CS246972
S-ar putea să vă placă și
- Chest Wall DeformitiesDocument19 paginiChest Wall Deformitiesmmkavitha9860% (5)
- Get Vaccinated-Argument EssayDocument4 paginiGet Vaccinated-Argument Essayapi-489674055Încă nu există evaluări
- 1ST Term SummaryDocument5 pagini1ST Term SummarySJane FeriaÎncă nu există evaluări
- MS LAB ReviewerDocument2 paginiMS LAB Reviewershachima0713Încă nu există evaluări
- SuctioningDocument2 paginiSuctioningHERRERA, ANGELAÎncă nu există evaluări
- ENT InstrumentsDocument32 paginiENT InstrumentsPathikritÎncă nu există evaluări
- Naso Oro SuctioningDocument4 paginiNaso Oro SuctioningandreabreeÎncă nu există evaluări
- ORO-NASO SuctioningDocument20 paginiORO-NASO Suctioningcaitie miracleÎncă nu există evaluări
- 10 Oropharyngeal and Nasopharyngeal SuctioningDocument8 pagini10 Oropharyngeal and Nasopharyngeal SuctioningKen Morales Alcantara100% (1)
- Immunosero Lab Pipetting Techniques 2Document2 paginiImmunosero Lab Pipetting Techniques 2Justine Claire M. NamocatcatÎncă nu există evaluări
- Updates Printed in RedDocument2 paginiUpdates Printed in RedAna-Mihaela BalanuțaÎncă nu există evaluări
- Suctioning: Loreiyne Grace MDocument100 paginiSuctioning: Loreiyne Grace MYasmin Macadatar GumamaÎncă nu există evaluări
- Tracheostomy (Skills)Document8 paginiTracheostomy (Skills)Luna JadeÎncă nu există evaluări
- General Guidance:: Gloves N-95 RespiratorDocument2 paginiGeneral Guidance:: Gloves N-95 RespiratorTaufan LutfiÎncă nu există evaluări
- TRACHEOSTOMY and Wound CareDocument9 paginiTRACHEOSTOMY and Wound CareSUREEN MAY ANG REGULARÎncă nu există evaluări
- Hai & Infection Control PracticesDocument134 paginiHai & Infection Control PracticesAnna 964250Încă nu există evaluări
- Endotracheal/Tracheal Suctioning ProcedureDocument4 paginiEndotracheal/Tracheal Suctioning ProcedureGiselle EstoquiaÎncă nu există evaluări
- Tracheostomy Set ContainingDocument10 paginiTracheostomy Set ContainingShivani DhillonÎncă nu există evaluări
- Tracheostomy DemoDocument3 paginiTracheostomy DemoMae lea AndoloyÎncă nu există evaluări
- Nasopharyngeal Swab Test ChecklistDocument3 paginiNasopharyngeal Swab Test ChecklistNoronisa D. CabugatanÎncă nu există evaluări
- Mycv Prelims Finals LabDocument21 paginiMycv Prelims Finals LabJuzhley PerezÎncă nu există evaluări
- Cology-5 BookDocument10 paginiCology-5 BookSmart MindÎncă nu există evaluări
- Tracheostomy Care - Docx 1Document2 paginiTracheostomy Care - Docx 1Shreyas WalvekarÎncă nu există evaluări
- Airway SuctioningDocument28 paginiAirway SuctioningMỹ HạnhÎncă nu există evaluări
- Step-By-Step Guide: Take Swab SampleDocument1 paginăStep-By-Step Guide: Take Swab SampleJo B. BorromeoÎncă nu există evaluări
- SUCTIONINGDocument50 paginiSUCTIONINGisaacpapicaÎncă nu există evaluări
- Inhaler Technique Checklist - NPS Medicinewise - 2020Document2 paginiInhaler Technique Checklist - NPS Medicinewise - 2020pdladvaÎncă nu există evaluări
- Unit 4 - Culture Transfer ShortenDocument3 paginiUnit 4 - Culture Transfer ShortenMinh DuyÎncă nu există evaluări
- Airway Management 2023-2024Document5 paginiAirway Management 2023-2024M7MD SHOWÎncă nu există evaluări
- ABON Poster FinalDocument1 paginăABON Poster FinalAngelina LugoÎncă nu există evaluări
- Chapter 7 Ofi Procedure ManualDocument47 paginiChapter 7 Ofi Procedure ManualAndree GalloÎncă nu există evaluări
- Ob PackDocument18 paginiOb PackElizabeth Louwel ConchaÎncă nu există evaluări
- Pap Test Endocervical Brush Spatula ProtocolDocument2 paginiPap Test Endocervical Brush Spatula ProtocolsamleverkÎncă nu există evaluări
- Oropharyngeal and Nasopharyngeal SuctioningDocument2 paginiOropharyngeal and Nasopharyngeal SuctioningAlana Caballero100% (1)
- PRELIM-NCM109 B-Specimen CollectionDocument6 paginiPRELIM-NCM109 B-Specimen CollectionJay EstrellaÎncă nu există evaluări
- Nasogastric Tube (INSERTION, FEEDING, & WITHDRAW)Document8 paginiNasogastric Tube (INSERTION, FEEDING, & WITHDRAW)Maricar VillelaÎncă nu există evaluări
- College of Health Care Technology: SuctioningDocument3 paginiCollege of Health Care Technology: SuctioningSheila Marie GanÎncă nu există evaluări
- MS2760 HiViral Mediu de Transport KitDocument2 paginiMS2760 HiViral Mediu de Transport KitLidia NarbÎncă nu există evaluări
- Catheterization Insertion and RemovalDocument5 paginiCatheterization Insertion and RemovalArmySapphireÎncă nu există evaluări
- Insertion of A Nasogastric TubeDocument3 paginiInsertion of A Nasogastric TubeJames Andre P. MoralesÎncă nu există evaluări
- Skill 29 (1) ..Urinary CatheterizationDocument2 paginiSkill 29 (1) ..Urinary CatheterizationnetsquadÎncă nu există evaluări
- Performing Oropharyngeal and Nasopharyngeal SuctioningDocument3 paginiPerforming Oropharyngeal and Nasopharyngeal SuctioningYa Mei LiÎncă nu există evaluări
- Genbody Covid - 19 Ag: Intended Use Explanation of The TestDocument2 paginiGenbody Covid - 19 Ag: Intended Use Explanation of The TestirvanagheÎncă nu există evaluări
- Skill 47 (1) ..Tracheostomy Tube Change PDFDocument2 paginiSkill 47 (1) ..Tracheostomy Tube Change PDFRadhika SethuÎncă nu există evaluări
- Inserting A Nasogastric Tube ChecklistDocument7 paginiInserting A Nasogastric Tube ChecklistAlyssa DeleonÎncă nu există evaluări
- Group 9 - Policy and Procedure FormulationDocument4 paginiGroup 9 - Policy and Procedure FormulationZYRAH LUKE E. VILBARÎncă nu există evaluări
- Manual PROPHI JET SET 2 - HOLEDocument4 paginiManual PROPHI JET SET 2 - HOLESanté IngenieríaÎncă nu există evaluări
- Station Exam - Pharm and MedsurgDocument9 paginiStation Exam - Pharm and MedsurgbriannareardonÎncă nu există evaluări
- Oro & Nasopharyngeal SuctioningDocument16 paginiOro & Nasopharyngeal SuctioningHERLIN HOBAYANÎncă nu există evaluări
- Final Term - 2nd Sem MCN PPT NotesDocument42 paginiFinal Term - 2nd Sem MCN PPT NotesJulie EstebanÎncă nu există evaluări
- Nasotracheal SuctioningDocument2 paginiNasotracheal Suctioningmarie100% (3)
- Oro-Naso DemoDocument4 paginiOro-Naso DemoMae lea AndoloyÎncă nu există evaluări
- Tracheostomy Care ChecklistDocument6 paginiTracheostomy Care ChecklistZero TwoÎncă nu există evaluări
- Ped. Oro-Nasopharyngeal Suctioning (E-Tool)Document2 paginiPed. Oro-Nasopharyngeal Suctioning (E-Tool)May RodeoÎncă nu există evaluări
- OxygenationDocument12 paginiOxygenationCherry Lou Guanzing100% (1)
- Ward Prep NotesDocument7 paginiWard Prep NotesReese Alessandra GandulfoÎncă nu există evaluări
- Trach Care ProcedureDocument1 paginăTrach Care ProcedureDavidÎncă nu există evaluări
- Outpatient Exam Room Cleaning Resource Guide: Non-Aerosol Generating Procedure Aerosol Generating Procedure (AGP)Document1 paginăOutpatient Exam Room Cleaning Resource Guide: Non-Aerosol Generating Procedure Aerosol Generating Procedure (AGP)Nissan OredataÎncă nu există evaluări
- Tracheostomy CareDocument34 paginiTracheostomy CareZgherea MihaiÎncă nu există evaluări
- Anaerobic Culture CollectionDocument3 paginiAnaerobic Culture CollectionADIÎncă nu există evaluări
- Nasopharyngeal Airway SuctioningDocument3 paginiNasopharyngeal Airway SuctioningHaitham ZraigatÎncă nu există evaluări
- TapScanner 24-03-2021-15.19Document1 paginăTapScanner 24-03-2021-15.19568563Încă nu există evaluări
- Vol1 No1 4Document5 paginiVol1 No1 4568563Încă nu există evaluări
- Tatalaksana Hipoksemia Akut CovidDocument31 paginiTatalaksana Hipoksemia Akut CovidLilis Sumiyati100% (1)
- c05790186 PDFDocument137 paginic05790186 PDFAngelli GuingueÎncă nu există evaluări
- List Obat BP Lanal SorongDocument3 paginiList Obat BP Lanal Sorong568563Încă nu există evaluări
- Vertigo Blok15Document23 paginiVertigo Blok15Mul YaniÎncă nu există evaluări
- Curriculum VitaeDocument2 paginiCurriculum Vitae568563Încă nu există evaluări
- Gastroenterol. Rep. 2014 Parikh Gastro Gou081Document3 paginiGastroenterol. Rep. 2014 Parikh Gastro Gou081568563Încă nu există evaluări
- Congestive Heart FailureDocument43 paginiCongestive Heart Failure568563100% (1)
- JaundiceDocument16 paginiJaundicemanognaaaaÎncă nu există evaluări
- 4713-7 8.16 Afzaninawati Suria YusofDocument5 pagini4713-7 8.16 Afzaninawati Suria Yusof568563Încă nu există evaluări
- Gut 2011 Morris 806 13Document9 paginiGut 2011 Morris 806 13568563Încă nu există evaluări
- HI! I'm Emily and Here To Tell You To Pay Attention... This Isa Really Great Talk!!!!!!!!!! Have You Seen My Brother? He's Sooooa AnnoyingDocument18 paginiHI! I'm Emily and Here To Tell You To Pay Attention... This Isa Really Great Talk!!!!!!!!!! Have You Seen My Brother? He's Sooooa Annoying568563Încă nu există evaluări
- 2013 Acc Aha Guideline On The Treatment of Blood Cholesterol To Reduce Athersclerotic Risk 2Document67 pagini2013 Acc Aha Guideline On The Treatment of Blood Cholesterol To Reduce Athersclerotic Risk 2HidayadÎncă nu există evaluări
- PERIODE GASAL 2013/2014 Tugas: Program Studi ArsitekturDocument1 paginăPERIODE GASAL 2013/2014 Tugas: Program Studi Arsitektur568563Încă nu există evaluări
- ALS Drug SummaryDocument1 paginăALS Drug Summary568563Încă nu există evaluări
- PERIODE GASAL 2013/2014 Tugas: Program Studi ArsitekturDocument1 paginăPERIODE GASAL 2013/2014 Tugas: Program Studi Arsitektur568563Încă nu există evaluări
- Hyperbilirubinemia FinalDocument26 paginiHyperbilirubinemia Final568563Încă nu există evaluări
- Acute Decompensated Heart FailureDocument31 paginiAcute Decompensated Heart Failure568563Încă nu există evaluări
- Severe Erythroderma As A Complication of Continuous Epoprostenol TherapyDocument3 paginiSevere Erythroderma As A Complication of Continuous Epoprostenol Therapy568563Încă nu există evaluări
- 68 Year Old Man Pags 71 A 75Document5 pagini68 Year Old Man Pags 71 A 75568563Încă nu există evaluări
- Erythroderma: A Clinicopathological Study of 102 Cases: Original ArticleDocument6 paginiErythroderma: A Clinicopathological Study of 102 Cases: Original Article568563Încă nu există evaluări
- PD1011 DPT ResidentsDocument3 paginiPD1011 DPT Residents568563Încă nu există evaluări
- 283 560 1 PBDocument5 pagini283 560 1 PB568563Încă nu există evaluări
- Combined Intravitreal Ranibizumab and Verteporfin Photodynamic Therapy Versus Ranibizumab Alone For The Treatment of Age-Related Macular DegenerationDocument5 paginiCombined Intravitreal Ranibizumab and Verteporfin Photodynamic Therapy Versus Ranibizumab Alone For The Treatment of Age-Related Macular Degeneration568563Încă nu există evaluări
- Critical Appraisal PrognosisDocument4 paginiCritical Appraisal Prognosis568563Încă nu există evaluări
- Erythroderma Caused Drug Allergies: Case ReportDocument7 paginiErythroderma Caused Drug Allergies: Case Report568563Încă nu există evaluări
- Short-Term Outcomes of Tonsillectomy in Adult Patients With Recurrent Pharyngitis: A Randomized Controlled TrialDocument1 paginăShort-Term Outcomes of Tonsillectomy in Adult Patients With Recurrent Pharyngitis: A Randomized Controlled Trial568563Încă nu există evaluări
- Short-Term Outcomes of Tonsillectomy in Adult Patients With Recurrent Pharyngitis: A Randomized Controlled TrialDocument1 paginăShort-Term Outcomes of Tonsillectomy in Adult Patients With Recurrent Pharyngitis: A Randomized Controlled Trial568563Încă nu există evaluări
- Yellow 627 Isolation PolicyDocument24 paginiYellow 627 Isolation PolicyPuspita Eka Kurnia SariÎncă nu există evaluări
- PLOTKINDocument6 paginiPLOTKINElPaisUyÎncă nu există evaluări
- Light CriteriaDocument4 paginiLight CriteriaKei Adam KurataÎncă nu există evaluări
- Balantidium ColiDocument12 paginiBalantidium Colidaenil_oliverÎncă nu există evaluări
- IWGDF Guideline On The Classification of Diabetic Foot UlcersDocument15 paginiIWGDF Guideline On The Classification of Diabetic Foot Ulcersdr. Ellen FernandaÎncă nu există evaluări
- Respiratory History PDFDocument4 paginiRespiratory History PDFsandeepÎncă nu există evaluări
- Splenic Injuries PDFDocument2 paginiSplenic Injuries PDFdessy pradessyaÎncă nu există evaluări
- Ambaji Nursing College, Ganeshpura: 2 Year B.Sc. (N) Medical Surgical Nursing-IDocument2 paginiAmbaji Nursing College, Ganeshpura: 2 Year B.Sc. (N) Medical Surgical Nursing-INEHA PANDEYÎncă nu există evaluări
- Ard - Pre - and Post-Exposure Prophylaxis For HIVDocument31 paginiArd - Pre - and Post-Exposure Prophylaxis For HIVChurschmann SpiralÎncă nu există evaluări
- Hubungan Antara Pemberian Asi Eksklusif Dengan Kejadian Ispa Pada Bayi Usia 6-12 Bulan Di Puskesmas Purwokerto BaratDocument9 paginiHubungan Antara Pemberian Asi Eksklusif Dengan Kejadian Ispa Pada Bayi Usia 6-12 Bulan Di Puskesmas Purwokerto BaratFindari RahmanÎncă nu există evaluări
- Filarial Worms AssignmentDocument3 paginiFilarial Worms AssignmentNokuthaba SiyafaÎncă nu există evaluări
- VASCULITISDocument79 paginiVASCULITISSol CamusÎncă nu există evaluări
- Duodenal UlcerDocument1 paginăDuodenal UlcerNeferterieManguinaoVillanueva100% (1)
- GMDSS Exam Schedule For Year 2022Document7 paginiGMDSS Exam Schedule For Year 2022Mani ThapaÎncă nu există evaluări
- 564-Article Text-3098-1-10-20220123Document7 pagini564-Article Text-3098-1-10-20220123Fatin syahirahÎncă nu există evaluări
- An Early Description of Painful Neuropathy in Hittite TabletsDocument1 paginăAn Early Description of Painful Neuropathy in Hittite TabletsHalim KılıçÎncă nu există evaluări
- Presentation 1Document15 paginiPresentation 1Hala YousefÎncă nu există evaluări
- Day 2 Concept MapDocument2 paginiDay 2 Concept Mapkyle frascoÎncă nu există evaluări
- Eo No. 013 - 2018 Reorganization of BhertDocument2 paginiEo No. 013 - 2018 Reorganization of BhertAnne Kimberly Peñalba BabaanÎncă nu există evaluări
- DiabeticRetPPP2014 SummaryBenchmarkPagesDocument2 paginiDiabeticRetPPP2014 SummaryBenchmarkPageskomite medikÎncă nu există evaluări
- Grade 8 HEALTH Q3 - M1Document17 paginiGrade 8 HEALTH Q3 - M1Virginia IniegoÎncă nu există evaluări
- The Decompressive CraniectomyDocument32 paginiThe Decompressive CraniectomyAmerican English CourseÎncă nu există evaluări
- Diphtheria and MeaslesDocument35 paginiDiphtheria and MeaslesMurugesanÎncă nu există evaluări
- Adhikari 2018Document7 paginiAdhikari 2018Ilham HidayatÎncă nu există evaluări
- Hypertension (Oxford American Cardiology Library) (PDFDrive)Document190 paginiHypertension (Oxford American Cardiology Library) (PDFDrive)Feyisa Abeshu100% (1)
- Neural Therapy in Practice Vol 01 No 02 2006 MayDocument1 paginăNeural Therapy in Practice Vol 01 No 02 2006 MaySamÎncă nu există evaluări
- Suharyono Stikes Rs Baptis KediriDocument69 paginiSuharyono Stikes Rs Baptis KediriDhea LaksonoÎncă nu există evaluări
- Gangguan Elminasi Pada Ibu HamilDocument10 paginiGangguan Elminasi Pada Ibu HamilRismawatiÎncă nu există evaluări