Documente Academic
Documente Profesional
Documente Cultură
De Ac Eterm Cidic Mina Solu Ation Ution Noff Ns Ferric C Ion in
Încărcat de
Henrique PiaggioTitlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
De Ac Eterm Cidic Mina Solu Ation Ution Noff Ns Ferric C Ion in
Încărcat de
Henrique PiaggioDrepturi de autor:
Formate disponibile
Titrration Applica
A tion No
ote H–119
Deetermminaation
n of ferric
f c ion in
accidic solu
ution
ns
Deteermination n of ferric ion in acidic and a coppeer-free soolutions using
u
therrmometricc titration.. The ferr ic ion is reduced
r b iodide. The released
by
iodinne reacts exothermically wheen titrated with thioosulfate soolution.
Method description
Principle * if determinations are run, where fluoride is present in
the solutions, then use the fluoride-resistant HF
Ferric ion is reduced to the ferrous state by iodide in Thermoprobe for Titrotherm (6.9011.040).
mildly acidic solutions. The iodine released reacts
exothermically when titrated by standard sodium
thiosulfate solution. Solutions
Titrant c(Na2S2O3) = 1 mol/L
[Fe3+ + e– → Fe2+] × 2
sodium thiosulfate solution
2 I– → I2 + 2 e–
glacial acetic acid
I2 + 2 e– → 2 I– c(KI) = 50% (w/v) potassium
2 S2O32– → S4O62– + 2 e– iodide
------------------------------ c(KI) = 50% (w/v) potassium
2 Fe3+ + 2 S2O32– → 2 Fe2+ + S4O62– iodide
Standard potassium iodate, KIO3,
Thus, 1 mol Fe3+ corresponds to 1 mol S2O32–
A.R., freshly dried at 110 oC
Samples Standard solution c(KIO3) = 0.1 mol/L in
«Sample solutions» were prepared from reagent grade freshly-prepared dist. water
HCl, Fe(NO3)3•9H2O, and Al(NO3)3•9H2O to approxi-
mate those that a customer desired to have analyzed. Analysis
Due to the highly concentrated nature of the customer's
own solutions, it was necessary to prepare them in such Based on the anticipated Fe3+ content of the solution,
a manner that they represented a 1:4 dilution. employ an aliquot size calculated to give an endpoint
volume of at least 1 mL, preferably 3–4 mL. Pipette this
Nominal concentration of «sample solutions»: aliquot into a titration vessel, add 2 mL glacial acetic
acid, and make to approximately 25 mL with dist. water.
HCl g/L Fe3+ g/L Al3+ g/L In this instance, 5 and 10 mL of the 1:4 diluted solutions
Sample A 36.4** * 56.9*** were used as aliquots. Use a titration program where
Sample B 30.6** * 0.5*** 5 mL of 50% (w/v) KI solution is added under stirring,
Sample C 27.1** * 56.6*** and a «wait» period of 20 seconds is allowed before the
addition of titrant commences. The titration is suitable
* Results reported below for inclusion of an «automatic stop» parameter.
** Experimental details reported in AN-H-118
*** Experimental details reported in AN-H-120 Preparation and standardization of 1 mol/L Na2S2O3
solution:
Sample preparation
Make an approximately 1 mol/L Na2S2O3 solution with
See above freshly prepared deionized water. Store and dispense
from a brown glass reagent bottle. Keep in use.
Configuration Standardization is most accurately and conveniently
Basic equipment list for automated titration performed using an automated tiamo™ program.
Aliquots ranging from 2 to 10 mL may be automatically
814 USB Sample Processor 2.814.0030 dispensed.
859 Titrotherm 2.859.0010 The tiamo™ program automatically computes the
strength of the Na2S2O3 solution by regression analysis,
Sample rack 24 x 75 mL 6.2041.340 as well as the coefficient of determination of the
Thermoprobe* 6.9011.020 analysis.
Sample beaker 75 mL 6.1459.400
802 Rod Stirrer 2.802.0010
Stirring propeller 104 mm 6.1909.020
2 × 800 Dosino 2.800.0010
Titration Application Note H–119
1 × Dosing unit 10 mL 6.3032.210
1 × Dosing unit 5 mL 6.3032.150
tiamo™ 6.6056.222
Version 1
Me
ethod descript
d ion
Param
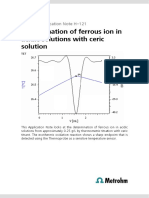
meters Titration Plots
Basic eexperimental paarameters
Titran
nt dose rate
4
(mL/mmin)
ERC EP1 (exothermic) –10
Data smooting
40
(“filteer factor”)
ng speed
Stirrin
14
(802 Rod Stirrer)
Evalu
uation start (mLL) 0.5
Damping until (mL) 0.5
Calculations
Titration of sample solution
s A
g/L Fe33+ = ((EP1 – blank) × C001 × C002)/C00
EP1 = endpoint in mL
m
C00 = sample weigh ht in mL
C001 = concentration
n of titrant in mol/L
m
C002 = molecular weeight of Fe (55.8845 g/mol)
Resultts
Standaardization of 1 mol/L Na2S2O3:
Molariity: 1.0017
1 mol/L
Blank: 0.069
0 mL
mination (R2): 1.0000
Coefficcient of determ 1
Titration of sample solution
s B
Fe3+ in g/L
ple A
Samp 76.0 ± 0.07
0
Samp
ple B 114.9 ± 0.16
0
Samp
ple C 78.3 ± 0.15
0
Titration of sample solution
s C
Titration Application Note H–119
Version 1
S-ar putea să vă placă și
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- Introduction To Analytical Chemistry: Rosemarie Ann Cuevas, R.CH., M.Sc. InstructorDocument44 paginiIntroduction To Analytical Chemistry: Rosemarie Ann Cuevas, R.CH., M.Sc. InstructorKurt BiduaÎncă nu există evaluări
- Analytical Method Development and Validation A Concise Review 2155 9872-5-233Document5 paginiAnalytical Method Development and Validation A Concise Review 2155 9872-5-233Nisrina Hasna MuthiaÎncă nu există evaluări
- SEPARATION METHODS - AnimDocument58 paginiSEPARATION METHODS - AnimSandi Mahesa0% (1)
- APV Membrane Systems For Multi Dairy ApplicationsDocument43 paginiAPV Membrane Systems For Multi Dairy ApplicationsGaytan DaygoroÎncă nu există evaluări
- Stp944-Eb 1420Document146 paginiStp944-Eb 1420delta lab sangli100% (1)
- General: Method: M3 - Titer Determination of Alkaline TitrantsDocument3 paginiGeneral: Method: M3 - Titer Determination of Alkaline TitrantsHenrique PiaggioÎncă nu există evaluări
- De Ac So Eterm Cidic Olutio Mina Solu On Ation Ution Noff Ns Wi Ferro TH Ce Us Io Eric On in NDocument3 paginiDe Ac So Eterm Cidic Olutio Mina Solu On Ation Ution Noff Ns Wi Ferro TH Ce Us Io Eric On in NHenrique PiaggioÎncă nu există evaluări
- General: Method: M1 - Calibrating The PH Glass ElectrodeDocument6 paginiGeneral: Method: M1 - Calibrating The PH Glass ElectrodeHenrique PiaggioÎncă nu există evaluări
- T-64 Ti Application Note No.: Title: Titanium and Iron in MixturesDocument1 paginăT-64 Ti Application Note No.: Title: Titanium and Iron in MixturesHenrique PiaggioÎncă nu există evaluări
- H-012 Thermo. Titr. Application Note No.: Title: Determination of Ferrous Ion Content of Heat Exchanger Wash SolutionsDocument3 paginiH-012 Thermo. Titr. Application Note No.: Title: Determination of Ferrous Ion Content of Heat Exchanger Wash SolutionsHenrique PiaggioÎncă nu există evaluări
- Metrohm - Corrosion StudiesDocument15 paginiMetrohm - Corrosion StudiesHenrique PiaggioÎncă nu există evaluări
- Autolab - EC02 - Reference Electrodes PDFDocument3 paginiAutolab - EC02 - Reference Electrodes PDFHenrique PiaggioÎncă nu există evaluări
- Electrochemical Properties of Graphene/epoxy CoatingDocument1 paginăElectrochemical Properties of Graphene/epoxy CoatingHenrique PiaggioÎncă nu există evaluări
- Full Report - Reverse Osmosis FIXDocument39 paginiFull Report - Reverse Osmosis FIXendang dian lestariÎncă nu există evaluări
- PDA-7000 Series: Shimadzu Optical Emission SpectrometerDocument16 paginiPDA-7000 Series: Shimadzu Optical Emission Spectrometerbozza85Încă nu există evaluări
- Fast Protein Liquid ChromatographyDocument5 paginiFast Protein Liquid Chromatographyd.lindabradley.1996Încă nu există evaluări
- USP-NF Atorvastatin Calcium TabletsDocument9 paginiUSP-NF Atorvastatin Calcium TabletsPhạm Đức LộcÎncă nu există evaluări
- A Microscale Experiment For Organic Chemistry: Column Chromatog Raphy of Pigments of Capsicum FrutescensDocument2 paginiA Microscale Experiment For Organic Chemistry: Column Chromatog Raphy of Pigments of Capsicum FrutescensAshleyFigueraÎncă nu există evaluări
- Cefpodoxime Proxetil PFOS ROWDocument1 paginăCefpodoxime Proxetil PFOS ROWAnonymous rHqEgpBÎncă nu există evaluări
- MSE 250: Structure and Properties of MaterialsDocument24 paginiMSE 250: Structure and Properties of MaterialsdubdubÎncă nu există evaluări
- Methods of Analysis For FluconazoleDocument6 paginiMethods of Analysis For FluconazoleJuan PerezÎncă nu există evaluări
- Acetogenins From Annonaceae Family. Their Potential Biological ApplicationsDocument43 paginiAcetogenins From Annonaceae Family. Their Potential Biological ApplicationsQuyen Do100% (1)
- C - Sol - Ch-07 - Redox Reactions and Volumetric AnalysisDocument3 paginiC - Sol - Ch-07 - Redox Reactions and Volumetric Analysismysoftinfo.incÎncă nu există evaluări
- Lab 3 Determination of Equilibrium Constant For A Chemical ReactionDocument4 paginiLab 3 Determination of Equilibrium Constant For A Chemical ReactionMarlette RaveloÎncă nu există evaluări
- Chapter 14 QuestionsDocument56 paginiChapter 14 QuestionsCaitlene Lee Uy100% (1)
- Project ThesisDocument73 paginiProject Thesissumaiya jalalÎncă nu există evaluări
- Tutorial On Laboratory CalculationDocument2 paginiTutorial On Laboratory Calculation13bellsÎncă nu există evaluări
- Analytical Profiles of Drug Substances Excipients and Related Methodology Volume 31Document380 paginiAnalytical Profiles of Drug Substances Excipients and Related Methodology Volume 31Narsinh M. Dodia67% (3)
- Polychlorinated Biphenyls PCB's by Gas Chromatography: Capillary Column Technique (EPA Method 8082/ 608)Document9 paginiPolychlorinated Biphenyls PCB's by Gas Chromatography: Capillary Column Technique (EPA Method 8082/ 608)Oktaviandri SaputraÎncă nu există evaluări
- Electrogravimetry & CoulometryDocument17 paginiElectrogravimetry & Coulometrymonica chrisdayanti100% (1)
- HydrolysisDocument18 paginiHydrolysisnav.aulakh933Încă nu există evaluări
- 201 L 4 Gravimetric AnalysisDocument24 pagini201 L 4 Gravimetric Analysismasitule nkombisaÎncă nu există evaluări
- B11 - B12 - B13 - 0301 - CHY1001 - Engineering ChemistryDocument1 paginăB11 - B12 - B13 - 0301 - CHY1001 - Engineering ChemistryUtkarsh KumarÎncă nu există evaluări
- Tyson. What Do Analytical Chemists DoDocument8 paginiTyson. What Do Analytical Chemists Dojeal92Încă nu există evaluări
- AOAC Official Method 939.05 Fat Acidity Grains Titrimetric MethodDocument1 paginăAOAC Official Method 939.05 Fat Acidity Grains Titrimetric MethodLaura TrujilloÎncă nu există evaluări
- LC-MS/MS Assay of Methylphenidate: Stability and Pharmacokinetics in HumanDocument6 paginiLC-MS/MS Assay of Methylphenidate: Stability and Pharmacokinetics in HumanasdgasdfasdfassdfasdfÎncă nu există evaluări
- Access Answers To NCERT Solutions For Class 7 Science Chapter 5Document7 paginiAccess Answers To NCERT Solutions For Class 7 Science Chapter 5Harsh KumarÎncă nu există evaluări
- DistillationDocument6 paginiDistillationpremise5274Încă nu există evaluări