Documente Academic
Documente Profesional
Documente Cultură
HardyCHROM™ Staph Aureus - Chromogenic Media For Staphylococcus Aureus Identification
Încărcat de
Seun Bandele DadaDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
HardyCHROM™ Staph Aureus - Chromogenic Media For Staphylococcus Aureus Identification
Încărcat de
Seun Bandele DadaDrepturi de autor:
Formate disponibile
HARDYCHROM STAPH AUREUS
Cat. no. G311 HardyCHROM Staph aureus, 15x100mm Plate, 18ml 10 plates/bag
INTENDED USE
HardyCHROM Staph aureus is a chromogenic medium recommended for the isolation, differentiation, and enumeration of Staphylococcus aureus by colony color.
SUMMARY
Staphylococcus aureus is a gram-positive, coagulase-positive cocci that has been well documented as a human pathogen. S. aureus has also been implicated in nosocomial infections and food poisoning outbreaks. Many S. aureus strains produce enterotoxins that cause food poisoning when ingested. Food poisoning, bacteremia, pneumonia, toxic shock syndrome, and meningitis are some of the more serious infections that can be caused by S. aureus. HardyCHROM Staph aureus allows for the rapid and reliable detection of S. aureus from both clinical and food specimens within 24 hours. Peptones in the medium supply the necessary nutrients. Selective agents inhibit the growth of gram-negative organisms, yeast, and some gram-positive cocci. Artificial substrates (chromogens) are broken down by specific microbial enzymes which release insoluble colored compounds. S. aureus uses only one of the chromogens and will produce deep pink to fuchsia colored colonies. Bacteria other than S. aureus may utilize the other chromogenic substrates and produce blue or turquoise colonies. If none of the substrates are utilized, natural or white colored colonies will be present. This medium can also be utilized in spread plate enumeration techniques.
FORMULA
Ingredients per liter of deionized water:*
Peptones Sodium Chloride Chromogenic Mixture Selective Agents Agar 40.0gm 25.0gm 4.0gm 1.0gm 15.0gm
Final pH 7.1 +/- 0.2 at 25 degrees C. * Adjusted and/or supplemented as required to meet performance criteria.
STORAGE AND SHELF LIFE
Storage: Upon receipt store at 2-8 degrees C. away from direct light. Media should not be used if there are any signs of deterioration (shrinking, cracking, or discoloration), contamination, or if the expiration date has passed. Product is light and temperature sensitive; protect from light, excessive heat, moisture, and freezing. The expiration date applies to the product in its intact packaging when stored as directed. This product has the following shelf life from the date of manufacture:
90 Days: G311 HardyCHROM Staph aureus
Refer to the keyword "Storage, in the Hardy Diagnostics software program HUGO, for more information on
storing culture media.
PRECAUTIONS
This product is in vitro diagnostic use only and is to be used only by adequately trained and qualified laboratory personnel. Observe approved biohazard precautions and aseptic techniques. All laboratory specimens should be considered infectious and handled according to "standard precautions". The "Guideline for Isolation Precautions" is available from the Centers of Disease Control and Prevention at www.cdc.gov/ncidod/dhqp/gl_isolation.html. For additional information regarding specific precautions for the prevention of the transmission of all infectious agents from laboratory instruments and materials, and for recommendations for the management of exposure to infectious disease, refer to CLSI document M29. Sterilize all biohazard waste before disposal. Refer to the keyword "Precautions", in the Hardy Diagnostics software program HUGO, for more information regarding general precautions when using culture media. Refer to the keyword "MSDS", in the Hardy Diagnostics software program HUGO, for more information on handling potentially hazardous material.
PROCEDURE
Clinical Procedure: Specimen Collection: Infectious material should be submitted directly to the laboratory without delay and protected from excessive heat and cold. If there is to be a delay in processing, the specimen should be inoculated onto an appropriate transport media and refrigerated until inoculation. Consult listed references for information on specimen collection. (2-5) Method of Use: Allow the plates to warm to room temperature. The agar surface should be dry prior to inoculating. Inoculate the specimen onto the media as soon as possible after it is received in the laboratory. If the material is being cultured from a swab, roll the swab over a small area of the agar surface and streak for isolation. Incubate plates aerobically at 35-37 degrees C. for 20 to 24 hours. Industrial Procedure: Specimen Collection: Consult listed references for information on specimen collection and processing of food, dairy, water samples and other materials of sanitary significance. (6-8) The plates should be warmed to room temperature and the agar surface should be dry before inoculating. Spread Plate Method: 1. Prepare serial dilutions in sterile diluent to obtain 30-300 CFU per plate. 2. Aseptically inoculate agar surface with 0.1ml of well mixed diluted sample. 3. Using a sterile spreader device (Cat. no. 174CS01), distribute the inoculum evenly over the agar surface. 4. Incubate plates aerobically for 20 to 28 hours at 35 degrees C.
INTERPRETATION OF RESULTS
After incubation (20-28 hours), read plates against a white background. Staphylococcus aureus will appear as smooth, deep pink to fuchsia colored colonies. Most other organisms, including Staphylococcus epidermidis will be partially to completely inhibited. Other organisms that may grow on HardyCHROM Staph aureus may appear as cream, blue, or colorless colonies. Staphylococcus saprophyticus will appear as turquoise colored colonies. Some gram-positive organisms other than S. aureus may appear as blue colonies. Spread Plate Method: Following incubation, examine the plates for growth of S. aureus. Count the number of colonies and express in number of colony forming units (CFU) per gram or milliliter of sample; take into account the dilution factor. If duplicate plates were set-up, express the average for the two plates in terms of the number of microorganisms per gram or milliliter of sample. Consult listed references for additional information on interpretation and enumeration of microbial growth on this medium. (6-8)
LIMITATIONS
Color-blind individuals may encounter difficulty in distinguishing the color differences on HardyCHROM Staph aureus.
Some non-S. aureus colonies may develop a light pink color after 48 hours. Do not incubate plates more than 24 to 28 hours. Some other staphylococcal strains may produce fuchsia colored colonies within 24 hours. Coagulase testing or latex agglutination testing should be used to confirm results. Refer to the keyword "Limitations", in the Hardy Diagnostics software program HUGO, for more information regarding general limitations on culture media.
MATERIALS REQUIRED BUT NOT PROVIDED
Standard microbiological supplies and equipment such as loops, swabs, applicator sticks, other culture media, incinerators, and incubators, etc., as well as serological and biochemical reagents, are not provided.
QUALITY CONTROL
The following organisms are routinely used for testing at Hardy Diagnostics:
Incubation Results Time Temperature Atmosphere
Test Organisms Staphylococcus aureus ATC C 25923** Staphylococcus saprophyticus ATC C 15305** Staphylococcus epidermidis ATC C 12228 Enterococcus faecalis ATC C 29212 Escherichia coli ATC C 25922**
Inoculation Method*
24hr
35C
Aerobic
Growth; smooth, deep pink to fuchsia colonies
24hr
35C
Aerobic
Growth; turquoise colonies
24hr
35C
Aerobic
Partial to complete inhibition; light pink colonies may appear after extended incubation (48 hours)
24hr
35C
Aerobic
Partial to complete inhibition; blue colonies may appear
24hr
35C
Aerobic
Partial to complete inhibition
** Recommended QC strains for User Quality Control according to the CLSI document M22 when applicable. USER QUALITY CONTROL Check for signs of contamination and deterioration. Users of commercially prepared media may be required to perform quality control testing with at least one known organism to demonstrate growth or a positive reaction; and at least one organism to demonstrate inhibition or a negative reaction (where applicable). Refer to the following keywords, in the Hardy Diagnostics software program HUGO, for more information on QC: "Introduction to QC", "QC of Finished Product", and "The CLSI (NCCLS) Standard and Recommendations for User QC of Media". Also see listed references for more information. * Refer to the keyword "Inoculation Procedures", in the Hardy Diagnostics software program HUGO, for a description of inoculation procedures.
PHYSICAL APPEARANCE
HardyCHROM Staph aureus should appear translucent, and light amber in color.
Staphylococcus aureus (ATC C 25923) colonies growing on HardyC HROM Staph aureus (C at. no. G311). Incubated aerobically for 24 hours at 35 deg. C .
Staphylococcus saprophyticus (ATC C 15305) colonies growing on HardyC HROM Staph aureus (C at. no. G311). Incubated aerobically for 24 hours at 35 deg. C .
REFERENCES
1. Anderson, N.L., et al. 2005. Cumitech 3B; Quality Systems in the Clinical Microbiology Laboratory, Coordinating ed., A.S. Weissfeld. American Society for Microbiology, Washington, D.C. 2. Murray, P.R., et al. 2003. Manual of Clinical Microbiology, 8th ed. American Society for Microbiology, Washington, D.C. 3. Forbes, B.A., et al. 2007. Bailey and Scott's Diagnostic Microbiology, 12th ed. C.V. Mosby Company, St. Louis, MO. 4. Isenberg, H.D. Clinical Microbiology Procedures Handbook, Vol. I, II & III. American Society for Microbiology, Washington, D.C. 5. Koneman, E.W., et al. 2006. Color Atlas and Textbook of Diagnostic Microbiology, 6th ed. J.B. Lippincott Company, Philadelphia, PA. 6. Standard Methods for the Examination of Dairy Products, 16th ed. 1992. APHA, Washington, D.C. 7. Compendium of Methods for the Microbiological Examination of Foods, 4th ed. 2001. APHA, Washington, D.C. 8. U.S. Food and Drug Administration. 1995. Bacteriological Analytical Manual, 8th ed. AOAC, Arlington, VA.
ATCC is a registered trademark of the American Type Culture Collection. 120909ha
HARDY DIAGNOSTICS
1430 West McCoy Lane, Santa Maria, CA 93455, USA Phone: (805) 346-2766 ext. 5658 Fax: (805) 346-2760 Website: www .HardyDiagnostics.com Email: TechService@HardyDiagnostics.com Distribution Centers: California Washington Utah Arizona Texas Ohio Florida The Hardy Diagnostics manufacturing facility and quality management system is certified to ISO 13485.
C opyright 1996 - 2011 by Ha rdy Diagnostics. All rights rese rve d.
S-ar putea să vă placă și
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Ingredients: Ep 18 - Rhubarb Trifle With Champagne Jelly and MascarponeDocument6 paginiIngredients: Ep 18 - Rhubarb Trifle With Champagne Jelly and Mascarponegrupo077Încă nu există evaluări
- Simple Present Tense and Simple Past TenseDocument7 paginiSimple Present Tense and Simple Past TenseResta MahesaÎncă nu există evaluări
- Aavin QuestionnaireDocument7 paginiAavin QuestionnaireRama padmavathi75% (8)
- Unit 4 - Holidays and CelebrationsDocument5 paginiUnit 4 - Holidays and CelebrationsMauricio Esteban Castro BastíasÎncă nu există evaluări
- Creature Feature Burskan GundarkDocument3 paginiCreature Feature Burskan GundarkSW-FanÎncă nu există evaluări
- Nesma BrochureDocument26 paginiNesma BrochurehalidÎncă nu există evaluări
- Recipe For A Healthy Fruit Salad Activity CardDocument4 paginiRecipe For A Healthy Fruit Salad Activity CardboobooÎncă nu există evaluări
- Rules of Subject Verb Agreement Are All You NeedDocument8 paginiRules of Subject Verb Agreement Are All You Needalexandra jacobÎncă nu există evaluări
- Coc2: Conduct of Competency Assessment Assessors Script: A. PrologueDocument4 paginiCoc2: Conduct of Competency Assessment Assessors Script: A. PrologueKringmehÎncă nu există evaluări
- Jelovnik / MenuDocument15 paginiJelovnik / MenuervinpoljakÎncă nu există evaluări
- 100 Things To Do in Columbia SCDocument1 pagină100 Things To Do in Columbia SCKirk VigilÎncă nu există evaluări
- F2F Workbook - AnkiDocument89 paginiF2F Workbook - AnkiTiago Silva100% (1)
- US Food System FactsheetDocument2 paginiUS Food System Factsheetmatthew_hoffman_19Încă nu există evaluări
- Chicas Poderosas-Revised VersionDocument104 paginiChicas Poderosas-Revised Versionmiple04Încă nu există evaluări
- Company Profile PT - HMSDocument10 paginiCompany Profile PT - HMSmasindraÎncă nu există evaluări
- Lab 2 Assignment: Cnidaria and Porifera BIOL 2P92 - 09 Romil Patel (5844196) TA: Fiona Tuesday, January 24, 2017Document8 paginiLab 2 Assignment: Cnidaria and Porifera BIOL 2P92 - 09 Romil Patel (5844196) TA: Fiona Tuesday, January 24, 2017RomilPatelÎncă nu există evaluări
- Baked Mac and CheeseDocument7 paginiBaked Mac and Cheeseapi-551082382Încă nu există evaluări
- CVDocument2 paginiCVJohn AttahÎncă nu există evaluări
- Energy Drink Exploritory PaperDocument7 paginiEnergy Drink Exploritory PaperJohn GoodmanÎncă nu există evaluări
- Talking Tesco: How We CompeteDocument28 paginiTalking Tesco: How We Competepjr007Încă nu există evaluări
- Coordinating Conjunctions: For and Nor But or Yet SoDocument10 paginiCoordinating Conjunctions: For and Nor But or Yet SoMariviv Rachell0% (1)
- 2ND Summative Test in Tle 10Document3 pagini2ND Summative Test in Tle 10FeGenGelsanoSarong100% (1)
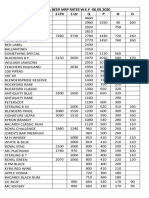
- Liquor & Beer MRP Rates Wef 17.12.2019 (1) - 1Document2 paginiLiquor & Beer MRP Rates Wef 17.12.2019 (1) - 1Kondu Krishna Vamsi ChowdaryÎncă nu există evaluări
- Tomato Based Spaghetti SauceDocument9 paginiTomato Based Spaghetti Sauceعلي محمدÎncă nu există evaluări
- Sample Integrated Marketing Communications Plan Happy Family Ice Cream MachinesDocument29 paginiSample Integrated Marketing Communications Plan Happy Family Ice Cream MachinesrodolfoÎncă nu există evaluări
- DEMO Mam LynDocument6 paginiDEMO Mam LynJhen NatividadÎncă nu există evaluări
- MM Module 1 A Prelude To The World of MarketingDocument11 paginiMM Module 1 A Prelude To The World of MarketingAbhishek MukherjeeÎncă nu există evaluări
- DietaDocument10 paginiDietaAlexa VasileÎncă nu există evaluări
- Collection of Easy To Follow Salad RecipesDocument52 paginiCollection of Easy To Follow Salad RecipesmasurimasoodÎncă nu există evaluări
- 4ds0315e P2 (F)Document1 pagină4ds0315e P2 (F)nagravÎncă nu există evaluări