Documente Academic
Documente Profesional
Documente Cultură
Plant Tissue Culture: GENE 251/351
Încărcat de
Mahesh KhyadeDescriere originală:
Titlu original
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Plant Tissue Culture: GENE 251/351
Încărcat de
Mahesh KhyadeDrepturi de autor:
Formate disponibile
GENE 251/351
Plant tissue culture
Definitions The sterile culture of plant cells, tissues or organs under conditions which lead to cell multiplication or regeneration of organs or whole plants. Tissue culture relies on three fundamental abilities of pla nts: 1- Totipotency is the potential or inherent capacity of a plant cell or tissue to develop into an entire plant if suitably stimulated. Totipotency implies that all the information necessary for growth and reproduction of the organism is contained in the cell. Although theoretically all plant cells are totipotent the meristematic cells are best able to express it. 2- Dedifferentiation is the capacity of mature cells to return to meristematic condition and development of a new growing point 3- Competency describes the endogenous potential of a given cell or tissue to develop in a particular way. For example, as embryogenically competent cells are capable of developing into fully functional embryos. The opposite is non-competent or morphogenetically incapable. Types of in vitro culture: culture of intact plants e.g. seed culture in orchids 2. embryo culture e.g. immature embryo culture 3. organ culture e.g. meristem culture, shoot tip culture root culture anther culture 4. callus culture 5. single cell culture 6. Protoplast culture. Historical perspectives: from theory of cell totipotency to molecular biotechnology Advantages of tissue culture: genetically identical virus - indexed germplasm maintenance hybrid production for incompatible species haploid plants year round production difficult to propagate species New variants research tool Disadvantages: requires expensive facilities particular skills are required error in maintenance of identity introduction of unknown pathogens or appearance of an unobserved mutant 1.
Acram Taji - 2003
GENE 251/351
Aspects of pollination biology and in vitro seed set
1. IN VITRO POLLINATION AND FERTILISATION
Success of in vitro pollination depends upon two basic considerations: (i) (ii) correct stage of development of pollen grain and ovule nutrient medium
Success of in vitro fertilisation (measured by production of viable seeds) is dependent upon the following factors: (i) (ii) (iii) (iv) (v) age of the explants - particularly that of the ovule adequate pollen germination successful microsporogenesis success in pollen tube entry into ovules others
Application of in vitro pollination and fertilisation: (i) (ii) (iii) (iv) 2. production of hybrids overcoming sexual self-incompatibility (homomorphic incompatibility) induction of haploid plants research tool to study pollen physiology and fertilisation
EMBRYO CULTURE
The underlying principle of embryo rescue is the aseptic excision of the embryo and its transfer to a suitable medium for development under optimum conditions. Applications of embryo culture: (i) (ii) (iii) (iv) (v) (vi) (vii) overcoming embryo inviability germination of seeds of obligatory parasite without the presence of host overcoming seed dormancy monoploid production in barley prevention of embryo abortion with early ripening stone fruits vegetative propagation tool for research in experimental embryogenesis
Problem of embryo culture. precocious germination
Acram Taji, 2003
GENE 251/351
Aspects of androgenesis
1. METHODS OF HAPLOID PLANT PRODUCTION IN VIVO (i) Gynogenesis Development from eggs penetrated by sperm but not fertilised. (ii) Androgenesis Development of a haploid individual from a pollen grain. (iii) Genome elimination Fertilisation occurs but soon afterwards one genome is eliminated. (iv) Semigamy In this case the nucleus of the egg-cell and the generative nucleus of the germinated pollen grain divide independently,resulting in a haploid chimera. (v) Chemical treatment Chromosome elimination can be induced by addition of certain chemicals. (vi) Shock treatment With high or low temperatures. (vii) Irradiation With X-rays or UV light. 2. METHOD OF HAPLOID PLANT PRODUCTION IN VITRO
Androgenesis is the production of haploid plants originating from very young pollen cell. In this process, which virtually takes place in vitro, the vegetative or generative nucleus of a pollen grain is stimulated to develop into a haploid individual without undergoing fertilisation. 3. SUCCESS OF ANDROGENESIS INVOLVES TWO IMPORTANT CONSIDERATIONS (i) (ii) physiological state of the donor plant the aptitude and the conditions necessary to modify the normal development of the pollen and to force it towards embryogenesis. This embryogenesis may be: (a) direct (b) indirect
4.
CONDITION OF DONOR PLANT (i) nutrition (ii) light regime (iii) watering (iv) fungicide and pesticide application (v) plant variety CONDITIONS AFFECTING ANDROGENESIS (i) (ii) low temperature treatment nutrition of medium
5.
(iii) (iv) (v)
major elements minor elements sugar plant growth regulators environmental conditions liquid vs solid medium light quality activated charcoal
6.
REGENERATION OF DIPLOID PLANTS (i) (ii) spontaneous doubling of chromosomes by endomitosis treatment with colchicine (a) at the time of the first pollen mitosis, just before the in vitro culture (b) after the production of haloid plants in vitro
7.
IMPORTANCE OF ANDROGENESIS
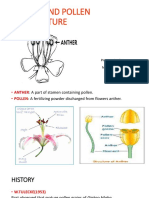
The time necessary to have an isogenic line is reduced from 5 or 6 generations to 1 or 2 using the technique of androgenesis. Acram Taji, 2003 ______________________________________________________________________ Anther Squash Te chnique To determine the stage of microspore (pollen) development within the anther it is necessary to examine the microspores. 1. 2. Collect anthers from flower buds at various stages of development. Place anther on a clean slide, add a drop of 0.8% aceto-orcein stain in 40% acetic acid. (Use1% orcein in 50% acetic acid or 1 g orcein in 50 mL glacial acetic acid. Boil in a flask with a funnel on it, and immediately remove from heat. Cool; add 50 mL distilled water. Stock: to 100 mL of preceding solution add 25 mL water). Gently macerate tissue with scalpel. Push large pieces of anther wall to the side using a scalpel. Gently heat slide, being careful not to scorch it. (Touch the slide to the back of your hand to test how hot it is. If it is too hot for your hand, it is too hot!) As stain evaporates, add more; continue for 5-10 minutes. This allows nuclear material to take up stain. Let slide sit for 5-10 minutes. Place cover slip over material.
3. 4. 5.
6. 7.
8.
Place slide on paper towel, and fold towel over slide; gently and evenly press cover slip. Do not break cover slip. Observe under microscope.
9.
S-ar putea să vă placă și
- 5B-Plant Tissue Culture PDFDocument50 pagini5B-Plant Tissue Culture PDFSharvind Kumar Sharvind Kumar100% (1)
- Anther Pollen Ovule Culture TechniquesDocument11 paginiAnther Pollen Ovule Culture TechniquesVARTIKA KHATI100% (1)
- Unit 4 - Haploid, Embryo, and Protoplast CultureDocument54 paginiUnit 4 - Haploid, Embryo, and Protoplast CultureAnonymous 3j6NRaSt267% (3)
- Anther CultureDocument14 paginiAnther CultureJyotishna SinghÎncă nu există evaluări
- Botany Unit 1Document16 paginiBotany Unit 1Hunter Gosai100% (1)
- ANTHER CULTURE TECHNIQUESDocument5 paginiANTHER CULTURE TECHNIQUESYogish HegdeÎncă nu există evaluări
- MicropropagationDocument6 paginiMicropropagationEmmanuel OcanseyÎncă nu există evaluări
- Kultur Jaringan Tumbuhan Kel 2Document26 paginiKultur Jaringan Tumbuhan Kel 2sitimeilviÎncă nu există evaluări
- Cultivation of VirusDocument45 paginiCultivation of VirusPRANAB DEYÎncă nu există evaluări
- Lectut BTN 303 PDF Micropropagation PDFDocument22 paginiLectut BTN 303 PDF Micropropagation PDFqwertÎncă nu există evaluări
- 2# Plant Cell and Tissue CultureDocument33 pagini2# Plant Cell and Tissue CultureNuria Azmi Fadhila100% (1)
- In Vitro Plant Propagation - A Review - Kumar 2011Document12 paginiIn Vitro Plant Propagation - A Review - Kumar 2011Santiago RojasÎncă nu există evaluări
- Lect 8 in Vitro Pollination Fertilization and Embryo Culture PDFDocument83 paginiLect 8 in Vitro Pollination Fertilization and Embryo Culture PDFSachin Maurya100% (1)
- Lect-8 in Vitro Pollination, Fertilization and Embryo CultureDocument83 paginiLect-8 in Vitro Pollination, Fertilization and Embryo Culturethinkkethi710187% (23)
- Lecture 2.1 GEB-2103Document46 paginiLecture 2.1 GEB-2103Niloy GhoshÎncă nu există evaluări
- Anther and Pollen CultureDocument25 paginiAnther and Pollen CultureMoharnab Sandillya100% (2)
- Aula 5Document38 paginiAula 5Ana Patricia MartinsÎncă nu există evaluări
- In Vitro Clonal Propagation TechniquesDocument16 paginiIn Vitro Clonal Propagation TechniquesArsh Raj SinghÎncă nu există evaluări
- Micropropagation: " The Art and Science of Multiplying Plants in Vitro."Document14 paginiMicropropagation: " The Art and Science of Multiplying Plants in Vitro."N DÎncă nu există evaluări
- 12 Production of Haploid PlantsDocument4 pagini12 Production of Haploid PlantsMonishaÎncă nu există evaluări
- Chapter # 5 Lecture # 1 Callus CultureDocument7 paginiChapter # 5 Lecture # 1 Callus CulturekhakyÎncă nu există evaluări
- Cultivationofviruses 151110102212 Lva1 App6892Document38 paginiCultivationofviruses 151110102212 Lva1 App6892Diana Intan Gabriella LusianasuwandiÎncă nu există evaluări
- Fundamentals of Cell CultureDocument10 paginiFundamentals of Cell CultureGimhani RaigamaÎncă nu există evaluări
- Get Discounts and FREE Study Material at www.PICKMYCOACHING.comDocument35 paginiGet Discounts and FREE Study Material at www.PICKMYCOACHING.comMohitÎncă nu există evaluări
- زراعة انسجة نباتيةDocument127 paginiزراعة انسجة نباتيةHewa HusenÎncă nu există evaluări
- Plant Tissue CultureDocument71 paginiPlant Tissue CultureShree Krishna Adhikari100% (6)
- By-Md. Zikurullah Shamim M.Sc. Agril. Biotech & Mol - Biology Instructor - Dr. Harsh Kumar Uni. Professor Dep. of Agril. Biotech & Mol - BiologyDocument20 paginiBy-Md. Zikurullah Shamim M.Sc. Agril. Biotech & Mol - Biology Instructor - Dr. Harsh Kumar Uni. Professor Dep. of Agril. Biotech & Mol - Biology12345676543Încă nu există evaluări
- 12th STD Bio-Botany Lesson-5 EM Book Back Answers-1Document3 pagini12th STD Bio-Botany Lesson-5 EM Book Back Answers-1D Ashok KumarÎncă nu există evaluări
- PLANT TISSUE CULTURE: HAPLOID CULTUREDocument13 paginiPLANT TISSUE CULTURE: HAPLOID CULTURErajiv pathak100% (1)
- Application of Tissue Culture in Plant BreedingDocument7 paginiApplication of Tissue Culture in Plant BreedingNasir Hussain Faraz89% (9)
- Viral Isolation Methods: RuhsyahadatiDocument37 paginiViral Isolation Methods: RuhsyahadatiHanung PujanggaÎncă nu există evaluări
- Chapter 11Document15 paginiChapter 11Zeeshan UsmaniÎncă nu există evaluări
- Introduction To Plant Tissue Culture PDFDocument5 paginiIntroduction To Plant Tissue Culture PDFDrAmit VermaÎncă nu există evaluări
- Techniques in Biotechnology: Ma. Joy O. Jocosol Eastern Samar State UniversityDocument56 paginiTechniques in Biotechnology: Ma. Joy O. Jocosol Eastern Samar State UniversityRazsel ColladoÎncă nu există evaluări
- Basics of Plant Tissue Culture Lab ReportDocument5 paginiBasics of Plant Tissue Culture Lab ReportFatimaKhadija AbdulÎncă nu există evaluări
- Haploid Production and UsesDocument11 paginiHaploid Production and Usesjaber AzimÎncă nu există evaluări
- Anther and Pollen CultureDocument20 paginiAnther and Pollen CultureMDZIKRULLAHSHAMIMÎncă nu există evaluări
- An Introduction To Plant Tissue Culture: Advances and PerspectivesDocument12 paginiAn Introduction To Plant Tissue Culture: Advances and PerspectivesFernanda QuelÎncă nu există evaluări
- MicropropagationDocument30 paginiMicropropagationBrigitte ReyesÎncă nu există evaluări
- Plant Tissue Culture: Biology-100 Section: 7 DR - Jebunnahar KhandakarDocument17 paginiPlant Tissue Culture: Biology-100 Section: 7 DR - Jebunnahar Khandakarijaj imran100% (2)
- Plant Tissue Culture TechniquesDocument17 paginiPlant Tissue Culture Techniquesrajiv pathakÎncă nu există evaluări
- In Vitro Fertilization (IVF) : PresentationDocument25 paginiIn Vitro Fertilization (IVF) : PresentationAnshul RanaÎncă nu există evaluări
- Micro Propagation: Some of The Important Plants That Have Been Micro Propagated On Large Scales AreDocument59 paginiMicro Propagation: Some of The Important Plants That Have Been Micro Propagated On Large Scales AreVijayÎncă nu există evaluări
- Types of plant tissue culture techniquesDocument2 paginiTypes of plant tissue culture techniquesRuss Dantes100% (2)
- Cultivation and Isolation of VirusDocument4 paginiCultivation and Isolation of VirusAlison 123 Parajuli 123Încă nu există evaluări
- Plant tissue culture and protoplast culture techniquesDocument5 paginiPlant tissue culture and protoplast culture techniquesSohail AfzalÎncă nu există evaluări
- Presentation1 171022084035Document16 paginiPresentation1 171022084035Akkipero123Încă nu există evaluări
- UntitledDocument17 paginiUntitledAnanya ChauhanÎncă nu există evaluări
- Anther and Pollen Culture: Presentation by Mantesh - SM PALM 7018Document23 paginiAnther and Pollen Culture: Presentation by Mantesh - SM PALM 7018Creative Mind100% (1)
- Additional Content - Zoology - Biotechnology and Its ApplicationDocument7 paginiAdditional Content - Zoology - Biotechnology and Its ApplicationPreethi SekarÎncă nu există evaluări
- Plant Tissue CultureDocument26 paginiPlant Tissue CultureNAVEENAÎncă nu există evaluări
- TC Question 5 M.NDocument3 paginiTC Question 5 M.NDoaa gamalÎncă nu există evaluări
- Explants: Irisan Organ Tanaman Yang Telah Disteril Yang Digunakan Sebagai Bahan Tanam Awal Dalam Kultur in VitroDocument48 paginiExplants: Irisan Organ Tanaman Yang Telah Disteril Yang Digunakan Sebagai Bahan Tanam Awal Dalam Kultur in VitroGerii PitaÎncă nu există evaluări
- Pl. Tissue Culture 3Document23 paginiPl. Tissue Culture 3Akash RoyÎncă nu există evaluări
- Plant Tissue Culture LaboratoryDocument25 paginiPlant Tissue Culture Laboratoryjay mishra0% (1)
- UntitledDocument70 paginiUntitledMr T KillworthÎncă nu există evaluări
- OrganogenesisDocument65 paginiOrganogenesisNavodit GoelÎncă nu există evaluări
- Invitro Pollination and FertilizationDocument18 paginiInvitro Pollination and FertilizationSenthil Alagarswamy100% (2)
- Experimental Biology with Micro-Organisms: Students' ManualDe la EverandExperimental Biology with Micro-Organisms: Students' ManualÎncă nu există evaluări
- Quantitative PhytochemicalsDocument8 paginiQuantitative PhytochemicalsMahesh KhyadeÎncă nu există evaluări
- Quantitative PhytochemicalsDocument8 paginiQuantitative PhytochemicalsMahesh KhyadeÎncă nu există evaluări
- SCP CompleteDocument13 paginiSCP Completeviveka_vgo182471100% (3)
- Plant Biotechnology LabDocument35 paginiPlant Biotechnology Labvenkatc100% (2)
- PTCDocument4 paginiPTCMahesh KhyadeÎncă nu există evaluări
- 3018Document4 pagini3018Mahesh KhyadeÎncă nu există evaluări
- Pomegranate Flower TypeDocument10 paginiPomegranate Flower TypeAki FujikawaÎncă nu există evaluări
- DCA - 5 - Marginal HollyDocument9 paginiDCA - 5 - Marginal HollyGuillermo BuragliaÎncă nu există evaluări
- Role of Seed Priming in Improving Seed G 07e993d4Document9 paginiRole of Seed Priming in Improving Seed G 07e993d4Muhammad AfnanÎncă nu există evaluări
- Geranium f1 Nano PelargoniumDocument2 paginiGeranium f1 Nano Pelargoniumgheorge alexÎncă nu există evaluări
- CS 414 - Weed Science: Section 2 Basic Weed BiologyDocument15 paginiCS 414 - Weed Science: Section 2 Basic Weed BiologyKimeu EstherÎncă nu există evaluări
- Effect of Grain Weight On Germination and Seed VigDocument4 paginiEffect of Grain Weight On Germination and Seed VigSalimo José ArmandoÎncă nu există evaluări
- 0610 w08 QP 01Document20 pagini0610 w08 QP 01Haider AliÎncă nu există evaluări
- Seeding and Transplanting GuideDocument5 paginiSeeding and Transplanting GuideAnuragÎncă nu există evaluări
- Seed Germination ProtocolDocument6 paginiSeed Germination ProtocolBrett GoetschiusÎncă nu există evaluări
- Rylott El Al 2006 the Arabidopsis Thaliana Multifunctional Protein Gene (MFP2) of Peroxisomal Β-oxidation is Essential for Seedling EstablishmentDocument12 paginiRylott El Al 2006 the Arabidopsis Thaliana Multifunctional Protein Gene (MFP2) of Peroxisomal Β-oxidation is Essential for Seedling EstablishmentLuisFrancisco BustamanteÎncă nu există evaluări
- Unit 1 Module 1 CombinedDocument10 paginiUnit 1 Module 1 Combinedapi-293001217Încă nu există evaluări
- Intergrated Science PDFDocument141 paginiIntergrated Science PDFAay Jay SequalsÎncă nu există evaluări
- How Plants SurviveDocument34 paginiHow Plants SurviveAlyzza Joy Albay100% (2)
- JPS PMR 2010 Science Skema JawapanDocument1 paginăJPS PMR 2010 Science Skema JawapanAmtazul AfeidatiÎncă nu există evaluări
- Selina Concise Biology Solutions Class 6 Chapter 2 The FlowerDocument17 paginiSelina Concise Biology Solutions Class 6 Chapter 2 The FlowerFLANTAMOCKÎncă nu există evaluări
- Domic 2017Document10 paginiDomic 2017Brenda Leidy Orosco ArangoÎncă nu există evaluări
- A 211 PlantsciDocument16 paginiA 211 Plantsciapi-230330590Încă nu există evaluări
- Virtual Garden Lab: Row Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Day 9Document6 paginiVirtual Garden Lab: Row Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Day 9Josalin RiveraÎncă nu există evaluări
- Seed Propagation of Arisaema Sikokianum AraceaeDocument5 paginiSeed Propagation of Arisaema Sikokianum AraceaeMick TalbotÎncă nu există evaluări
- AaaqsqDocument14 paginiAaaqsqaudy ricardoÎncă nu există evaluări
- Q'bic Ch. 37 Vegetative Plant DevelopmentDocument28 paginiQ'bic Ch. 37 Vegetative Plant Developmentdolt2111Încă nu există evaluări
- Produce Organic Vegetables 2013Document186 paginiProduce Organic Vegetables 2013mannalonak101Încă nu există evaluări
- Biology HL - Laboratory Worksheets - Second Edition - Pearson 2014Document48 paginiBiology HL - Laboratory Worksheets - Second Edition - Pearson 2014Gunay OmarovaÎncă nu există evaluări
- Hairy Root Induction by Agrobacterium Rhizogenes - Mediated Transformation in Paramignya Trimera For Root Biomass ProductionDocument36 paginiHairy Root Induction by Agrobacterium Rhizogenes - Mediated Transformation in Paramignya Trimera For Root Biomass ProductionHa Phuong LeÎncă nu există evaluări
- Germination Instructions PDFDocument1 paginăGermination Instructions PDFMustapha RédouaneÎncă nu există evaluări
- Reflection 3 - Fertilizer ExperimentDocument3 paginiReflection 3 - Fertilizer Experimentfern shirleyÎncă nu există evaluări
- Thinking Like A Plant - HoldregeDocument4 paginiThinking Like A Plant - HoldregeMartin BungeÎncă nu există evaluări
- Propagating OrchidsDocument4 paginiPropagating OrchidsHelenChung100% (1)
- Raising Bamboo From SeedDocument2 paginiRaising Bamboo From SeedGib CooperÎncă nu există evaluări
- Evaluating Science Experiments for StudentsDocument201 paginiEvaluating Science Experiments for Studentsobsrvr67% (3)