Documente Academic
Documente Profesional
Documente Cultură
Glomerular Filtration Rate
Încărcat de
Suresh KumarDescriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Glomerular Filtration Rate
Încărcat de
Suresh KumarDrepturi de autor:
Formate disponibile
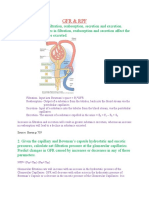
GLOMERULAR FILTRATION RATE
Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. Creatinine clearance rate (CCr or CrCl) is the volume of blood plasma that is cleared of creatinine per unit time and is a useful measure for approximating the GFR.
RENAL BLOOD FLOW & GLOMERULAR FILTRATION
Clearance
The concept of clearance is frequently used in measurements of renal blood flow and the glomerular filtration rate (GFR). The renal clearance of a substance is defined as the volume of blood that is completely cleared of that substance per unit of time (usually, per minute).
Renal Blood Flow
Renal plasma flow (RPF) is most commonly measured by p-aminohippurate (PAH) clearance. PAH at low plasma concentrations can be assumed to be completely cleared from plasma by filtration and secretion in one passage through the kidneys. Consequently
where [PAH]U = urinary concentration of PAH and [PAH]P = plasma PAH concentration.
If the hematocrit is known, then
RPF and RBF are normally about 660 and 1200 mL/min, respectively.
Glomerular Filtration Rate
The GFR is normally about 20% of RPF. Clearance of inulin, a fructose polysaccharide that is completely filtered but is neither secreted nor reabsorbed, is a good measure of GFR. Normal values for GFR are about 120 25 mL/min in men and 95 20 mL/min in women. Although less accurate than measuring inulin clearance, creatinine clearance is a much more practical measurement of GFR Creatinine clearance tends to overestimate GFR because some creatinine is normally secreted by renal tubules. Creatinine is a product of phosphocreatine breakdown in muscle. Creatinine clearance is calculated as follows:
where [creatinine]U = creatinine concentration in urine and [creatinine]P = creatinine concentration in plasma. The ratio of GFR to RPF is called the filtration fraction (FF) and is normally 20%. GFR is dependent on the relative tones of both the afferent and efferent arterioles. Afferent arteriolar dilatation or efferent arteriolar vasoconstriction can increase the FF and maintain GFR, even when RPF decreases. Afferent arteriolar tone appears to be responsible for maintaining GFR nearly constant over a wide range of blood pressures.
Control Mechanisms
Regulation of renal blood flow represents a complex interplay between intrinsic autoregulation, tubuloglomerular balance, and hormonal and neuronal influences.
INTRINSIC REGULATION Autoregulation of RBF normally occurs between mean arterial blood pressures of 80 and 180 mm Hg. Blood flow is generally decreased at mean arterial pressures less than 70 mm Hg.
Although the exact mechanism is not known, it is thought to be an intrinsic myogenic response of the afferent arterioles to changes in blood pressure. Within these limits, RBF (and GFR) can be kept relatively constant by afferent arteriolar vasoconstriction or vasodilation. Outside the autoregulation limits, RBF becomes pressure dependent. Glomerular filtration generally ceases when mean systemic arterial pressure is less than 4050 mm Hg.
TUBULOGLOMERULAR BALANCE AND FEEDBACK Changes in renal tubular flow rates affect GFR: Increases in tubular flow tend to reduce GFR, whereas decreases in flow tend to favor increases in GFR. Tubuloglomerular feedback probably plays an important role in maintaining GFR constant over a wide range of perfusion pressures. Although the mechanism is poorly understood, the macula densa appears to be responsible for tubuloglomerular feedback by inducing reflex changes in afferent arteriolar tone and possibly glomerular capillary permeability. Angiotensin II probably plays a permissive role in this mechanism. Local release of adenosine (which occurs in response to volume expansion) may inhibit renin release and dilate the afferent arteriole. The phenomenon of pressure natriuresis, or decreased sodium reabsorption in response to increases in blood pressure, likely reflects tubuloglomerular feedback. HORMONAL REGULATION Increases in afferent arteriolar pressure stimulate renin release and formation of angiotensin II. Angiotensin II causes generalized arterial vasoconstriction and secondarily reduces RBF. Both afferent and efferent arterioles are constricted but because the efferent arteriole is smaller, its resistance becomes greater than that of the afferent arteriole; GFR therefore tends to be relatively preserved. Very high levels of angiotensin II constrict both arterioles and can markedly decrease GFR. Adrenal catecholamines (epinephrine and norepinephrine) directly and preferentially increase afferent arteriolar tone, but marked decreases in GFR are minimized indirectly through activation of renin release and angiotensin II formation.
Relative preservation of GFR during increased aldosterone or catecholamine secretion appears to be at least partly mediated by angiotensin-induced prostaglandin synthesis and is blocked by inhibitors of prostaglandin synthesis (nonsteroidal antiinflammatory drugs). Renal synthesis of vasodilating prostaglandins (PGD2, PGE2, and PGI2) is an important protective mechanism during periods of systemic hypotension and renal ischemia. ANP is released from atrial myocytes in response to distention. ANP is a direct smooth muscle dilator and antagonizes the vasoconstrictive action of norepinephrine and angiotensin II. It appears to preferentially dilate the afferent arteriole, may constrict the efferent arteriole, and relaxes mesangial cells, effectively increasing GFR. ANP also inhibits both the release of renin and angiotensin-induced secretion of aldosterone, and antagonizes the action of aldosterone in the distal and collecting tubules.
NEURONAL REGULATION Sympathetic outflow from the spinal cord at T4L1 reaches the kidneys via the celiac and renal plexuses. Sympathetic nerves innervate the juxtaglomerular apparatus ( 1) as well as the renal vasculature ( 1). This innervation is probably responsible for stress-induced reductions in RBF.
1
-Adrenergic receptors enhance sodium reabsorption in proximal tubules, whereas
receptors decrease such reabsorption and promote water excretion. Dopamine and fenoldopam dilate afferent and efferent arterioles via D1-receptor activation. Unlike dopamine, fenoldopam is selective for the D1-receptor. Fenoldopam and low-dose dopamine infusion can at least partially reverse norepinephrineinduced renal vasoconstriction. Activation of D2-receptors on presynaptic postganglionic sympathetic neurons can also vasodilate arterioles through inhibition of norepinephrine secretion (negative feedback). Dopamine, which is formed in the proximal tubules as well as released from nerve endings, reduces proximal reabsorption of Na+. Some cholinergic vagal fibers are also present, but their role is poorly understood.
S-ar putea să vă placă și
- Renin - Angiotensin System RAS Renin - Angiotensin-Aldosterone System RaasDocument3 paginiRenin - Angiotensin System RAS Renin - Angiotensin-Aldosterone System RaasReynaKatÎncă nu există evaluări
- Anaesthetic Management of Ihd Patients For Non Cardiac SurgeryDocument18 paginiAnaesthetic Management of Ihd Patients For Non Cardiac SurgerySuresh KumarÎncă nu există evaluări
- NMCN Updated Past Question - 2012-2019-1Document37 paginiNMCN Updated Past Question - 2012-2019-1ChinaemeremÎncă nu există evaluări
- Renal PhysiologyDocument75 paginiRenal PhysiologyAmanuel Maru100% (1)
- Labour Analgesia - Recent ConceptsDocument18 paginiLabour Analgesia - Recent ConceptsSuresh KumarÎncă nu există evaluări
- Lecture-4.Drug Metabolism and EliminationDocument47 paginiLecture-4.Drug Metabolism and EliminationC/fataax C/xakiimÎncă nu există evaluări
- Latest Fcps McqsDocument205 paginiLatest Fcps McqsMuntaha JavedÎncă nu există evaluări
- Mechanism of Urine FormationDocument35 paginiMechanism of Urine FormationHemanth PrakashÎncă nu există evaluări
- Expt. 29 Urine FormationDocument15 paginiExpt. 29 Urine Formationapi-3769252100% (5)
- AUBF QuetionsDocument34 paginiAUBF QuetionsAprille Patol100% (1)
- Geriatric NursingDocument30 paginiGeriatric NursingVyasan Joshi100% (2)
- Glomerular Filtration & Factors Affecting GFRDocument55 paginiGlomerular Filtration & Factors Affecting GFRkhalito1991100% (1)
- Acute Renal FailureDocument6 paginiAcute Renal Failurearif kurnia timurÎncă nu există evaluări
- KDIGO 2021 BP Guideline Speakers GuideDocument60 paginiKDIGO 2021 BP Guideline Speakers GuideMuhammad Yusuf Arief Akbar100% (1)
- Bio560 OsmoregulationDocument110 paginiBio560 OsmoregulationNuratika OthmanÎncă nu există evaluări
- Alleviating Cancer PainDocument9 paginiAlleviating Cancer PainSuresh KumarÎncă nu există evaluări
- Experiment 9 - The Chemistry of UrineDocument7 paginiExperiment 9 - The Chemistry of UrineMark Ryan TripoleÎncă nu există evaluări
- Renal Blood FlowDocument17 paginiRenal Blood FlowAbu RidhuwanÎncă nu există evaluări
- L2-Regulation of GFRDocument15 paginiL2-Regulation of GFRSanjay NatarajanÎncă nu există evaluări
- 101 OHara PDFDocument11 pagini101 OHara PDFAntonio Lopez HernandezÎncă nu există evaluări
- Glomerular Filtration RateDocument5 paginiGlomerular Filtration RateMatin Ahmad KhanÎncă nu există evaluări
- Glomerular Filtration RateDocument21 paginiGlomerular Filtration RateIndra UroÎncă nu există evaluări
- Lo 1.2Document2 paginiLo 1.2robert leeÎncă nu există evaluări
- Nephron Protection in Diabetic Kidney DiseaseDocument3 paginiNephron Protection in Diabetic Kidney Diseasekhangsiean89Încă nu există evaluări
- Atrial Natriuretic Peptide (ANP) Is A 28-Amino Acid Peptide That Is Synthesized, Stored, and ReleasedDocument3 paginiAtrial Natriuretic Peptide (ANP) Is A 28-Amino Acid Peptide That Is Synthesized, Stored, and Releasedfadilah mutiaÎncă nu există evaluări
- Screenshot 2023-03-17 at 12.32.32 AMDocument1 paginăScreenshot 2023-03-17 at 12.32.32 AMRenadÎncă nu există evaluări
- Physiology RAASDocument3 paginiPhysiology RAASAulia Mahya FaradisaÎncă nu există evaluări
- Renal Notes Unit 1Document66 paginiRenal Notes Unit 1Zha-Raa'iaam L SamoriÎncă nu există evaluări
- D - Renal PhysiologyDocument26 paginiD - Renal PhysiologyMohamad Zekry ZuhairyÎncă nu există evaluări
- 25.8 Endocrine Regulation of Kidney Function - Anatomy and Physiology 2e - OpenStaxDocument3 pagini25.8 Endocrine Regulation of Kidney Function - Anatomy and Physiology 2e - OpenStaxMarlene AngwaforÎncă nu există evaluări
- UntitledDocument1 paginăUntitledugonna okoronkwoÎncă nu există evaluări
- GFR & RPF: Source: Boron p.759Document19 paginiGFR & RPF: Source: Boron p.759lazyschmoeÎncă nu există evaluări
- GUS1 - K5 - Renal Blood Flow and Glomerular FiltrationDocument23 paginiGUS1 - K5 - Renal Blood Flow and Glomerular FiltrationRudinÎncă nu există evaluări
- 25.7 Regulation of Renal Blood Flow - Anatomy and Physiology 2e - OpenStaxDocument2 pagini25.7 Regulation of Renal Blood Flow - Anatomy and Physiology 2e - OpenStaxMarlene AngwaforÎncă nu există evaluări
- Regulasi Renal & Pengaruh HormonDocument17 paginiRegulasi Renal & Pengaruh HormonAdhy al galaÎncă nu există evaluări
- Glomerular Filtration: Membrane, Which Consists of A Meshwork ofDocument3 paginiGlomerular Filtration: Membrane, Which Consists of A Meshwork ofPriscilia FooÎncă nu există evaluări
- Farmakoterapi Terapan Program Pendidikan Profesi Apoteker Medan 2011Document55 paginiFarmakoterapi Terapan Program Pendidikan Profesi Apoteker Medan 2011Ika Fitri RamadhanaÎncă nu există evaluări
- Hepato and Cardiorenal SyndromeDocument31 paginiHepato and Cardiorenal SyndromeanandababuÎncă nu există evaluări
- Patho RefDocument15 paginiPatho Ref3D - AURELIO, Lyca Mae M.Încă nu există evaluări
- Anaesthesia in Renal Failure: Nadia Van Heerden Kimberley Hospital Complex 30 January 2015Document56 paginiAnaesthesia in Renal Failure: Nadia Van Heerden Kimberley Hospital Complex 30 January 2015Khaeril IrfanÎncă nu există evaluări
- Urinary System: - Ridho IslamieDocument27 paginiUrinary System: - Ridho IslamieLaksmi DwiÎncă nu există evaluări
- Renin-Angiotensin System: I: (The Juxtaglomerular Apparatus)Document1 paginăRenin-Angiotensin System: I: (The Juxtaglomerular Apparatus)reioctabianoÎncă nu există evaluări
- Renal Considerations in Angiotensin Converting Enzyme Inhibitor TherapyDocument8 paginiRenal Considerations in Angiotensin Converting Enzyme Inhibitor TherapyCondurache Ilie-AndreiÎncă nu există evaluări
- Atrial Natriuretic PeptideDocument3 paginiAtrial Natriuretic PeptideMarri Sai SudheerÎncă nu există evaluări
- Sodium and Water Retention in Heart Failure and Diuretic Therapy: Basic MechanismsDocument6 paginiSodium and Water Retention in Heart Failure and Diuretic Therapy: Basic MechanismsAchmad DainuriÎncă nu există evaluări
- Renal Autoregulation: New Perspectives Regarding The Protective and Regulatory Roles of The Underlying MechanismsDocument27 paginiRenal Autoregulation: New Perspectives Regarding The Protective and Regulatory Roles of The Underlying MechanismsYoAmoNYCÎncă nu există evaluări
- Azotemia: Dept BiochemistryDocument25 paginiAzotemia: Dept BiochemistryPrajna PÎncă nu există evaluări
- Renal Physiology: Glomerular Filtration Andrenal Blood Flow: Emmanuel A. Gutierrez, MDDocument73 paginiRenal Physiology: Glomerular Filtration Andrenal Blood Flow: Emmanuel A. Gutierrez, MDEmmanuel GutierrezÎncă nu există evaluări
- Fluxul de Sânge RenalDocument2 paginiFluxul de Sânge RenalAurelia AlexandraÎncă nu există evaluări
- هام Vasoactive Peptides-PHL351Document4 paginiهام Vasoactive Peptides-PHL351ALNAKIÎncă nu există evaluări
- Drugs and The Kidney: Continuing Medical EducationDocument6 paginiDrugs and The Kidney: Continuing Medical EducationJANINE PASIONÎncă nu există evaluări
- Regulation of GFR Micturition ReflexDocument54 paginiRegulation of GFR Micturition ReflexadystiÎncă nu există evaluări
- Jurnal CardioDocument11 paginiJurnal Cardiomohamad safiiÎncă nu există evaluări
- Acute Renal FailureDocument5 paginiAcute Renal FailureAchmad ZainudinÎncă nu există evaluări
- Name: Class: Department: Roll No.: Subject: Course Code: Cr. HR.: Submitted To: Date of SubmissionDocument10 paginiName: Class: Department: Roll No.: Subject: Course Code: Cr. HR.: Submitted To: Date of SubmissionAnoosha FarooquiÎncă nu există evaluări
- Renin-Angiotensin SystemDocument5 paginiRenin-Angiotensin SystemZiedTrikiÎncă nu există evaluări
- 2 - The Physiology of The Afferent and Efferent ArteriolesDocument8 pagini2 - The Physiology of The Afferent and Efferent ArteriolesSharvind PrakashÎncă nu există evaluări
- Renal Blood Flow SheetDocument16 paginiRenal Blood Flow SheetAmjad AmjadÎncă nu există evaluări
- Renovascular HypertensionDocument20 paginiRenovascular Hypertensionalul847474Încă nu există evaluări
- Angiotensin Receptor Blockers (O)Document26 paginiAngiotensin Receptor Blockers (O)farmasi_hmÎncă nu există evaluări
- Short-Term Regulation of Blood PressureDocument5 paginiShort-Term Regulation of Blood PressureRae OkonÎncă nu există evaluări
- Renal PhysiologyDocument12 paginiRenal Physiologymina mounirÎncă nu există evaluări
- Cardiorenal Syndrome Schrier R (Curr Cardiol Rep 2013)Document9 paginiCardiorenal Syndrome Schrier R (Curr Cardiol Rep 2013)Luis Gerardo Alcalá GonzálezÎncă nu există evaluări
- Mechanisms of Blood Pressure Reduction With Sodium GlucoseDocument4 paginiMechanisms of Blood Pressure Reduction With Sodium GlucoseArturo GuerreroÎncă nu există evaluări
- Renal Physiology 2Document39 paginiRenal Physiology 2Hema KamatÎncă nu există evaluări
- Artigo - Hellen 01Document6 paginiArtigo - Hellen 01YuchungLeeÎncă nu există evaluări
- Hyponatremia in Cirrhosis: An Update: Joseph J. Alukal, MD, Savio John, MD and Paul J. Thuluvath, MD, FRCPDocument11 paginiHyponatremia in Cirrhosis: An Update: Joseph J. Alukal, MD, Savio John, MD and Paul J. Thuluvath, MD, FRCPMuzaffar MehdiÎncă nu există evaluări
- Mineral o CorticoidsDocument20 paginiMineral o CorticoidsAayush AdhikariÎncă nu există evaluări
- Renal Funcetion Part 3Document3 paginiRenal Funcetion Part 3Dr-Dalya ShakirÎncă nu există evaluări
- Urinary Module NotesDocument22 paginiUrinary Module NotesAthira SureshÎncă nu există evaluări
- Atrial Natriuretic PeptideDocument12 paginiAtrial Natriuretic Peptidesaad1qÎncă nu există evaluări
- Respiratory Monitoring in Mechanical Ventilation: Techniques and ApplicationsDe la EverandRespiratory Monitoring in Mechanical Ventilation: Techniques and ApplicationsJian-Xin ZhouÎncă nu există evaluări
- Medico-Legal Issues in AnaesthesiaDocument2 paginiMedico-Legal Issues in AnaesthesiaSuresh KumarÎncă nu există evaluări
- GO S For Tamilnadu DoctorsDocument160 paginiGO S For Tamilnadu DoctorsSuresh KumarÎncă nu există evaluări
- Ventilator Associated Pneumonia (Vap)Document11 paginiVentilator Associated Pneumonia (Vap)Suresh KumarÎncă nu există evaluări
- Local Anaesthetic AgentsDocument21 paginiLocal Anaesthetic AgentsSuresh KumarÎncă nu există evaluări
- Anaesthetic Management of Blunt Chest TraumaDocument11 paginiAnaesthetic Management of Blunt Chest TraumaSuresh KumarÎncă nu există evaluări
- Spinal Anesthesia For Caeserian Section: Comparison of 5.0% Lignocaine and 0.5% BupivacaineDocument4 paginiSpinal Anesthesia For Caeserian Section: Comparison of 5.0% Lignocaine and 0.5% BupivacaineSuresh KumarÎncă nu există evaluări
- Perioperative Management of A Patient With Left Ventricular FailureDocument6 paginiPerioperative Management of A Patient With Left Ventricular FailureSuresh KumarÎncă nu există evaluări
- Controversial Issues in NeuroanaesthesiaDocument12 paginiControversial Issues in NeuroanaesthesiaSuresh KumarÎncă nu există evaluări
- Cerebral Protection What Is New ?Document11 paginiCerebral Protection What Is New ?Suresh Kumar100% (1)
- Anaesthesia Care Beyond Operating Rooms: Newer Opportunities & Challenges.Document8 paginiAnaesthesia Care Beyond Operating Rooms: Newer Opportunities & Challenges.Suresh Kumar100% (1)
- Day Care AnesthesiaDocument4 paginiDay Care AnesthesiaSuresh KumarÎncă nu există evaluări
- Anaesthesia in The Gastrointestinal Endoscopy SuiteDocument3 paginiAnaesthesia in The Gastrointestinal Endoscopy SuiteSuresh KumarÎncă nu există evaluări
- Depth of Anesthesia & MonitoringDocument2 paginiDepth of Anesthesia & MonitoringSuresh KumarÎncă nu există evaluări
- Role of The Anaesthesiologists in The Management of BurnsDocument12 paginiRole of The Anaesthesiologists in The Management of BurnsSuresh KumarÎncă nu există evaluări
- Surgical Patients at Risk For Renal FailureDocument35 paginiSurgical Patients at Risk For Renal FailureSuresh KumarÎncă nu există evaluări
- Muscle Relaxants in Current PracticeDocument6 paginiMuscle Relaxants in Current PracticeSuresh KumarÎncă nu există evaluări
- Anaesthetic Management of Bleeding Obstetric PatientDocument10 paginiAnaesthetic Management of Bleeding Obstetric PatientSuresh KumarÎncă nu există evaluări
- Preoperative Anaesthetic Risk Assessment and Risk Reduction Before SurgeryDocument17 paginiPreoperative Anaesthetic Risk Assessment and Risk Reduction Before SurgerySuresh KumarÎncă nu există evaluări
- Anaesthetic Management of Patients With Pacemakers and Implantable Cardioverter Defibrillator.Document16 paginiAnaesthetic Management of Patients With Pacemakers and Implantable Cardioverter Defibrillator.Suresh KumarÎncă nu există evaluări
- Anaesthesia For Bleeding TonsilDocument6 paginiAnaesthesia For Bleeding TonsilSave MedicalEducation Save HealthCareÎncă nu există evaluări
- Anesthesia For Fetal SurgeryDocument8 paginiAnesthesia For Fetal SurgerySuresh KumarÎncă nu există evaluări
- Pre-Operative Cardio-Pulmonary Exercise Testing (Cpet)Document14 paginiPre-Operative Cardio-Pulmonary Exercise Testing (Cpet)Suresh KumarÎncă nu există evaluări
- Fluid Resuscitation in TraumaDocument17 paginiFluid Resuscitation in TraumaSuresh Kumar100% (1)
- Analysis of Arterial Blood GasesDocument17 paginiAnalysis of Arterial Blood GasesSuresh KumarÎncă nu există evaluări
- Pulmonary Function Test in Pre Anaesthetic EvaluationDocument10 paginiPulmonary Function Test in Pre Anaesthetic EvaluationSuresh KumarÎncă nu există evaluări
- Anaesthesia Breathing SystemsDocument15 paginiAnaesthesia Breathing SystemsSuresh KumarÎncă nu există evaluări
- Basic Physics Applied To AnaesthesiologyDocument10 paginiBasic Physics Applied To AnaesthesiologySuresh KumarÎncă nu există evaluări
- Chapter 26 Urinary SystemDocument17 paginiChapter 26 Urinary SystemAmy Lalringhluani100% (1)
- Renal QuizDocument10 paginiRenal QuizChezer KiethÎncă nu există evaluări
- ReninDocument4 paginiReninSarah AndrianiÎncă nu există evaluări
- Nutrition Noteworthy, 4 (1) Garcia, Marcela EsperanzaDocument7 paginiNutrition Noteworthy, 4 (1) Garcia, Marcela EsperanzadidikekoÎncă nu există evaluări
- 6.1. AntihipertensiDocument52 pagini6.1. AntihipertensiDada DoniÎncă nu există evaluări
- DoH Guidelines For The Clinical Management of People Refusing Food in Immigration Removal Centres and PrisonsDocument51 paginiDoH Guidelines For The Clinical Management of People Refusing Food in Immigration Removal Centres and PrisonsPHR IsraelÎncă nu există evaluări
- Ilovepdf MergedDocument503 paginiIlovepdf MergedRitika NigamÎncă nu există evaluări
- Physiologic Changes With Aging: Genito - Urinary System Urinary SystemDocument4 paginiPhysiologic Changes With Aging: Genito - Urinary System Urinary Systemkassy yeonÎncă nu există evaluări
- Brown 2020Document21 paginiBrown 2020dega230989Încă nu există evaluări
- Grade 12 OkayDocument223 paginiGrade 12 OkayRenen Millo BantilloÎncă nu există evaluări
- Aorta Delivers Blood Bladder Lungs Kidney Nephron Renal Vein Returns Blood Skin Ureter UrethraDocument2 paginiAorta Delivers Blood Bladder Lungs Kidney Nephron Renal Vein Returns Blood Skin Ureter UrethraMarc Jeff GabasaÎncă nu există evaluări
- ABX Pentra Urinary Proteins CPDocument4 paginiABX Pentra Urinary Proteins CPSivaÎncă nu există evaluări
- AdvancesDocument218 paginiAdvancesCristina AlexaÎncă nu există evaluări
- LP CKD FiksDocument16 paginiLP CKD FiksWahyu7.8Încă nu există evaluări
- (5145) - 204 First Year B. Pharmacy 1.2.4. Human Anatomy Physiology - Ii (2013 Pattern) (Semester-II)Document2 pagini(5145) - 204 First Year B. Pharmacy 1.2.4. Human Anatomy Physiology - Ii (2013 Pattern) (Semester-II)Kunal DangatÎncă nu există evaluări
- Histology of The Urinary SystemDocument116 paginiHistology of The Urinary SystemSandra OnceÎncă nu există evaluări
- Kidney Cara Jaga Buah PinggangDocument17 paginiKidney Cara Jaga Buah PinggangmachevallieÎncă nu există evaluări
- KT/V CalculationDocument1 paginăKT/V CalculationNor Nadiah Mohd NawawiÎncă nu există evaluări
- Chapter 08 PDFDocument18 paginiChapter 08 PDFalisha khanÎncă nu există evaluări