Documente Academic
Documente Profesional
Documente Cultură
Physiology of Nigrostriatal Pathways
Încărcat de
putai2010Descriere originală:
Drepturi de autor
Formate disponibile
Partajați acest document
Partajați sau inserați document
Vi se pare util acest document?
Este necorespunzător acest conținut?
Raportați acest documentDrepturi de autor:
Formate disponibile
Physiology of Nigrostriatal Pathways
Încărcat de
putai2010Drepturi de autor:
Formate disponibile
Physiology of Nigrostriatal Pathways The basal ganglia have a crucial role in the regulation of purposeful movement and are
the site of the pathology in Parkinson's disease. The basal ganglia do not connect directly to spinal motor neurons, and thus do not directly control the individual movements of muscles. They function instead by assisting in learning coordinated patterns of movement and by facilitating the execution of learned motor patterns. Dopamine has a central role in the operation of this system by signaling when desired movements are executed successfully and driving the learning process.
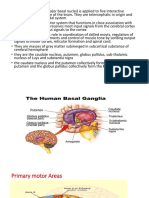
Figure The feedback circuit between the cerebral cortex and basal ganglia. Cortical inputs into the basal ganglia are excitatory (+), and the outputs of the basal ganglia back to the cortex are mediated through the thalamus. Because the outputs of the thalamus to the cortex are generally excitatory (+), the inhibitory inputs into the thalamus from the basal ganglia (-) serve to dampen thalamic excitation of cortical neurons. Anatomically, the basal ganglia form a re-entrant loop by receiving input from the cerebral cortex, processing this information in the context of dopaminergic input from the substantia nigra, and sending information back to the cortex by way of the thalamus. The internal circuitry of the basal ganglia consists of several components. The striatum (caudate and putamen) is the primary input nucleus of the system, while the globus pallidus pars interna (GPi) and substantia nigra pars reticulata (SNpr) are the output nuclei. These are interconnected through two internuclei, the subthalamic nucleus (STn) and the globus pallidus pars externa (GPe). Much of the information processing performed by the basal ganglia occurs in the striatum. The cortical inputs to this structure are excitatory and use glutamate as a transmitter. The striatum also receives dopaminergic input from substantia nigra pars compacta (SNpc). The neurons in the striatum are of several types. The majority of neurons are medium spiny neurons. These cells are studded with spines that receive
input from corticostriatal axons. These medium spiny neurons release the inhibitory transmitter GABA and send their projections to two downstream targets, forming the direct pathway and the indirect pathways. The striatum also contains several small but important populations of interneurons, including neurons that release acetylcholine. These interneurons participate in the intercommunication between the direct and indirect pathways. The balance of activity between the direct and indirect pathways regulates movement. The direct pathway, formed by striatal medium spiny neurons expressing primarily dopamine D1 receptors (they also coexpress neuropeptides such as dynorphin and substance P), projects directly to the output of the basal ganglia i.e the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNpr). The latter neurons tonically inhibit the thalamus, which in turn, sends excitatory projections to the cortex that initiate movement. In this manner, activation of the direct pathway disinhibits the thalamus; that is, the direct pathway stimulates movement. The indirect pathway, formed by striatal neurons expressing predominantly D2 receptors (they also coexpress the neuropeptide enkephalin), projects to the external segment of the globus pallidus (GPe), which in turn, inhibits neurons in the subthalamic nucleus (STn). The neurons in the subthalamic nucleus are excitatory glutamatergic neurons that project to the internal segment of the globus pallidus (GPi). As a result of this multistep pathway, activation of the indirect pathway disinhibits neurons of the subthalamic nucleus, which in turn, stimulate neurons in the internal segment of the globus pallidus to inhibit the thalamus; that is, the indirect pathway inhibits movement. The differential expression of D1 and D2 receptors within the two pathways leads to differing effects of dopaminergic stimulation. Increased levels of dopamine in the striatum tend to activate the D1-expressing neurons of the direct pathway while inhibiting the D2-expressing neurons of the indirect pathway. Notice that both of these effects promote movement. The opposite effect occurs in Parkinson's disease, a state of dopamine deficiency: the direct pathway shows reduced activity while the indirect pathway is overactive, leading to reduced movement. This model of basal ganglia function is greatly simplified, of course, but it has been useful in developing a deeper understanding of how the basal ganglia work. An important prediction of the model is that, in Parkinson's disease, the indirect pathway (and, in particular, the subthalamic nucleus) should be overactive. This prediction has been proven directly by electrical recordings in living patients with Parkinson's disease. Furthermore, surgical therapies that target the subthalamic nucleus, such as deep brain stimulation in this location, are now often used to treat Parkinson's disease when pharmacologic treatments are inadequate. Pathophysiology
Parkinson disease is caused by the death of dopamine neurons in the substantia nigra pars compacta, with consequent dopamine depletion in the caudate nucleus and putamen. Pathologic manifestations include degenerative changes, such as neuronal deterioration and depigmentation in the substantia nigra, and the appearance of intracellular inclusions called Lewy bodies in dopamine neurons. Death of dopaminergic neurons in the VTA and that of other monoaminergic neurons (eg, noradrenergic and serotonergic neurons in the brainstem) also may occur on a much smaller scale. The extent of dopamine loss is profound, with at least 80% of the neurons destroyed at the time symptoms first appear; often, 95% of the neurons are missing at autopsy. The destruction of these neurons results in the core features of the disease: bradykinesia, or slowness of movement; rigidity, a resistance to passive movement of the limbs; impaired postural balance, which predisposes to falling; and a characteristic tremor when the limbs are at rest. The mechanisms underlying the destruction of DA neurons in the substantia nigra in Parkinson's disease are not fully understood. Both environmental factors and genetic influences have been implicated. In 1983, the unexpected development of Parkinson's disease in abusers of the synthetic opioid meperidine yielded the first agent known to produce Parkinson's disease directly and the strongest evidence that environmental factors can cause Parkinson's disease. These individuals, who tended to be young and otherwise healthy, suddenly developed severe, levodopa-responsive parkinsonian symptoms. The cases were all linked to a single contaminated batch of meperidine that had been synthesized in a makeshift lab. The contaminant was found to be 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which forms as an impurity in the synthesis of meperidine when its manufacture is carried out for too long and at too high a temperature. Studies in nonhuman primates have shown that MPTP is oxidized in the brain to MPP+ (1-methyl-4-phenyl-pyridinium), which is selectively toxic to neurons in the substantia nigra. Despite extensive searches, it does not appear that there is any significant amount of MPTP present in the everyday environment, and MPTP itself is not the cause of most cases of Parkinson's disease. There may, however, be other environmental factors that have a more subtle effect on development of the disease, such as exposure to certain pesticides. Recent research has established that genetic factors may cause Parkinson's disease. The best studied examples are families with mutations in the protein -synuclein, which lead to an autosomal dominant form of Parkinson's disease. While the function of this protein is not clear, it appears to be involved in the formation of neurotransmitter vesicles and the release of dopamine in the brain. At least four other genes have been identified as causing Parkinson's disease in one or more families. These genetic discoveries have provided important clues into the biology of Parkinson's disease and have allowed the development of transgenic mouse and fruit fly models that serve as a platform for developing new treatments. Although these genetic discoveries have provided insight into the biology of Parkinson's disease, it is important to note that all of the different genetic causes identified so far account for less than 5% of cases, and most cases are still of
unknown cause. The etiology of Parkinson's disease in most patients is likely multifactorial, with contributions from both genetic and environmental factors. Role of cholinergic interneurones of the striatum There are many cholinergic neurons in the neostriatum, which are excitatory to neostriatal projection neurons that supply the external pallidal segment (indirect pathway). Therefore, it is possible that the hypokinetic disorder occurs in part because of the actions of these cholinergic neurons, whose effects become more pronounced by the reduced inhibitory actions of dopamine acting through D2 inhibitory receptors in the neostriatum. This line of reasoning represents the rationale for an approach that involves the use of anticholinergic drugs to reduce the imbalance between dopamine and acetylcholine levels in the neostriatum. In normal conditions, cholinergic and dopaminergic neurotransmission are in balance in the striatum, with a tonic dopaminergic inhibitory tone on ACh release. The loss of striatal dopaminergic terminals occurring in PD removes this tonic inhibitory control. This imbalance leads to an hyperactivity of cholinergic interneurons
Huntington's Disease/ Chorea In general, Chorea is characterized by wild, uncontrolled movements of the distal musculature, which appear as abrupt and jerky. Huntington's disease is an inherited autosomal dominant illness with the genetic defect located on the short arm of chromosome 4. The gene encodes a protein referred to as huntingtin. In the mutated form, it includes a much longer patch (than normal protein) of glutamine residues. Specifically, the DNA segment (CAG) that encodes glutamine is repeated more than 60 times in the mutated gene as opposed to approximately 20 repeats in the normal gene. Longer repeats in individuals with Huntington disease are associated with an earlier age of onset and, in some cases, with more severe symptoms. The mechanism underlying Huntington disease represents a classic example of a gain-offunction mutation. The disease is caused by a single copy of a mutant gene that leads to a new biologic effect, rather than a simple increase or decrease in the normal effect of the gene. The new function of mutant huntingtin remains incompletely understood. However, according to an emerging hypothesis, the longer the glutamine repeat is, the more likely the huntingtin protein is to accumulate within vulnerable neurons as intranuclear inclusion bodies, which are seen in the striatum of patients with Huntington disease. Such inclusion bodies lead to disruptions in critical cellular functions, including RNA processing, and eventually cause cell death. It has been hypothesized that the gain of function in mutant huntingtin is the neural toxicity of the expanded polyglutamine domain of the mutant protein.
Consistent with this theory are recent findings from mice that overexpress a mutant huntingtin gene; these animals exhibit nuclear inclusion bodies and morphologic changes in vulnerable neuronsearly signs of apoptosis that parallel the patterns of cell death associated with Huntington disease. Such mice represent models that can be used to develop new therapies. Interestingly, the huntingtin gene is widely expressed throughout the brain and most other organs; therefore, why mutant huntingtin leads to a relatively selective pathologic process within striatal GABAergic neurons remains a mystery. The normal function of huntingtin is also something of a mystery. Knockout mice that lack huntingtin die early during embryonic development, exhibiting features of apoptotic cell death. Such findings indicate that huntingtin plays a critical role in many cell types, yet investigators have yet to uncover clues to its function Neurological features Degeneration is quite extensive. It involves the neostriatum, where there is significant loss of GABA. As the disease becomes more progressive, it also involves the cerebral cortex and, in particular, the frontal and prefrontal regions, as well as a number of other structures. Damage to these regions causes not only motor damage, but also loss of intellectual functions. The disease is progressive with an onset in the fifth and sixth decades of life. There is also a juvenile form of the disease; individuals usually die before the age of 21 years. The manifestation of the hyperkinetic motor effects inherent in Huntington's disease may be understood in terms of the effects of the loss of GABAergic neurons in the neostriatum on the indirect pathway. It is believed that there is a preferential loss of GABAergic neurons that project to the external pallidal segment (GPe) (and thus the indirect pathway) compared with the GABAergic neurons that project elsewhere. As a result of the loss of inhibitory GABAergic inputs to the external pallidal segment from the neostriatum, this segment of the pallidum can now generate greater inhibition on the subthalamic nucleus. This will lead to a consequent loss of excitatory input from the subthalamic nucleus onto the internal pallidal segment (GPi). Since the internal pallidal segment normally inhibits the thalamic projection nuclei to the motor regions of the cortex, loss of subthalamic excitatory input to the internal pallidal segment will result in a consequent reduced inhibitory input onto these thalamic nuclei. This would ultimately result in excess excitation of the motor regions of the cortex, causing the choreiform movements seen in Huntington's disease.
S-ar putea să vă placă și
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe la EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeEvaluare: 4 din 5 stele4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDe la EverandShoe Dog: A Memoir by the Creator of NikeEvaluare: 4.5 din 5 stele4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)De la EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Evaluare: 4 din 5 stele4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe la EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceEvaluare: 4 din 5 stele4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe la EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersEvaluare: 4.5 din 5 stele4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe la EverandThe Little Book of Hygge: Danish Secrets to Happy LivingEvaluare: 3.5 din 5 stele3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDe la EverandGrit: The Power of Passion and PerseveranceEvaluare: 4 din 5 stele4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDe la EverandThe Emperor of All Maladies: A Biography of CancerEvaluare: 4.5 din 5 stele4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe la EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaEvaluare: 4.5 din 5 stele4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe la EverandNever Split the Difference: Negotiating As If Your Life Depended On ItEvaluare: 4.5 din 5 stele4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe la EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryEvaluare: 3.5 din 5 stele3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDe la EverandOn Fire: The (Burning) Case for a Green New DealEvaluare: 4 din 5 stele4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe la EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureEvaluare: 4.5 din 5 stele4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnDe la EverandTeam of Rivals: The Political Genius of Abraham LincolnEvaluare: 4.5 din 5 stele4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe la EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyEvaluare: 3.5 din 5 stele3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaDe la EverandThe Unwinding: An Inner History of the New AmericaEvaluare: 4 din 5 stele4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe la EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreEvaluare: 4 din 5 stele4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De la EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Evaluare: 4.5 din 5 stele4.5/5 (120)
- Her Body and Other Parties: StoriesDe la EverandHer Body and Other Parties: StoriesEvaluare: 4 din 5 stele4/5 (821)
- Adenosine and Its Receptors As Therapeutic Targets An OverviewDocument9 paginiAdenosine and Its Receptors As Therapeutic Targets An OverviewLeonelLedezmaEstradaÎncă nu există evaluări
- Internal Capsule and Horizontal Slices of ForebrainDocument5 paginiInternal Capsule and Horizontal Slices of ForebrainDenis QosjaÎncă nu există evaluări
- 1.psychiatric Neuroscience - Incorporating Pathophysiology Into Clinical Case Formulation - ClinicalKeyDocument43 pagini1.psychiatric Neuroscience - Incorporating Pathophysiology Into Clinical Case Formulation - ClinicalKeyClaudia0% (1)
- Brain Bee R1 P2Document9 paginiBrain Bee R1 P2sarahsyedazakiÎncă nu există evaluări
- Heubner ArteryDocument1 paginăHeubner Arterytiririca1901Încă nu există evaluări
- What Makes Us Tick? Functional and Neural Mechanisms Oe Interval TimincDocument12 paginiWhat Makes Us Tick? Functional and Neural Mechanisms Oe Interval TimincJuan Fran CoachÎncă nu există evaluări
- Basal GangliaDocument4 paginiBasal GangliaOvolime UbodiomÎncă nu există evaluări
- Magic Book DR Nikita 2.0 OnlineDocument263 paginiMagic Book DR Nikita 2.0 OnlineDinesh MorÎncă nu există evaluări
- Chorea in Children: Etiology, Diagnostic Approach and ManagementDocument20 paginiChorea in Children: Etiology, Diagnostic Approach and ManagementArepalli Santhosh100% (1)
- Snell Thalamus and HypothalamusDocument19 paginiSnell Thalamus and HypothalamusFrnz RiveraÎncă nu există evaluări
- Excitotoxins The Taste That KillsDocument324 paginiExcitotoxins The Taste That Killsvladaems100% (2)
- Basal Ganglia-KreitzerDocument51 paginiBasal Ganglia-KreitzerresearchphysioÎncă nu există evaluări
- Dopamine Serotonin Interactions in Attention Deficit Hyperactivity Disorder ADHDDocument30 paginiDopamine Serotonin Interactions in Attention Deficit Hyperactivity Disorder ADHDpcfernandezdiazÎncă nu există evaluări
- Unit 2 Study GuideDocument10 paginiUnit 2 Study GuideangieswensonÎncă nu există evaluări
- DopamineDocument28 paginiDopamineNTA UGC-NETÎncă nu există evaluări
- Fuad Lechin, Bertha Van Der Dijs, Gerardo Herna Ndez-Adria NDocument21 paginiFuad Lechin, Bertha Van Der Dijs, Gerardo Herna Ndez-Adria NMaria Lucrecia CrespoÎncă nu există evaluări
- Stahl's 1st Ed Child & AdolDocument388 paginiStahl's 1st Ed Child & AdolfpÎncă nu există evaluări
- Kstrapiramidal Syndrome: Pembimbing: Dr. Sigit Hari Nursjamsu, Sp. S Dr. Intan Nurswida, Sp. S Dr. Nella Lusti W., Sp. SDocument42 paginiKstrapiramidal Syndrome: Pembimbing: Dr. Sigit Hari Nursjamsu, Sp. S Dr. Intan Nurswida, Sp. S Dr. Nella Lusti W., Sp. SAmira TauhidaÎncă nu există evaluări
- Basal GangliaDocument20 paginiBasal GangliaUloko Christopher100% (1)
- Functional Neuroanatomy of BrainDocument50 paginiFunctional Neuroanatomy of BrainAsim ShresthaÎncă nu există evaluări
- American Association For The Advancement of ScienceDocument7 paginiAmerican Association For The Advancement of SciencecaeliumÎncă nu există evaluări
- Pocket Neurology (Pocket Notebook Series) (PDFDrive)Document659 paginiPocket Neurology (Pocket Notebook Series) (PDFDrive)Kirstine Gail Zingalawa-EfacÎncă nu există evaluări
- 2020 - 10 - 29 - Εγκεφαλικά Ημισφαίρια και Αγγειακά Σύνδρομα - ΜήτσιαςDocument67 pagini2020 - 10 - 29 - Εγκεφαλικά Ημισφαίρια και Αγγειακά Σύνδρομα - ΜήτσιαςΖέτα ΤσίρκαÎncă nu există evaluări
- Circuitos de La AdiccionDocument28 paginiCircuitos de La AdiccionJose Andres AlvarezÎncă nu există evaluări
- Greipl 2021-Instructor AnnotationsDocument12 paginiGreipl 2021-Instructor Annotationsprairie_fairyÎncă nu există evaluări
- Neurology MCQ IMODocument6 paginiNeurology MCQ IMONabila DaraÎncă nu există evaluări
- Neuroanatomy MCQDocument7 paginiNeuroanatomy MCQi can always make u smile :D78% (9)
- A5 CBT Inter 2016Document7 paginiA5 CBT Inter 2016lalalalalililililiÎncă nu există evaluări
- Dopamine AgonistsDocument9 paginiDopamine AgonistsCarmen-Elena DinculescuÎncă nu există evaluări
- Adhd Workshop Gpa 2016 041016c2Document128 paginiAdhd Workshop Gpa 2016 041016c2meiraimÎncă nu există evaluări